Sustainable Management of Major Fungal Phytopathogens in Sorghum (Sorghum bicolor L.) for Food Security: A Comprehensive Review
Abstract
:1. Introduction
2. Overview of Sorghum Cultivation
2.1. Distribution of Sorghum Production Worldwide
2.2. Primary Production Practices and Their Relation to Disease Prevalence
2.3. Importance of Fungal Phytopathogen Management in Sorghum Farming Systems
3. Main Fungal Diseases in Sorghum
3.1. Anthracnose in Sorghum
3.2. Grain Mold Complex (Fusarium spp., Curvularia spp., and Others)
3.3. Charcoal Rot (Macrophomina phaseolina)
3.4. Downy Mildew (Peronosclerospora sorghi)
3.5. Rust (Puccinia purpurea)
3.6. Other Significant Diseases: Brief Descriptions and Impacts
3.7. Pathogenesis and Symptomatology
4. Mycotoxins
5. Current Strategies for Managing Fungal Phytopathogens
5.1. Use of Fungicides: Advantages and Drawbacks
5.2. Biological Control Measures: Efficacy and Limitations
5.3. Efficacy of Biological Control in Sorghum
5.4. Crop Rotation and Intercropping: Benefits and Challenges
5.5. Breeding for Disease Resistance: Progress and Potential
6. Advances in Research and Fungal Phytopathogen Management
6.1. Advances in Sorghum Breeding for Disease Resistance: MAS, CRISPR, and Transformation
6.2. Predictive Modeling and Digital Agriculture: Role in Early Warning and Disease Management
6.3. Fungal Phytopathogen Management Through Digital Agriculture
7. Future Directions and Opportunities
7.1. Potential Improvements in Fungal Phytopathogen Management Strategies
7.2. Role of Technology and Digital Transformation in Improving Disease Detection and Management
7.3. Policy Recommendations for Supporting Farmers and Research in Disease Management
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medraoui, L.; Rabeh, K.; Ater, M.; Filali-Maltouf, A. Genetic diversity analysis of sorghum (Sorghum bicolor L. Moench) landraces from northwestern Morocco using ISSR and AFLP markers. Genet. Resour. Crop Evol. 2024, 71, 835–850. [Google Scholar] [CrossRef]
- Kazungu, F.K.; Muindi, E.M.; Mulinge, J.M. Overview of sorghum (Sorghum bicolor L.), its economic importance, ecological requirements and production constraints in Kenya. Int. J. Plant Soil Sci. 2023, 35, 62–71. [Google Scholar] [CrossRef]
- Assefa, Y.; Holman, J.D.; Obour, A.K.; O’Brien, D.; Prasad, P.V.V. Historic grain sorghum production, value, yield gap, and weather relation trends. Agronomy 2024, 14, 2582. [Google Scholar] [CrossRef]
- FAO. Agricultural Production Statistics. 2023. Available online: https://openknowledge.fao.org (accessed on 10 January 2024).
- Mundia, C.W.; Secchi, S.; Akamani, K.; Wang, G. A regional comparison of factors affecting global sorghum production: The case of North America, Asia and Africa’s Sahel. Sustainability 2019, 11, 2135. [Google Scholar] [CrossRef]
- Weng, W.; Yan, J.; Zhou, M.; Yao, X.; Gao, A.; Ma, C.; Cheng, J.; Ruan, J. Roles of arbuscular mycorrhizal fungi as a biocontrol agent in the control of plant diseases. Microorganisms 2022, 10, 1266. [Google Scholar] [CrossRef]
- Dutta, G.; Paramanik, B.; Bhabai, B.; Layek, J.; Choudhury, A.; Dutta, S.; Bhattacharjee, S.; Rahman, F.H. Climate change impacts and adaptation strategies for agronomic crops. In Climate Change Impacts on Soil-Plant-Atmosphere Continuum; Springer: Berlin/Heidelberg, Germany, 2024; pp. 383–404. [Google Scholar] [CrossRef]
- Abegaz, S.B. Local sorghum (Sorghum bicolor (L.) Moench) on-farm genetic diversity status and possible determinants from wollo lowland areas, northeastern Ethiopia. Agrosyst. Geosci. Environ. 2024, 7, e20518. [Google Scholar] [CrossRef]
- Pragya; Sharma, K.K.; Kumar, A.; Singh, D.; Kumar, V.; Singh, B. Immobilized phytases: An overview of different strategies, support material, and their applications in improving food and feed nutrition. Crit. Rev. Food Sci. Nutr. 2023, 63, 5465–5487. [Google Scholar] [CrossRef]
- Johansson, E.; Kuktaite, R.; Labuschagne, M.; Lama, S.; Lan, Y.; Nakimbugwe, D.; Repo-Carrasco-Valencia, R.; Tafesse, F.; Tesfaye, K.; Vazquez, D. Adaptation to abiotic stress factors and their effects on cereal and pseudocereal grain quality. In Developing Sustainable and Health Promoting Cereals and Pseudocereals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 339–358. [Google Scholar] [CrossRef]
- Novotni, D.; Gamel, T.H.; Helou, C.; Rocha, J.M. Transferring theoretical principles into practical applications: Cereals, pseudocereals, and their applications in breadmaking and other agri-food. In Developing Sustainable and Health Promoting Cereals and Pseudocereals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 399–431. [Google Scholar] [CrossRef]
- Rameez, M.; Khan, N.; Ahmad, S.; Ahmad, M.M. Bio nanocomposites: A new approach for fungal disease management. Biocatal. Agric. Biotechnol. 2024, 2024, 103115. [Google Scholar] [CrossRef]
- Mubarak Alqahtani, A. Sweet sorghum and bagasse: A comprehensive review of feedstock traits, conversion processes, and economic viability for bioethanol and biogas production. Biofuels 2024, 15, 575–585. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Jadam, R.S.; Kumar, A. Quality management system in millet and sorghum. In Millets and Millet Technology; Springer: Singapore, 2021; pp. 363–379. [Google Scholar] [CrossRef]
- Zarei, M.; Amirkolaei, A.K.; Trushenski, J.T.; Sealey, W.M.; Schwarz, M.H.; Ovissipour, R. Sorghum as a potential valuable aquafeed ingredient: Nutritional quality and digestibility. Agriculture 2022, 12, 669. [Google Scholar] [CrossRef]
- Srivastava, P.; Sangeetha, C.; Baskar, P.; Mondal, K.; Bharti, S.D.; Singh, B.V.; Agnihotri, N. Unleashing the potential of millets promoting nutritious grains as vital cereal staples during the international year of millets: A review. Int. J. Plant Soil Sci. 2023, 35, 1860–1871. [Google Scholar] [CrossRef]
- Sileshi, G.W.; Dagar, J.C.; Akinnifesi, F.K.; Mng’omba, S.A. Potentials of indigenous fruit trees in enhancing nutrition, income and biodiversity conservation in African agroforestry. In Agroforestry for Sustainable Intensification of Agriculture in Asia and Africa; Springer: Berlin/Heidelberg, Germany, 2023; pp. 321–361. [Google Scholar] [CrossRef]
- Albahri, G.; Alyamani, A.A.; Badran, A.; Hijazi, A.; Nasser, M.; Maresca, M.; Baydoun, E. Enhancing essential grains yield for sustainable food security and bio-safe agriculture through latest innovative approaches. Agronomy 2023, 13, 1709. [Google Scholar] [CrossRef]
- Yan, P.; Song, Y.-H.; Zhang, K.-Y.; Zhang, F.; Tang, Y.-J.; Zhao, X.-N.; Wang, N.; Ke, F.-L.; Gao, F.-J.; Li, J.-H. Interaction of genotype-ecological type-plant spacing configuration in sorghum (Sorghum bicolor (L.) Moench] in china. Front. Plant. Sci. 2023, 13, 1076854. [Google Scholar] [CrossRef] [PubMed]
- Periakaruppan, R.; Palanimuthu, V.; Abed, S.A.; Danaraj, J. New perception about the use of Nano fungicides in sustainable agriculture practices. Arch. Microbiol. 2023, 205, 4. [Google Scholar] [CrossRef]
- Nath, S. A vision of precision agriculture: Balance between agricultural sustainability and environmental stewardship. Agron. J. 2024, 116, 1126–1143. [Google Scholar] [CrossRef]
- Sorghum Market Size, Share & Trends Analysis Report by Type (Grain Sorghum, Forage Sorghum, Biomass Sorghum, Sweet Sorghum), by End-Use (b2b, b2c), by Region, and Segment Forecasts. 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/sorghum-market-report (accessed on 24 December 2024).
- Mayank, C. Millet Market Size, Share & Industry Analysis, by Millet Types (Finger Millet, Pearl Millet, Foxtail Millet, Sorghum), by Form (Whole Seeds, Flour), by Application (Bakery & Confectionary, Snacks & Flakes & Cereals, Ready to Cook (Noodles & Pasta, Porridge) and Regional Analysis. 2024–2031. Available online: https://www.kingsresearch.com/millet-market-568 (accessed on 24 December 2024).
- Alston, J.M.; G Pardey, P.; Rao, X. Payoffs to a half century of CGIAR research. Am. J. Agric. Econ. 2022, 104, 502–529. [Google Scholar] [CrossRef]
- De Almeida Moreira, B.R.; Hine, D.; Godwin, I.; Yadav, S. Sorghum Straw Pellets: A Dispatch Able Energy Source for Australia’s Renewable Energy Transition. 2024. Available online: https://ssrn.com/abstract=4748400 (accessed on 28 October 2024).
- Rizvi, A.; Ahmed, B.; Umar, S.; Khan, M.S. Comprehensive insights into sorghum (Sorghum bicolor) defense mechanisms unveiled: Plant growth-promoting rhizobacteria in combating Burkholderia-induced bacterial leaf stripe disease. Plant Stress 2024, 11, 100397. [Google Scholar] [CrossRef]
- Laidig, F.; Feike, T.; Klocke, B.; Macholdt, J.; Miedaner, T.; Rentel, D.; Piepho, H. Yield reduction due to diseases and lodging and impact of input intensity on yield in variety trials in five cereal crops. Euphytica 2022, 218, 150. [Google Scholar] [CrossRef]
- Benjamin, J.; Idowu, O.; Babalola, O.K.; Oziegbe, E.V.; Oyedokun, D.O.; Akinyemi, A.M.; Adebayo, A. Cereal production in Africa: The threat of certain pests and weeds in a changing climate—A review. Agric. Food Secur. 2024, 13, 18. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Sautua, F.J.; Szparaga, A.; Bohata, A.; Kocira, S.; Carmona, M.A. New tools for the management of fungal pathogens in extensive cropping systems for friendly environments. Crit. Rev. Plant Sci. 2024, 43, 63–93. [Google Scholar] [CrossRef]
- Koima, I.N.; Kilalo, D.C.; Orek, C.O.; Wagacha, J.M.; Nyaboga, E.N. Identification and characterization of Colletotrichum species causing sorghum anthracnose in Kenya and screening of sorghum germplasm for resistance to anthracnose. J. Fungi 2023, 9, 100. [Google Scholar] [CrossRef]
- Diatta, C.; Tovignan, T.K.; Sine, B.; Ifie, B.E.; Faye, J.M.; Diatta-Holgate, E.; Sylla, F.A.; Bodian, S.; Aidara, O.; Danquah, E.Y. Farmers’ production constraints, preferred varietal traits and perceptions on sorghum grain mold in Senegal. Heliyon 2024, 10, e30221. [Google Scholar] [CrossRef] [PubMed]
- Wenndt, A.; Boyles, R.; Ackerman, A.; Sapkota, S.; Repka, A.; Nelson, R. Host determinants of fungal species composition and symptom manifestation in the sorghum grain mold disease complex. Plant Dis. 2023, 107, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Gálvez, L.; Guasch, L.; Palmero, D. Fungal pathogens and seed storage in the dry state. Plants 2022, 11, 3167. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.; Shafique, M.; Khan, M.D. Understanding the interactions between plants and pathogenic microorganisms and their impact on plant health and productivity. Indus J. Biosci. Res. 2023, 1, 23–28. [Google Scholar]
- Stukenbrock, E.; Gurr, S. Address the growing urgency of fungal disease in crops. Nature 2023, 617, 31–34. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Bello, S.K. Biological control of soil-borne pathogens in arid lands: A review. J. Plant Dis. Prot. 2024, 131, 293–313. [Google Scholar] [CrossRef]
- Dahir, M.; Abera, M.; Abdullahi, H. Management of covered kernel smut (Sporisorium sorghi) disease of sorghum (Sorghum bicolor) at gabilley district in Somaliland. Int. J. Phytopathol. 2023, 12, 283–294. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Mustafa, M.; Charles, K.; Wagara, I.W.; Kappel, N. Selected emerging and reemerging plant pathogens affecting the food basket: A threat to food security. J. Agric. Food Res. 2023, 14, 100827. [Google Scholar] [CrossRef]
- Tanwar, R.; Panghal, A.; Chaudhary, G.; Kumari, A.; Chhikara, N. Nutritional, phytochemical and functional potential of sorghum: A review. Food Chem. Adv. 2023, 3, 100501. [Google Scholar] [CrossRef]
- De Nijs, E.; Maas, L.; Bol, R.; Tietema, A. Assessing the potential of co-composting rose waste as a sustainable waste management strategy: Nutrient availability and disease control. J. Clean. Prod. 2023, 399, 136685. [Google Scholar] [CrossRef]
- Fatondji, D.; Ajeigbe, H.A.; Ibrahim, A. Modern crop management practices for pearl millet cultivation in semi-arid Africa. In Pearl Millet in the 21st Century: Food-Nutrition-Climate Resilience-Improved Livelihoods; Springer: Singapore, 2024; pp. 445–477. [Google Scholar] [CrossRef]
- Ali, M.A.; Abdellah, I.M.; Eletmany, M.R. Towards sustainable management of insect pests: Protecting food security through ecological intensification. Int. J. Chem. Biochem. Sci. 2023, 24, 386–394. [Google Scholar]
- Khatri, P.; Kumar, P.; Shakya, K.S.; Kirlas, M.C.; Tiwari, K.K. Understanding the intertwined nature of rising multiple risks in modern agriculture and food system. Environ. Dev. Sustain. 2023, 26, 24107–24150. [Google Scholar] [CrossRef]
- Diancoumba, M.; Kholová, J.; Adam, M.; Famanta, M.; Clerget, B.; Traore, P.C.; Weltzien, E.; Vacksmann, M.; McLean, G.; Hammer, G.L. APSIM-based modeling approach to understand sorghum production environments in Mali. Agron. Sustain. Dev. 2024, 44, 25. [Google Scholar] [CrossRef]
- Yadav, S.S.; Arya, A.; Singh, V.; Singh, Y. Elicitation of native bio protective microbial agents associated systemic defense responses and plant growth promotion against bacterial stalk rot pathogen in sorghum (Sorghum bicolor). Phytopathol. Res. 2023, 5, 47. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Yang, L.; Rahman, S.; Sriboonchitta, S. Agricultural productivity growth and its determinants in south and southeast Asian countries. Sustainability 2020, 12, 4981. [Google Scholar] [CrossRef]
- Demarco, P.A.; Mayor, L.; Rotundo, J.L.; Prasad, P.V.; Morris, G.P.; Fernandez, J.A.; Tamagno, S.; Hammer, G.; Messina, C.D.; Ciampitti, I.A. Retrospective study in us commercial sorghum breeding: II. Physiological changes associated to yield gain. Crop Sci. 2023, 63, 867–878. [Google Scholar] [CrossRef]
- USDA-NASS. Crop Production 2022 Summary. 2022. Available online: https://www.nass.usda.gov/Quick_Stats/index.php (accessed on 15 January 2024).
- Raymundo, R.; Mclean, G.; Sexton-Bowser, S.; Lipka, A.E.; Morris, G.P. Crop modeling suggests limited transpiration would increase yield of sorghum across drought-prone regions of the United States. Front. Plant. Sci. 2024, 14, 1283339. [Google Scholar] [CrossRef]
- Khalifa, M.; Eltahir, E.A. Assessment of global sorghum production, tolerance, and climate risk. Front. Sustain. Food Syst. 2023, 7, 1184373. [Google Scholar] [CrossRef]
- Kessler-Mathieu, M.S.; Tilley, M.; Prakash, S.; Bean, S.R.; Peiris, K.H.; Aramouni, F.M. Taqman-based duplex real-time PCR approach for analysis of grain composition (Zea mays-Sorghum bicolor) in feedstock flour mixes for bioethanol production. ACS Agric. Sci. Technol. 2023, 3, 232–240. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Rejman, K.; Ganczewski, G.; Kwiatkowski, B. Economic importance of nutritional and healthy cereals and/or cereal products. In Developing Sustainable and Health Promoting Cereals and Pseudocereals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 433–450. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Alletto, L.; Cong, W.-F.; Dayoub, E.; Maury, P.; Plaza-Bonilla, D.; Reckling, M.; Saia, S.; Soltani, E.; Tison, G. Relay cropping for sustainable intensification of agriculture across temperate regions: Crop management challenges and future research priorities. Field Crops Res. 2023, 291, 108795. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, J.; Du, T.; Ju, X.; Gan, Y.; Li, S.; Xia, L.; Shen, Y.; Pacenka, S.; Steenhuis, T.S. Diversifying crop rotation increases food production, reduces net greenhouse gas emissions and improves soil health. Nat. Commun. 2024, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.K. Challenges and opportunities in managing diseases in no-till farming systems. In No-Till Farming Systems for Sustainable Agriculture: Challenges and Opportunities; Springer: Cham, Switzerland, 2020; pp. 141–154. [Google Scholar] [CrossRef]
- Jinger, D.; Kaushal, R.; Kumar, R.; Paramesh, V.; Verma, A.; Shukla, M.; Chavan, S.B.; Kakade, V.; Dobhal, S.; Uthappa, A.R. Degraded land rehabilitation through agroforestry in India: Achievements, current understanding, and future prospectives. Front. Ecol. Evol. 2023, 11, 1088796. [Google Scholar] [CrossRef]
- Wei, G.; Zhao, W.; Hu, A.; Ren, M.; Huang, Y.; Xu, H. Identification of a new pathogenic fungi causing sorghum leaf spot disease and its management using natural product and microorganisms. Microorganisms 2023, 11, 1431. [Google Scholar] [CrossRef]
- Begam, A.; Pramanick, M.; Dutta, S.; Paramanik, B.; Dutta, G.; Patra, P.S.; Kundu, A.; Biswas, A. Inter-cropping patterns and nutrient management effects on maize growth, yield and quality. Field Crops Res. 2024, 310, 109363. [Google Scholar] [CrossRef]
- Fetene, D.Y. Review of the rice blast diseases (Pyricularia oryzae) response to nitrogen and silicon fertilizers. Int. J. Res. Stud. Agric. Sci. 2019, 5, 37–44. [Google Scholar] [CrossRef]
- Angon, P.B.; Mondal, S.; Jahan, I.; Datto, M.; Antu, U.B.; Ayshi, F.J.; Islam, M.S. Integrated pest management (IPM) in agriculture and its role in maintaining ecological balance and biodiversity. Adv. Agric. 2023, 2023, 5546373. [Google Scholar] [CrossRef]
- Tonle, F.B.; Niassy, S.; Ndadji, M.M.; Tchendji, M.T.; Nzeukou, A.; Mudereri, B.T.; Senagi, K.; Tonnang, H.E. A road map for developing novel decision support system (DSS) for disseminating integrated pest management (IPM) technologies. Comput. Electron. Agric. 2024, 217, 108526. [Google Scholar] [CrossRef]
- Archer, L.; Crane, J.H.; Albrecht, U. Trunk injection as a tool to deliver plant protection materials—An overview of basic principles and practical considerations. Horticulturae 2022, 8, 552. [Google Scholar] [CrossRef]
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological control and integrated pest management in organic and conventional systems. Biol. Control. 2020, 140, 104095. [Google Scholar] [CrossRef]
- Gunny, A.A.N.; Leem, S.J.; Makhtar, M.M.Z.; Zainuddin, N.I.; Mohd Roslim, M.H.; Raja Hashim, R.H.; Pusphanathan, K.; Siddiqui, M.R.; Alam, M.; Rafatullah, M. The use of essential oil embedded in Polylactic acid/chitosan-based film for mango post-harvest application against pathogenic fungi. Polymers 2023, 15, 2722. [Google Scholar] [CrossRef] [PubMed]
- Thierfelder, C.; Mhlanga, B.; Ngoma, H.; Marenya, P.; Matin, A.; Tufa, A.; Alene, A.; Chikoye, D. Unanswered questions and unquestioned answers: The challenges of crop residue retention and weed control in conservation agriculture systems of southern Africa. Renew. Agric. Food Syst. 2024, 39, e7. [Google Scholar] [CrossRef]
- Gerling, M.; Pätzig, M.; Hempel, L.; Büttner, C.; Müller, M.E. Arable weeds at the edges of kettle holes as overwintering habitat for phytopathogenic fungi. Agronomy 2022, 12, 823. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Cunniffe, N.J.; Fedoroff, N.V.; Finegold, C.; Garrett, K.A.; Gilligan, C.A.; Jones, C.M. The persistent threat of emerging plant disease pandemics to global food security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef]
- Almogdad, M.; Jonavičienė, A.; Semaškienė, R. Bruchus rufimanus Boh. Effect on broad bean seed quality and the infection level of seed-borne fungal pathogens. Plants 2023, 12, 1825. [Google Scholar] [CrossRef]
- Shukla, S.; Upadhyay, D.; Mishra, A.; Jindal, T.; Shukla, K. Challenges faced by farmers in crops production due to fungal pathogens and their effect on Indian economy. In Fungal Diversity, Ecology and Control Management; Springer: Berlin/Heidelberg, Germany, 2022; pp. 495–505. [Google Scholar] [CrossRef]
- Oyetunji, O.E.; Ojuederie, O.B.; Thonda, O.A.; Kotun, B.; Glick, B.R.; Babalola, O.O. The expediency of fungi as biocontrol agents for the enhancement of food security. In Biocontrol Agents for Improved Agriculture; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–28. [Google Scholar] [CrossRef]
- Joshi, T.; Mandal, S.K.; Asati, V.; Deepa, P.R.; Sharma, P.K. Arid/semi-arid flora as a treasure trove of bioactives and bioenergy: The case for underutilized desert legumes towards environmental sustainability. Environ. Sci. Pollut. Res. 2023, 31, 39025–39036. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Gill, A.R.; Jewell, N.; Brien, C.J.; Berger, B.; Tran, B.T.; Mace, E.; Cruickshank, A.W.; Jordan, D.R.; Garnett, T. Enhancement of sorghum grain yield and nutrition: A role for arbuscular mycorrhizal fungi regardless of soil phosphorus availability. Plants People Planet 2022, 4, 143–156. [Google Scholar] [CrossRef]
- Patel, N.; Desai, P.; Patel, N.; Jha, A.; Gautam, H.K. Agro nanotechnology for plant fungal disease management: A review. Int. J. Curr. Microbiol. App. Sci 2014, 3, 71–84. [Google Scholar]
- Ayesha, S.; Abideen, Z.; Haider, G.; Zulfiqar, F.; El-Keblawy, A.; Rasheed, A.; Siddique, K.H.; Khan, M.B.; Radicetti, E. Enhancing sustainable plant production and food security: Understanding the mechanisms and impacts of electromagnetic fields. Plant Stress 2023, 9, 100198. [Google Scholar] [CrossRef]
- Shelare, S.D.; Belkhode, P.N.; Nikam, K.C.; Jathar, L.D.; Shahapurkar, K.; Soudagar, M.E.M.; Veza, I.; Khan, T.Y.; Kalam, M.; Nizami, A.-S. Biofuels for a sustainable future: Examining the role of nano-additives, economics, policy, internet of things, artificial intelligence and machine learning technology in biodiesel production. Energy 2023, 282, 128874. [Google Scholar] [CrossRef]
- Sharma, I.; Kumari, N.; Sharma, V. Sorghum fungal diseases. In Sustainable Agriculture Reviews. Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2015; pp. 141–172. [Google Scholar] [CrossRef]
- Abreha, K.B.; Ortiz, R.; Carlsson, A.S.; Geleta, M. Understanding the sorghum–Colletotrichum sublineola interactions for enhanced host resistance. Front. Plant. Sci. 2021, 12, 641969. [Google Scholar] [CrossRef] [PubMed]
- Mekonen, M.; Tesfaye, K.; Mengiste, T.; Chala, A.; Nida, H.; Mekonnen, T.; Abreha, K.B.; Geleta, M. Pathotype determination of sorghum anthracnose (Colletotrichum sublineola) isolates from ethiopia using sorghum differentials. Front. Microbiol. 2024, 15, 1458450. [Google Scholar] [CrossRef] [PubMed]
- Tsedaley, B.; Alemu, K. Temporal and spatial dynamics of anthracnose (Colletotrichum sublineolum) disease on selected sorghum genotypes at assosa zone, western Ethiopia. F1000Research 2024, 13, 1290. [Google Scholar] [CrossRef]
- Priyashantha, A.H.; Dai, D.-Q.; Bhat, D.J.; Stephenson, S.L.; Promputtha, I.; Kaushik, P.; Tibpromma, S.; Karunarathna, S.C. Plant–fungi interactions: Where it goes? Biology 2023, 12, 809. [Google Scholar] [CrossRef]
- Salotti, I.; Liang, Y.-J.; Ji, T.; Rossi, V. Development of a model for Colletotrichum diseases with calibration for phylogenetic clades on different host plants. Front. Plant. Sci. 2023, 14, 1069092. [Google Scholar] [CrossRef]
- Hossain, M.S.; Islam, M.N.; Rahman, M.M.; Mostofa, M.G.; Khan, M.A.R. Sorghum: A prospective crop for climatic vulnerability, food and nutritional security. J. Agric. Food Res. 2022, 8, 100300. [Google Scholar] [CrossRef]
- Berraies, S.; Walkowiak, S.; Buchwaldt, L.; Menzies, J.G. Ergot (Claviceps spp.) of cereals in western Canada. Plant Health Cases 2023, 73, 1301–1316. [Google Scholar] [CrossRef]
- Singh, Y.; Sharma, D.; Kharayat, B.S. Major diseases of sorghum and their management. In Diseases of Field Crops Diagnosis and Management; Apple Academic Press: Cambridge, MA, USA, 2020; pp. 153–182. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Wu, A.; Sun, A.; Dong, E.; Wang, Y.; Huang, X.; Hu, H.; Jiao, X. Plant-associated microorganisms during the reproductive period best predict sorghum yield and quality. Field Crops Res. 2023, 304, 109167. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A. Endophytic fungal communities and their biotechnological implications for agro-environmental sustainability. Folia Microbiol. 2022, 67, 203–232. [Google Scholar] [CrossRef]
- Du, Y.; Qi, Z.; Yu, J.; Yu, M.; Cao, H.; Zhang, R.; Yong, M.; Yin, X.; Pan, X.; Song, T. Effects of panicle development stage and temperature on rice panicle blast infection by Magnaporthe oryzae and visualization of its infection process. Plant. Pathol. 2021, 70, 1436–1444. [Google Scholar] [CrossRef]
- Abrol, S.; Singh, S.; Singh, V.; Basu, U.; Singh, R.; Singh, A.; Ahanger, S.A.; Mehta, A.; Singh, S.; Kakraliya, D.P. Effect of agro-met conditions on the progression of brown leaf spot disease in basmati-370 rice. Asian J. Microbiol. Biotechnol. Environ. Sci. Pap. 2022, 24, 335–340. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.; Vicente, S.; Castellari, C.; Mousegne, F.; Jecke, F.; Cornejo, P.; Ibañez, V.; Sansinena, M.; Vago, M.; Stenglein, S. Timing is everything: How planting period shapes nutritional quality, mycobiota characteristics, and mycotoxin contamination in maize (Zea mays) grains. Eur. J. Plant Pathol. 2024, 169, 201–217. [Google Scholar] [CrossRef]
- Ariong, R.M.; Okello, D.M.; Otim, M.H.; Paparu, P. The cost of inadequate postharvest management of pulse grain: Farmer losses due to handling and storage practices in Uganda. Agric. Food Secur. 2023, 12, 20. [Google Scholar] [CrossRef]
- Julian Maywald, N.; Francioli, D.; Mang, M.; Ludewig, U. Role of mineral nitrogen nutrition in fungal plant diseases of cereal crops. Crit. Rev. Plant Sci. 2023, 42, 93–123. [Google Scholar] [CrossRef]
- Khanal, S.; Gaire, S.P.; Zhou, X.-G. Kernel smut and false smut: The old-emerging diseases of rice—A review. Phytopathology® 2023, 113, 931–944. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Tan, Y.; Zhong, J.; Zhang, Z.; Zhu, J.Z. First report of Colletotrichum siamense causing anthracnose on Dioscorea alata in China. Plant Dis. 2023, 107, 2848. [Google Scholar] [CrossRef]
- Badmos, F.O.; Muhammad, H.L.; Dabara, A.; Adefolalu, F.; Salubuyi, S.; Abdulkadir, A.; Oyetunji, V.T.; Apeh, D.O.; Muhammad, H.K.; Mwanza, M. Assessment of dietary exposure and levels of mycotoxins in sorghum from Niger state of Nigeria. Food Addit. Contam. Part A 2024, 41, 74–90. [Google Scholar] [CrossRef]
- Singh, S.; Prashad, H.; Gautam, H. Management of Macrophomina phaseolina by cultural practices. In Macrophomina phaseolina; Elsevier: Amsterdam, The Netherlands, 2023; pp. 241–250. [Google Scholar] [CrossRef]
- Kumar, S.; Vishnoi, V.K.; Kumar, P.; Dubey, R.C. Survival of Macrophomina phaseolina in plant tissues and soil. In Macrophomina Phaseolina; Elsevier: Amsterdam, The Netherlands, 2023; pp. 205–224. [Google Scholar] [CrossRef]
- Ahn, E.; Fall, C.; Botkin, J.; Curtin, S.; Prom, L.K.; Magill, C. Inoculation and screening methods for major sorghum diseases caused by fungal pathogens: Claviceps africana, Colletotrichum sublineola, Sporisorium reilianum, Peronosclerospora sorghi and Macrophomina phaseolina. Plants 2023, 12, 1906. [Google Scholar] [CrossRef]
- Priyashantha, A.K.H.; Karunarathna, S.C.; Lu, L.; Tibpromma, S. Fungal endophytes: An alternative biocontrol agent against phytopathogenic fungi. Encyclopedia 2023, 3, 759–780. [Google Scholar] [CrossRef]
- Bellaloui, N.; Mengistu, A.; Smith, J.R.; Abbas, H.K.; Accinelli, C.; Shier, W.T. Effects of charcoal rot on soybean seed composition in soybean genotypes that differ in charcoal rot resistance under irrigated and non-irrigated conditions. Plants 2021, 10, 1801. [Google Scholar] [CrossRef]
- Punja, Z.K.; Sutton, D.B.; Kim, T. Glandular trichome development, morphology, and maturation are influenced by plant age and genotype in high thc-containing cannabis (Cannabis sativa L.) inflorescences. J. Cannabis Res. 2023, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-U.; Webber, H.; Adiku, S.G.; Júnior, R.d.S.N.; Deswarte, J.-C.; Asseng, S.; Ewert, F. Mechanisms and modelling approaches for excessive rainfall stress on cereals: Waterlogging, submergence, lodging, pests and diseases. Agric. For. Meteorol. 2024, 344, 109819. [Google Scholar] [CrossRef]
- Priya, R.S.; Yuvaraj, M.; Sharmila, R.; Jagathjothi, N.; Saranya, M.; Suganthi, N.; Subramanian, K.; Deivamani, M.; Cyriac, J.; Arthanari, P.M. Effects of climate change on plant diseases. In Plant Quarantine Challenges Under Climate Change Anxiety; Springer: Berlin/Heidelberg, Germany, 2024; pp. 183–225. [Google Scholar] [CrossRef]
- Gaur, A.; Kumar, A.; Kiran, R.; Kumari, P. Importance of seed-borne diseases of agricultural crops: Economic losses and impact on society. In Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Springer: Singapore, 2020; pp. 3–23. [Google Scholar] [CrossRef]
- Yin, H.; Tian, M.; Peng, Y.; Qin, N.; Lü, H.; Ren, L.; Zhao, X. First report on Choanephora cucurbitarum causing Choanephora rot in Chenopodium plants and its sensitivity to fungicide. J. Fungi 2023, 9, 881. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.E.; El-Tabakh, M.A.; Khattab, A.M.; Mohamed, Y.A.; Saleh, A.M.; El-Abeid, S.E. Recent advances of using innovative strategies in management of millet plant pathogens. In Genetic Improvement of Small Millets; Springer: Singapore, 2024; pp. 297–328. [Google Scholar] [CrossRef]
- Schumacher, L.A.; Grabau, Z.J.; Wright, D.L.; Small, I.M.; Liao, H.-L. Nematicide influence on cotton yield and plant-parasitic nematodes in conventional and sod-based crop rotation. J. Nematol. 2020, 52, e2020-34. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nano biopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef]
- Hernandez, A.P.; Bublitz, D.M.; Wenzel, T.J.; Ruth, S.K.; Bloomingdale, C.; Mettler, D.C.; Bloomquist, M.W.; Hanson, L.E.; Willbur, J.F. An in-field heat treatment to reduce Cercospora beticola survival in plant residue and improve Cercospora leaf spot management in sugarbeet. Front. Plant. Sci. 2023, 14, 1100595. [Google Scholar] [CrossRef]
- Drakopoulos, D.; Gimeno, A.; Kägi, A.; Jenny, E.; Bänziger, I.; Musa, T.; Forrer, H.-R.; Vogelgsang, S. Innovative cropping systems to reduce fusarium mycotoxins in wheat. Agrar. Schweiz 2021, 12, 16–23. [Google Scholar] [CrossRef]
- Benaissa, A. Rhizosphere: Role of bacteria to manage plant diseases and sustainable agriculture—A review. J. Basic Microbiol. 2024, 64, 2300361. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Sawangphruk, M. Sustainable reuse and recycling of spent Li-Ion batteries from electric vehicles: Chemical, environmental, and economical perspectives. Glob. Chall. 2023, 7, 2200212. [Google Scholar] [CrossRef]
- Muis, A.; Ryley, M.J.; Tan, Y.P.; Suharjo, R.; Nonci, N.; Danaatmadja, Y.; Hidayat, I.; Widiastuti, A.; Widinugraheni, S.; Shivas, R.G. Peronosclerospora neglecta sp. Nov.—A widespread and overlooked threat to corn (maize) production in the tropics. Mycol. Prog. 2023, 22, 12. [Google Scholar] [CrossRef]
- Crouch, J.; Davis, W.; Shishkoff, N.; Castroagudín, V.; Martin, F.; Michelmore, R.; Thines, M. Peronosporaceae species causing downy mildew diseases of Poaceae, including nomenclature revisions and diagnostic resources. Fungal Syst. Evol. 2022, 9, 43–86. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.T.O.; Prasanna, B.; Setty, T.; Rathore, R. Genetic variability for resistance to sorghum downy mildew (Peronosclerospora sorghi) and rajasthan downy mildew (P. Heteropogoni) in the tropical/sub-tropical Asian maize germplasm. Euphytica 2004, 138, 23–31. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Lakshmidevi, P.; Satya, V.; Gopalakrishnan, C. Plant Pathology and Disease Management: Principles and Practices; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar] [CrossRef]
- Gvozdenac, S.; Dedić, B.; Mikić, S.; Ovuka, J.; Miladinović, D. Impact of climate change on integrated pest management strategies. In Climate Change and Agriculture: Perspectives, Sustainability and Resilience; Wiley: Hoboken, NJ, USA, 2022; pp. 311–372. [Google Scholar] [CrossRef]
- Taibi, O.; Salotti, I.; Rossi, V. Plant resistance inducers affect multiple epidemiological components of Plasmopara viticola on grapevine leaves. Plants 2023, 12, 2938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Z.; Yong, S.; Yu, S.; Feng, H.; Yin, M.; Ye, W.; Wang, Y.; Qiu, M. An oomycete-specific leucine-rich repeat-containing protein is involved in zoospore flagellum development in Phytophthora sojae. Phytopathology® 2022, 112, 2351–2359. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Alamri, S.A.; Alrumman, S.A.; Hashem, M.; Baka, Z.A. Green synthesis of silver nanoparticles using pomegranate and orange peel extracts and their antifungal activity against Alternaria solani, the causal agent of early blight disease of tomato. Plants 2021, 10, 2363. [Google Scholar] [CrossRef]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E. Current scenario and future prospects of endophytic microbes: Promising candidates for abiotic and biotic stress management for agricultural and environmental sustainability. Microb. Ecol. 2023, 86, 1455–1486. [Google Scholar] [CrossRef]
- Choi, S.; Yang, J.W.; Kim, J.-E.; Jeon, H.; Shin, S.; Wui, D.; Kim, L.S.; Kim, B.J.; Son, H.; Min, K. Infectivity and stress tolerance traits affect community assembly of plant pathogenic fungi. Front. Microbiol. 2023, 14, 1234724. [Google Scholar] [CrossRef]
- Aslam, H.M.U.; Peters, N.T.; Killiny, N.; Riaz, H.; Hameed, A.; Aslam, S.; Shakeel, Q. An insight into infectious and noninfectious diseases of sorghum species. In Sustainable Summer Fodder; CRC Press: Boca Raton, FL, USA, 2024; pp. 58–69. [Google Scholar]
- Robert, M.K.; Charles, O.; Koima, I.N.; Muli, B.; Evans, N. Effects of major fungal pathogens on growth and yield of improved and local sorghum genotypes under field trials in lower eastern Kenya. Annu. Res. Rev. Biol. 2023, 38, 25–44. [Google Scholar] [CrossRef]
- Perumal, R.; Nimmakayala, P.; Erattaimuthu, S.R.; No, E.-G.; Reddy, U.K.; Prom, L.K.; Odvody, G.N.; Luster, D.G.; Magill, C.W. Simple sequence repeat markers useful for sorghum downy mildew (Peronosclerospora sorghi) and related species. BMC Genet. 2008, 9, 77. [Google Scholar] [CrossRef]
- Proietti, I.; Frazzoli, C.; Mantovani, A. Exploiting nutritional value of staple Foods in the World’s Semi-Arid Areas: Risks, benefits, challenges and opportunities of sorghum. Healthcare 2015, 3, 172–193. [Google Scholar] [CrossRef]
- Anitha, K.; Das, I.; Holajjer, P.; Sivaraj, N.; Reddy, C.R.; Balijepalli, S.B. Sorghum diseases: Diagnosis and management. In Sorghum in the 21st Century: Food–Fodder–Feed–Fuel for a Rapidly Changing World; Springer: Berlin/Heidelberg, Germany, 2020; pp. 565–619. [Google Scholar] [CrossRef]
- Kapanigowda, M.H.; Perumal, R.; Djanaguiraman, M.; Aiken, R.M.; Tesso, T.; Prasad, P.V.; Little, C.R. Genotypic variation in sorghum [Sorghum bicolor (L.) Moench] exotic germplasm collections for drought and disease tolerance. SpringerPlus 2013, 2, 650. [Google Scholar] [CrossRef] [PubMed]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: A review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Rawat, L.; Bisht, T.; Naithani, D.C. Plant disease management in organic farming system: Strategies and challenges. In Emerging Trends in Plant Pathology; Springer: Singapore, 2021; pp. 611–642. [Google Scholar] [CrossRef]
- Nazlı, R.İ.; Polat, M. Agronomic practices for sorghum production. In Omics and Biotechnological Approaches for Product Profile-Driven Sorghum Improvement; Springer: Berlin/Heidelberg, Germany, 2024; pp. 21–53. [Google Scholar] [CrossRef]
- Sharma, R.; Humayun, P.; Anitha, K.; Balijepalli, S.B. Harmonization of quarantine regulation and legislation for global exchange of sorghum germplasm. In Sorghum in the 21st Century: Food–Fodder–Feed–Fuel for a Rapidly Changing World; Springer: Berlin/Heidelberg, Germany, 2020; pp. 621–637. [Google Scholar] [CrossRef]
- Martins, F.C.; Batista, A.D.; Melchert, W.R. Current overview and perspectives in environmentally friendly microextractions of carbamates and dithiocarbamates. Compr. Rev. Food Sci. Food Saf. 2021, 20, 6116–6145. [Google Scholar] [CrossRef] [PubMed]
- Thind, T. Role of fungicides in crop health management: Prospects and challenges. In Developments in Fungal Biology and Applied Mycology; Springer: Singapore, 2017; pp. 433–447. [Google Scholar] [CrossRef]
- Holan, K.L.; White, C.H.; Whitham, S.A. Application of a u-net neural network to the Puccinia sorghi–maize Pathosystem. Phytopathology® 2024, 114, 990–999. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.-H.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Shafiq, M.; Ali, F.; Yu, X.-Y.; Yu, Q.; Zhao, J.-T. Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challenges, and global perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Avasthi, S.; Gautam, A.K.; Niranjan, M.; Verma, R.K.; Karunarathna, S.C.; Kumar, A.; Suwannarach, N. Insights into diversity, distribution, and systematics of rust genus Puccinia. J. Fungi 2023, 9, 639. [Google Scholar] [CrossRef]
- Bansal, S.; Balamurugan, A.; Mallikarjuna, M.G.; Singh, S.P.; Nayaka, S.C.; Prakash, G. The major diseases of pearl millet in the Indian sub-continent: Current scenarios in resistance and management strategies. In Pearl Millet in the 21st Century: Food-Nutrition-Climate Resilience-Improved Livelihoods; Springer: Berlin/Heidelberg, Germany, 2024; pp. 305–330. [Google Scholar] [CrossRef]
- Krępski, T.; Piasecka, A.; Święcicka, M.; Kańczurzewska, M.; Sawikowska, A.; Dmochowska-Boguta, M.; Rakoczy-Trojanowska, M.; Matuszkiewicz, M. Leaf rust (Puccinia recondita F. Sp. Secalis) triggers substantial changes in rye (Secale cereale L.) at the transcriptome and metabolome levels. BMC Plant. Biol. 2024, 24, 107. [Google Scholar] [CrossRef]
- Wakatsuki, H.; Ju, H.; Nelson, G.C.; Farrell, A.D.; Deryng, D.; Meza, F.; Hasegawa, T. Research trends and gaps in climate change impacts and adaptation potentials in major crops. Curr. Opin. Environ. Sustain. 2023, 60, 101249. [Google Scholar] [CrossRef]
- Shekhawat, P.S.; Singh, S.P.; Bishnoi, S. Integrated management of barley diseases: Current status and future research priorities. In New Horizons in Wheat and Barley Research: Crop Protection and Resource Management; Springer: Berlin/Heidelberg, Germany, 2022; pp. 215–260. [Google Scholar] [CrossRef]
- Habte, N.; Girma, G.; Xu, X.; Liao, C.J.; Adeyanju, A.; Hailemariam, S.; Lee, S.; Okoye, P.; Ejeta, G.; Mengiste, T. Haplotypes at the sorghum arg4 and arg5 nlr loci confer resistance to anthracnose. Plant J. 2024, 118, 106–123. [Google Scholar] [CrossRef]
- Figueroa, M.; Dodds, P.N.; Henningsen, E.C.; Sperschneider, J. Global landscape of rust epidemics by Puccinia species: Current and future perspectives. In Plant Relationships: Fungal-Plant Interactions; Springer: Berlin/Heidelberg, Germany, 2022; pp. 391–423. [Google Scholar] [CrossRef]
- Fraser, S.; McTaggart, A.R.; Roux, J.; Nel, J.; Potgieter, J.; Shuey, L.S.; Somchit, C.; Wingfield, M.J. The life cycle and field epidemiology of Uromycladium acaciae (Pucciniales) on Acacia mearnsii in South Africa. Ann. Appl. Biol. 2021, 179, 21–33. [Google Scholar] [CrossRef]
- Sarker, P.K.; Paul, A.S.; Karmoker, D. Mitigating climate change and pandemic impacts on global food security: Dual sustainable agriculture approach (2s approach). Planta 2023, 258, 104. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.S. Saccharide sweet (SS) principles, classification and structural and functional details of SS sweeteners and plants. In Alternative Sweet and Supersweet Principles: Natural Sweeteners and Plants; Springer: Berlin/Heidelberg, Germany, 2022; pp. 113–223. [Google Scholar] [CrossRef]
- Kebede, D.; Dramadri, I.O.; Rubaihayo, P.; Odong, T.; Edema, R. Resistance of sorghum genotypes to ergot (Claviceps species). Agriculture 2023, 13, 1100. [Google Scholar] [CrossRef]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Abazied, S. Factors affecting phytochemical content in some sweet sorghum varieties. Food Technol. Res. J. 2024, 5, 52–63. [Google Scholar] [CrossRef]
- De Souza, J.M.S.; Neto, A.B.; da Rosa, M.A.B.; Tardin, F.D.; Galati, R.L.; Chaves, C.S.; Pereira, D.H. Nutritional value and fermentability of sorghum silages grown in the amazon biome. Grassl. Sci. 2024. [Google Scholar] [CrossRef]
- Mohammed, A.; Bekeko, Z.; Yusufe, M.; Sulyok, M.; Krska, R. Fungal species and multi-mycotoxin associated with post-harvest sorghum (Sorghum bicolor (L.) Moench) grain in eastern Ethiopia. Toxins 2022, 14, 473. [Google Scholar] [CrossRef]
- Koima, I.N.; Kilalo, D.C.; Orek, C.O.; Wagacha, J.M.; Nyaboga, E.N. Survey of fungal foliar and panicle diseases in smallholder sorghum cropping systems in different agro-ecologies of lower eastern Kenya. Microbiol. Res. 2022, 13, 765–787. [Google Scholar] [CrossRef]
- Bedru, N.; Matiwos, T.; Birhan, T.; Belete, T. Performance evaluation of different sorghum genotypes (Sorghum bicolor (L.) Moench) using gge bi-plot stability analysis. Int. J. Genet. 2024, 12, 19–30. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Khugaev, C.V.; Utkina, A.O.; Isaev, K.V.; Mohamed, E.S.; Kucher, D.E. Contribution of eco-friendly agricultural practices in improving and stabilizing wheat crop yield: A review. Agronomy 2023, 13, 2400. [Google Scholar] [CrossRef]
- Theron, J.S.; van Coller, G.J.; Rose, L.J.; Labuschagne, J.; Swanepoel, P.A. The effect of crop rotation and tillage practice on fusarium crown rot and agronomic parameters of wheat in South Africa. Crop. Protect. 2023, 166, 106175. [Google Scholar] [CrossRef]
- Patroti, P.; Madhusudhana, R.; Rakshit, S.; Sharma, K.; Samdur, M.; Nitesh, S.; Elangovan, M.; Tonapi, V. Analysis of genetic diversity in exotic sorghum (Sorghum bicolor) germplasm and identification of trait specific superior accessions for post-rainy situation. Crop Res. 2022, 57, 28–37. [Google Scholar] [CrossRef]
- Baloch, F.S.; Altaf, M.T.; Liaqat, W.; Bedir, M.; Nadeem, M.A.; Cömertpay, G.; Çoban, N.; Habyarimana, E.; Barutçular, C.; Cerit, I. Recent advancements in the breeding of sorghum crop: Current status and future strategies for marker-assisted breeding. Front. Genet. 2023, 14, 1150616. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Prom, L.K.; Park, S.; Hu, Z.; Magill, C.W. Genome-wide association analysis uncovers genes associated with resistance to head smut path type 5 in Senegalese sorghum accessions. Plants 2024, 13, 977. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, Y.; Tabor, G.; Nian, H.; Phillips, J.; Wolters, P.; Yang, Q.; Balint-Kurti, P. A leucine-rich repeat receptor kinase gene confers quantitative susceptibility to maize southern leaf blight. New Phytol. 2023, 238, 1182–1197. [Google Scholar] [CrossRef]
- Joshi, B.M.; Bhavsar, H. Plant leaf disease detection and control: A survey. J. Inf. Optim. Sci. 2020, 41, 475–487. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Niaz, B.; Rasheed, A.; Umar, M.; Hussain, M.; Nayik, G.A.; Ansari, M.J. Quality and safety aspects of cereal grains. In Cereal Grains; CRC Press: Boca Raton, FL, USA, 2023; pp. 297–308. [Google Scholar] [CrossRef]
- Nataraj, V.; Kumar, S.; Kumawat, G.; Shivakumar, M.; Rajput, L.S.; Ratnaparkhe, M.B.; Ramteke, R.; Gupta, S.; Satpute, G.K.; Rajesh, V. Charcoal rot resistance in soybean: Current understanding and future perspectives. In Disease Resistance in Crop Plants: Molecular, Genetic and Genomic Perspectives; Springer: Berlin/Heidelberg, Germany, 2019; pp. 241–259. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, H.; Wang, H.; He, Y. Research progress on the relationship between leaf senescence and quality, yield and stress resistance in horticultural plants. Front. Plant. Sci. 2022, 13, 1044500. [Google Scholar] [CrossRef]
- Szabó, B.K.; Körösi, K. Storage mycotoxin producing fungi in Hungarian sorghum (Sorghum bicolor L. Moench) samples—Molecular approach of Fusarium spp. J. Plant Pathol. 2024, 107, 109–115. [Google Scholar] [CrossRef]
- Gemede, H.F. Influence of pre-treatment on antioxidant and functional properties of Ethiopia endemic anchote (Coccinia abyssinica) tuber and seed flour. Cogent Food Agric. 2023, 9, 2221833. [Google Scholar] [CrossRef]
- Mesterhazy, A. Food safety aspects of breeding maize to multi-resistance against the major (Fusarium graminearum, F. Verticillioides, Aspergillus flavus) and minor toxigenic fungi (Fusarium spp.) as well as to toxin accumulation, trends, and solutions—A review. J. Fungi 2024, 10, 40. [Google Scholar] [CrossRef]
- Santiago, R.; Cao, A.; Malvar, R.A.; Butrón, A. Genomics of maize resistance to fusarium ear rot and fumonisin contamination. Toxins 2020, 12, 431. [Google Scholar] [CrossRef]
- Sadik, J.; Fentahun, N.; Brouwer, I.; Tessema, M.; van der Fels-Klerx, H. Preharvest and postharvest management practices related to mycotoxin contamination in maize in Ethiopia—A review. World Mycotoxin J. 2023, 1, 211–216. [Google Scholar] [CrossRef]
- Chilenga, C.; Masamba, K.; Kasapila, W.; Ndhlovu, B.; Munkhuwa, V.; Rafoneke, L.; Machira, K. Mycotoxin management in sub-Saharan Africa: A comprehensive systematic review of policies and strategies in Malawi. Toxicol. Rep. 2024, 14, 101871. [Google Scholar] [CrossRef] [PubMed]
- Frasiński, S.; Czembor, E.; Lalak-Kańczugowska, J. The impact of Fusarium ear rot in Poland and methods to reduce losses caused by the disease. Biul. Inst. Hod. I Aklim. Roślin 2020, 290, 43–50. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. Mycotoxins in food: Cancer risks and strategies for control. Foods 2024, 13, 3502. [Google Scholar] [CrossRef]
- Ogunniran, O.P.; Ayeni, K.I.; Shokunbi, O.S.; Krska, R.; Ezekiel, C.N. A 10-year (2014–2023) review of complementary food development in sub-Saharan Africa and the impact on child health. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70022. [Google Scholar] [CrossRef]
- McLaughlin, M.S.; Roy, M.; Abbasi, P.A.; Carisse, O.; Yurgel, S.N.; Ali, S. Why do we need alternative methods for fungal disease management in plants? Plants 2023, 12, 3822. [Google Scholar] [CrossRef]
- Prom, L.K.; Ahn, E.J.; Cuevas, H.E.; Liu, J.; Isakeit, T.S.; Magill, C.W. Association and interrelationship among agronomic traits and fungal diseases of sorghum, anthracnose and grain mold. Crops 2024, 4, 651–666. [Google Scholar] [CrossRef]
- Bhat, M.T.; Shishupala, S. Pathogenicity, phytotoxicity and enzymatic potential of sorghum grain mold pathogens. Int. J. Curr. Microbiol. App. Sci. 2024, 13, 80–88. [Google Scholar] [CrossRef]
- Hoenigl, M.; Arastehfar, A.; Arendrup, M.C.; Brüggemann, R.; Carvalho, A.; Chiller, T.; Chen, S.; Egger, M.; Feys, S.; Gangneux, J.-P. Novel antifungals and treatment approaches to tackle resistance and improve outcomes of invasive fungal disease. Clin. Microbiol. Rev. 2024, 37, e0007423. [Google Scholar] [CrossRef]
- Fisher, A., II; DeGrandi-Hoffman, G.; Liao, L.-H.; Tadei, R.; Harrison, J.F. The challenge of balancing fungicide use and pollinator health. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 64, pp. 117–190. [Google Scholar] [CrossRef]
- Jain, S.; Thind, T.; Sekhon, P.; Singh, A. Novel detection techniques for plant pathogens and their application in disease management. In Recent Advances in the Diagnosis and Management of Plant Diseases; Springer: New Delhi, India, 2015; pp. 243–251. [Google Scholar] [CrossRef]
- Craven, M.; Smith, K.; Berner, J.; Morey, L.; McLaren, N.W. Evaluation of fungicides for potential growth regulating properties on sorghum. Crop. Protect. 2017, 101, 43–49. [Google Scholar] [CrossRef]
- Tleuova, A.B.; Wielogorska, E.; Talluri, V.P.; Štěpánek, F.; Elliott, C.T.; Grigoriev, D.O. Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Dubery, I.A.; Nephali, L.P.; Tugizimana, F.; Steenkamp, P.A. Data-driven characterization of metabolome reprogramming during early development of sorghum seedlings. Metabolites 2024, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, H.; Qi, G.; Qian, Y.; Li, B.; Shi, L.; Liu, B. Combating fungal infections and resistance with a dual-mechanism Luminogen to disrupt membrane integrity and induce DNA damage. J. Am. Chem. Soc. 2024, 146, 31656–31664. [Google Scholar] [CrossRef]
- Little, C.; Bandara, A.; Perumal, R. Sorghum diseases and their management in cultivation: Seedling, seed, panicle and foliar diseases agricultural research center–hays, USA. In Achieving Sustainable Cultivation of Sorghum; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; Volume 1, pp. 397–446. [Google Scholar] [CrossRef]
- Christopher, F.; Mbega, E.; Ndakidemi, P.; Nyalala, S. Challenges and prospects for management of anthracnose caused by Colletotrichum species in tropical Africa. J. Anim. Plant Sci. 2022, 32, 891. [Google Scholar] [CrossRef]
- Barathikannan, K.; Chelliah, R.; Selvakumar, V.; Elahi, F.; Rubab, M.; Sanyal, S.; Yeon, S.-J.; Oh, D.-H. Plant-based metabolites and their uses in nanomaterials synthesis: An overview. In Secondary Metabolites Based Green Synthesis of Nanomaterials and Their Applications; Springer: Singapore, 2023; pp. 1–22. [Google Scholar] [CrossRef]
- Fenta, L.; Mekonnen, H. Microbial biofungicides as a substitute for chemical fungicides in the control of phytopathogens: Current perspectives and research directions. Scientifica 2024, 2024, 5322696. [Google Scholar] [CrossRef]
- Wani, S.; Rajput, A.; Pingale, P. Herbal nanoformulations: A magical remedy for management of fungal diseases. J. Herb. Med. 2023, 42, 100810. [Google Scholar] [CrossRef]
- Huang, T.; Li, X.; Maier, M.; O’Brien-Simpson, N.M.; Heath, D.E.; O’Connor, A.J. Using inorganic nanoparticles to fight fungal infections in the antimicrobial resistant era. Acta Biomater. 2023, 158, 56–79. [Google Scholar] [CrossRef]
- Olita, T.; Stankovic, M.; Sung, B.; Jones, M.; Gibberd, M. Growers’ perceptions and attitudes towards fungicide resistance extension services. Sci. Rep. 2024, 14, 6821. [Google Scholar] [CrossRef]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Moumni, M.; Brodal, G.; Romanazzi, G. Recent innovative seed treatment methods in the management of seed borne pathogens. Food Secur. 2023, 15, 1365–1382. [Google Scholar] [CrossRef]
- Taylor, N.P.; Cunniffe, N.J. Modelling quantitative fungicide resistance and breakdown of resistant cultivars: Designing integrated disease management strategies for Septoria of winter wheat. PLoS Comp. Biol. 2023, 19, e1010969. [Google Scholar] [CrossRef] [PubMed]
- Dassou, A.G.; Tovignan, S.; Vodouhè, F.; Vodouhè, S.D. Meta-analysis of agro ecological technologies and practices in the sustainable management of banana pests and diseases. Environ. Dev. Sustain. 2023, 26, 21937–21954. [Google Scholar] [CrossRef]
- Jayaraman, S.; Naorem, A.; Lal, R.; Dalal, R.C.; Sinha, N.; Patra, A.; Chaudhari, S. Disease-suppressive soils—Beyond food production: A critical review. J. Soil Sci. Plant Nutr. 2021, 21, 1437–1465. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, N.E.; Carter, D.A. Climate change and the emergence of fungal pathogens. PLoS Path. 2021, 17, e1009503. [Google Scholar] [CrossRef]
- Bulut, S.; Arslan, M. Plant protection methods in organic farming. Curr. Trends Natl. Sci. 2023, 12, 183–192. [Google Scholar] [CrossRef]
- Mohanty, L.K.; Singh, N.; Raj, P.; Prakash, A.; Tiwari, A.K.; Singh, V.; Sachan, P. Nurturing crops, enhancing soil health, and sustaining agricultural prosperity worldwide through agronomy. J. Exp. Agric. Int. 2024, 46, 46–67. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Lecina-Diaz, J.; Chas-Amil, M.-L.; Aquilué, N.; Sil, Â.; Brotons, L.; Regos, A.; Touza, J. Incorporating fire-smartness into agricultural policies reduces suppression costs and ecosystem services damages from wildfires. J. Environ. Manag. 2023, 337, 117707. [Google Scholar] [CrossRef]
- Resende, R.S.; Rodrigues, F.Á.; Soares, J.M.; Casela, C.R. Influence of silicon on some components of resistance to anthracnose in susceptible and resistant sorghum lines. Eur. J. Plant Pathol. 2009, 124, 533–541. [Google Scholar] [CrossRef]
- Resende, R.S.; Rodrigues, F.Á.; Costa, R.V.; Silva, D.D. Silicon and fungicide effects on anthracnose in moderately resistant and susceptible sorghum lines. J. Phytopathol. 2013, 161, 11–17. [Google Scholar] [CrossRef]
- Resende, R.; Rodrigues, F.; Gomes, R.; Nascimento, K. Microscopic and biochemical aspects of sorghum resistance to anthracnose mediated by silicon. Ann. Appl. Biol. 2013, 163, 114–123. [Google Scholar] [CrossRef]
- D’Aquino, S.; Palma, A. Reducing or replacing conventional postharvest fungicides with low toxicity acids and salts. In Postharvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2019; pp. 595–632. [Google Scholar] [CrossRef]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of fungicides and factors affecting their fate and removal efficacy: A review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Heick, T.M.; Justesen, A.F.; Jørgensen, L.N. Anti-resistance strategies for fungicides against wheat pathogen Zymoseptoria tritici with focus on dmi fungicides. Crop. Protect. 2017, 99, 108–117. [Google Scholar] [CrossRef]
- Dorigan, A.F.; Moreira, S.I.; da Silva Costa Guimarães, S.; Cruz-Magalhães, V.; Alves, E. Target and non-target site mechanisms of fungicide resistance and their implications for the management of crop pathogens. Pest Manag. Sci. 2023, 79, 4731–4753. [Google Scholar] [CrossRef] [PubMed]
- Pothiraj, G.; Hussain, Z.; Singh, A.K.; Solanke, A.U.; Aggarwal, R.; Ramesh, R.; Shanmugam, V. Characterization of Fusarium spp. Inciting vascular wilt of tomato and its management by a Chaetomium-based biocontrol consortium. Front. Plant. Sci. 2021, 12, 748013. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Arunkumar, P.; Joo, S.-W.; Gnanasekaran, L.; Kamyab, H.; Rajendran, S.; Balakrishnan, D.; Chelliapan, S.; Klemeš, J.J. Metal-organic framework-enabled pesticides are an emerging tool for sustainable cleaner production and environmental hazard reduction. J. Clean. Prod. 2022, 373, 133966. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; Umar, S.; Lee, J. Sorghum-phosphate solubilizers’ interactions: Crop nutrition, biotic stress alleviation, and yield optimization. Front. Plant. Sci. 2021, 12, 746780. [Google Scholar] [CrossRef]
- Pimentão, A.R.; Cuco, A.P.; Pascoal, C.; Cássio, F.; Castro, B.B. Current trends and mismatches on fungicide use and assessment of the ecological effects in freshwater ecosystems. Environ. Pollut. 2024, 347, 123678. [Google Scholar] [CrossRef]
- Sreevidya, S.; Sankarasubramanian, K.; Katre, Y.; Yadav, S.; Asthana, A.; Singh, A.K.; Alexis, F.; Carabineiro, S.A. Users opinion about synthetic, bio-and nano-biopesticides. J. Nat. Pestic. Res. 2023, 6, 100058. [Google Scholar] [CrossRef]
- Michelson, H.; Gourlay, S.; Lybbert, T.; Wollburg, P. Purchased agricultural input quality and small farms. Food Policy 2023, 116, 102424. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, A.; Mahato, D.K.; Pandhi, S.; Pandey, A.K.; Kargwal, R.; Mishra, S.; Suhag, R.; Sharma, N.; Saurabh, V. Aflatoxins in cereals and cereal-based products: Occurrence, toxicity, impact on human health, and their detoxification and management strategies. Toxins 2022, 14, 687. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.; Mostafa, Y.S.; Alamri, S.; Abbas, A.M.; Eid, E.M. Exploitation of agro-industrial residues for the formulation of a new active and cost effective biofungicide to control the root rot of vegetable crops. Sustainability 2021, 13, 9254. [Google Scholar] [CrossRef]
- Kumar, V.; Koul, B.; Taak, P.; Yadav, D.; Song, M. Journey of Trichoderma from pilot scale to mass production: A review. Agriculture 2023, 13, 2022. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Fotopoulos, V.; Saeid, A. Production of a rich fertilizer base for plants from waste organic residues by microbial formulation technology. Microorganisms 2024, 12, 541. [Google Scholar] [CrossRef]
- Martinez, Y.; Ribera, J.; Schwarze, F.W.; De France, K. Biotechnological development of Trichoderma-based formulations for biological control. Appl. Microbiol. Biotechnol. 2023, 107, 5595–5612. [Google Scholar] [CrossRef]
- Lyubenova, A.; Rusanova, M.; Nikolova, M.; Slavov, S.B. Plant extracts and Trichoderma spp.: Possibilities for implementation in agriculture as biopesticides. Biotechnol. Biotechnol. Equip. 2023, 37, 159–166. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, G.; Kalotra, S.; Verma, L.; Chauhan, A. Biocontrol agents for soil health management. In Advancements in Microbial Biotechnology for Soil Health; Springer: Berlin/Heidelberg, Germany, 2024; pp. 149–172. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Moreira, J.V.; Akamine, I.T.; Cardoso, V.S.; Mansoldo, F.R. Agricultural pest management: The role of microorganisms in biopesticides and soil bioremediation. Plants 2024, 13, 2762. [Google Scholar] [CrossRef]
- Saiyam, D.; Dubey, A.; Malla, M.A.; Kumar, A. Lipopeptides from Bacillus: Unveiling biotechnological prospects—Sources, properties, and diverse applications. Braz. J. Microbiol. 2024, 55, 281–295. [Google Scholar] [CrossRef]
- Atri, A.; Banyal, D.; Bhardwaj, N.; Roy, A. Exploring the integrated use of fungicides, bio-control agent and biopesticide for management of foliar diseases (Anthracnose, grey leaf spot and zonate leaf spot) of sorghum. Int. J. Pest Manag. 2022, 70, 789–800. [Google Scholar] [CrossRef]
- Basavaraj, K.; Simha, H.; Palaiah, P.; Manjunath, N.; Shruthi, N.; Bellad, S. Integrated crop management practices resulted in higher yield in sorghum (Sorghum bicolor L.). J. Krishi Vigyan 2022, 11, 211–216. [Google Scholar] [CrossRef]
- Rajini, S.B.; Nandhini, M.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Prakash, H.S. Diversity, plant growth-promoting traits, and biocontrol potential of fungal endophytes of Sorghum bicolor. Plant. Pathol. 2020, 69, 642–654. [Google Scholar] [CrossRef]
- Begna, T. Effect of striga species on sorghum (Sorghum bicolor L. Moench) production and its integrated management approaches. Int. J. Res. Stud. Agric. Sci. 2021, 7, 10–22. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Milošević, D.; Ignjatov, M.; Karačić, V.; Jakšić, S. Bio-priming with Bacillus isolates suppresses seed infection and improves the germination of garden peas in the presence of Fusarium strains. J. Fungi 2024, 10, 358. [Google Scholar] [CrossRef]
- Verma, R.; Singh, P.; Singh, J. Evaluation of different modules for the management of false smut of rice under field conditions. J. Pharmacogn. Phytochem. 2020, 9, 299–301. [Google Scholar] [CrossRef]
- Islam, T.; Haque, M.A.; Barai, H.R.; Istiaq, A.; Kim, J.-J. Antibiotic resistance in plant pathogenic bacteria: Recent data and environmental impact of unchecked use and the potential of biocontrol agents as an eco-friendly alternative. Plants 2024, 13, 1135. [Google Scholar] [CrossRef]
- Kandasamy, G.D.; Kathirvel, P. Insights into bacterial endophytic diversity and isolation with a focus on their potential applications—A review. Microbiol. Res. 2023, 266, 127256. [Google Scholar] [CrossRef]
- Segoli, M.; Abram, P.K.; Ellers, J.; Hardy, I.C.; Greenbaum, G.; Heimpel, G.E.; Keasar, T.; Ode, P.J.; Sadeh, A.; Wajnberg, E. Trait-based approaches to predicting biological control success: Challenges and prospects. Trends Ecol. Evol. 2023, 38, 802–811. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Mason, P.G.; Mc Kay, F.; Silvestri, L.C.; Hill, M.; Weyl, P.; Hinz, H.L.; Brodeur, J.; Vitorino, M.D.; Barratt, B.I. International agreement for the use and exchange of classical biological control genetic resources: A practical proposal. BioControl 2023, 68, 329–339. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Xia, X. Socioeconomic status, ambidextrous learning, and farmers’ adoption of biological control technology: Evidence from 650 kiwifruit growers in China. Pest Manag. Sci. 2022, 78, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Pambuka, G.T.; Kinge, T.R.; Ghosh, S.; Cason, E.D.; Nyaga, M.M.; Gryzenhout, M. Plant and soil core mycobiomes in a two-year sorghum–legume intercropping system of underutilized crops in south Africa. Microorganisms 2022, 10, 2079. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.D.; Ribeiro, F.P.; de Figueiredo, C.C.; Muller, A.G.; Malaquias, J.V.; Dos Santos, I.L.; de Sá, M.A.C.; Soares, J.P.G.; Dos Santos, M.V.A.; de Carvalho, A.M. Effects of soil management, rotation and sequence of crops on soil nitrous oxide emissions in the Cerrado: A multi-factor assessment. J. Environ. Manag. 2023, 348, 119295. [Google Scholar] [CrossRef]
- Peralta, A.L.; Sun, Y.; McDaniel, M.D.; Lennon, J.T. Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 2018, 9, e02235. [Google Scholar] [CrossRef]
- Pierre, H.M.; Kinama, J.M.; Olubayo, F.M.; Wanderi, S.W.; Muthomi, J.W.; Nzuve, F.M. Effect of intercropping maize and promiscuous soybean on growth and yield. J. Exp. Agric. Int. 2018, 18, 1–21. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Z.; Chen, Y.; Wang, Y.; Feng, S. Crop rotation and diversification in china: Enhancing sustainable agriculture and resilience. Agriculture 2024, 14, 1465. [Google Scholar] [CrossRef]
- Duo, L.; Yang, Y.; Gao, Y.; Zhao, S. Graphene oxide affects the symbiosis of legume-rhizobium and associated rhizosphere rhizobial communities. Chemosphere 2023, 342, 140166. [Google Scholar] [CrossRef]
- Jaisval, G.K.; Dwivedi, H.; Pandey, A.; Jaiswal, S.; Kumar, A.; Kushwaha, D.; Shukla, P. A comprehensive review on plant disease vectors and their management. Int. J. Environ. Clim. Change 2023, 13, 2518–2530. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, Z.; Cheng, J.; Ma, B.; Cong, W.-F.; Zhang, C.; Zhang, F.; van der Werf, W.; Groot, J.C. Designing diversified crop rotations to advance sustainability: A method and an application. Sustain. Prod. Consum. 2023, 40, 532–544. [Google Scholar] [CrossRef]
- Aggarwal, S.; Srinivas, R.; Puppala, H.; Magner, J. Integrated decision support for promoting crop rotation based sustainable agricultural management using geoinformatics and stochastic optimization. Comput. Electron. Agric. 2022, 200, 107213. [Google Scholar] [CrossRef]
- Volsi, B.; Higashi, G.E.; Bordin, I.; Telles, T.S. The diversification of species in crop rotation increases the profitability of grain production systems. Sci. Rep. 2022, 12, 19849. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Sahoo, U.; Sairam, M.; Gitari, H.I.; Rezaei-Chiyaneh, E.; Battaglia, M.L.; Hossain, A. Cultivating sustainability: A comprehensive review on intercropping in a changing climate. Res. Crops 2023, 24, 702–715. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.; Abd El-Mageed, T.A.; Negm, S.H. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant. Sci. 2022, 13, 923880. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Deng, M.; Lin, Y.; Liu, P.; Wang, X.; Yang, S.; Ren, X.; Chen, X.; Liu, T. Effects of the border on yield and water use in wheat/maize intercropping in rain-fed areas with different nitrogen levels. Field Crops Res. 2023, 302, 109105. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Dinelli, G.; Negri, L. Towards an agro ecological approach to crop health: Reducing pest incidence through synergies between plant diversity and soil microbial ecology. Npj Sustain. Agric. 2024, 2, 6. [Google Scholar] [CrossRef]
- Huss, C.; Holmes, K.; Blubaugh, C. Benefits and risks of intercropping for crop resilience and pest management. J. Econ. Entomol. 2022, 115, 1350–1362. [Google Scholar] [CrossRef]
- Affichard, M.; Jacquelin, M.; Khalil, T.; Andrivon, D.; Le May, C. Consideration of the disease complexes, the missing link to correctly analyze the impact of intercropping on disease development. Agronomy 2024, 14, 1210. [Google Scholar] [CrossRef]
- Wu, J.; Bao, X.; Zhang, J.; Lu, B.; Zhang, W.; Callaway, R.M.; Li, L. Temporal stability of productivity is associated with complementarity and competitive intensities in intercropping. Ecol. Appl. 2023, 33, e2731. [Google Scholar] [CrossRef]
- Charania, I.; Li, X. Smart farming: Agriculture’s shift from a labor intensive to technology native industry. Internet Things 2020, 9, 100142. [Google Scholar] [CrossRef]
- Kabir, A.H.; Bennetzen, J.L. Molecular insights into the mutualism that induces iron deficiency tolerance in sorghum inoculated with Trichoderma harzianum. Microbiol. Res. 2024, 281, 127630. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Shiwani. Molecular breeding and marker-assisted selection for crop improvement. In Plant Genomics for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2022; pp. 129–164. [Google Scholar] [CrossRef]
- Feni, E.I.; Kasmiyati, S.; Meitiniarti, V.I. Resistance selection of local sorghum varieties in East Nusa Tenggara, Indonesia against Rhizoctonia solani. Biodiversitas J. Biol. Divers. 2023, 24, 5309–5318. [Google Scholar] [CrossRef]
- Singh, S.; Chaudhary, R.; Lokya, V.; Tiwari, S. Genome editing based trait improvement in crops: Current perspective, challenges and opportunities. Nucleus 2024, 67, 97–126. [Google Scholar] [CrossRef]
- Misganaw, A.; Feyissa, T.; Mekonnen, T.; Desalegne, O.; Disasa, T. Genetic diversity analysis of sorghum genotypes for sustainable genetic resource conservation and its implication for breeding program in Ethiopia. Genet. Resour. Crop Evol. 2023, 70, 1831–1852. [Google Scholar] [CrossRef]
- Singha, S.; Singha, R. Crop improvement strategies and principles of selective breeding. In Water-Soil-Plant-Animal Nexus in the era of Climate Change; IGI Global: Pennsylvania, PA, USA, 2024; pp. 93–113. [Google Scholar] [CrossRef]
- Boyles, R.E.; Ballén-Taborda, C.; Brown-Guedira, G.; Costa, J.; Cowger, C.; DeWitt, N.; Griffey, C.A.; Harrison, S.A.; Ibrahim, A.; Johnson, J. Approaching 25 years of progress towards fusarium head blight resistance in southern soft red winter wheat (Triticum aestivum L.). Plant Breed. 2024, 143, 66–81. [Google Scholar] [CrossRef]
- Grenni, P. Antimicrobial resistance in rivers: A review of the genes detected and new challenges. Environ. Toxicol. Chem. 2022, 41, 687–714. [Google Scholar] [CrossRef]
- Jabran, M.; Ali, M.A.; Zahoor, A.; Muhae-Ud-Din, G.; Liu, T.; Chen, W.; Gao, L. Intelligent reprogramming of wheat for enhancement of fungal and nematode disease resistance using advanced molecular techniques. Front. Plant. Sci. 2023, 14, 1132699. [Google Scholar] [CrossRef]
- Finger, R.; Zachmann, L.; McCallum, C. Short supply chains and the adoption of fungus-resistant grapevine varieties. Appl. Econ. Perspect. Policy 2023, 45, 1753–1775. [Google Scholar] [CrossRef]
- Samuel, L.; de Barcellos, M.D.; Watabaji, M.D.; De Steur, H. Factors affecting farmers’ acceptance and adoption of bio fortified crops: A systematic review. Outlook Agric. 2024, 53, 15–29. [Google Scholar] [CrossRef]
- Ndudzo, A.; Makuvise, A.S.; Moyo, S.; Bobo, E.D. Crispr-cas9 genome editing in crop breeding for climate change resilience: Implications for smallholder farmers in Africa. J. Agric. Food Res. 2024, 16, 101132. [Google Scholar] [CrossRef]
- Birhanu, C.; Girma, G.; Mekbib, F.; Nida, H.; Tirfessa, A.; Lule, D.; Bekeko, Z.; Ayana, G.; Bejiga, T.; Bedada, G. Exploring the genetic basis of anthracnose resistance in Ethiopian sorghum through a genome-wide association study. BMC Genom. 2024, 25, 677. [Google Scholar] [CrossRef] [PubMed]
- Mewa, D.B.; Lee, S.; Liao, C.J.; Adeyanju, A.; Helm, M.; Lisch, D.; Mengiste, T. ANTHRACNOSE RESISTANCE GENE2 confers fungal resistance in sorghum. Plant J. 2023, 113, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Chhapekar, S.S.; Vieira, C.C.; Da Silva, M.P.; Rojas, A.; Lee, D.; Liu, N.; Pardo, E.M.; Lee, Y.-C.; Dong, Z. Breeding for disease resistance in soybean: A global perspective. Theor. Appl. Genet. 2022, 135, 3773–3872. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, C.; Vailleau, F.; Berthomé, R.; Roux, F. Genome-wide association studies in plant pathosystems: Success or failure? Trends Plant Sci. 2023, 28, 471–485. [Google Scholar] [CrossRef]
- Zhu, Q.; Fei, Y.-J.; Wu, Y.-B.; Luo, D.-L.; Chen, M.; Sun, K.; Zhang, W.; Dai, C.-C. Endophytic fungus reshapes spikelet microbiome to reduce mycotoxin produced by Fusarium proliferatum through altering rice metabolites. J. Agric. Food Chem. 2023, 71, 11350–11364. [Google Scholar] [CrossRef]
- Liu, B.; Stevens-Green, R.; Johal, D.; Buchanan, R.; Geddes-McAlister, J. Fungal pathogens of cereal crops: Proteomic insights into fungal pathogenesis, host defense, and resistance. J. Plant Physiol. 2022, 269, 153593. [Google Scholar] [CrossRef]
- Wang, K.; Abid, M.A.; Rasheed, A.; Crossa, J.; Hearne, S.; Li, H. Dnngp, a deep neural network-based method for genomic prediction using multi-omics data in plants. Mol. Plant 2023, 16, 279–293. [Google Scholar] [CrossRef]
- Boubakri, H. Recent progress in Crispr/cas9-based genome editing for enhancing plant disease resistance. Gene 2023, 866, 147334. [Google Scholar] [CrossRef]
- Mengistu, G.; Shimelis, H.; Assefa, E.; Lule, D. Genome-wide association analysis of anthracnose resistance in sorghum [Sorghum bicolor (L.) Moench]. PLoS ONE 2021, 16, e0261461. [Google Scholar] [CrossRef]
- Borrelli, V.M.; Brambilla, V.; Rogowsky, P.; Marocco, A.; Lanubile, A. The enhancement of plant disease resistance using Crispr/CAS9 technology. Front. Plant. Sci. 2018, 9, 1245. [Google Scholar] [CrossRef]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.-J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J. Morphogenic regulators baby boom and Wuschel improve monocot transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Crop disease control and management. In Encyclopedia of Digital Agricultural Technologies; Springer: Berlin/Heidelberg, Germany, 2023; pp. 193–197. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. Crop health sensing: Disease, pest, nutrient, and water stresses. In Encyclopedia of Digital Agricultural Technologies; Springer: Berlin/Heidelberg, Germany, 2023; pp. 198–207. [Google Scholar] [CrossRef]
- Ali, F.; Rehman, A.; Hameed, A.; Sarfraz, S.; Rajput, N.A.; Atiq, M. Climate change impact on plant pathogen emergence: Artificial intelligence (AI) approach. In Plant Quarantine Challenges Under Climate Change Anxiety; Springer: Berlin/Heidelberg, Germany, 2024; pp. 281–303. [Google Scholar] [CrossRef]
- Purnama, I.; Syafrani, S.; Mutamima, A.; Saputra, R.; Nasution, N.; Amalia, A. Improving edible oilseed (oil palm) health and productivity: Integration of sustainable pest management, precision farming, and stakeholder collaboration. In Edible Oilseeds Research-Updates and Prospects; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Dhanaraju, M.; Chenniappan, P.; Ramalingam, K.; Pazhanivelan, S.; Kaliaperumal, R. Smart farming: Internet of things (IoT)-based sustainable agriculture. Agriculture 2022, 12, 1745. [Google Scholar] [CrossRef]
- Zhao, A.P.; Li, S.; Cao, Z.; Hu, P.J.-H.; Wang, J.; Xiang, Y.; Xie, D.; Lu, X. Ai for science: Predicting infectious diseases. J. Saf. Sci. Resil. 2024, 5, 130–146. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, J.; Andersson, B.; Wiik, L.; Zhan, J. Modelling potato yield losses caused by Phytophthora infestans: Aspects of disease growth rate, infection time and temperature under climate change. Field Crops Res. 2023, 299, 108977. [Google Scholar] [CrossRef]
- Ahmed, I.; Yadav, P.K. Plant disease detection using machine learning approaches. Expert Syst. 2023, 40, e13136. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Arnal Barbedo, J.G.; Chiang, K.-S.; Del Ponte, E.M.; Bock, C.H. From detection to protection: The role of optical sensors, robots, and artificial intelligence in modern plant disease management. Phytopathology 2024, 114, 1733–1741. [Google Scholar] [CrossRef]
- Garrett, K.; Bebber, D.; Etherton, B.; Gold, K.; Plex Sulá, A.; Selvaraj, M.G. Climate change effects on pathogen emergence: Artificial intelligence to translate big data for mitigation. Annu. Rev. Phytopathol. 2022, 60, 357–378. [Google Scholar] [CrossRef]
- Haque, S.; Mengersen, K.; Barr, I.; Wang, L.; Yang, W.; Vardoulakis, S.; Bambrick, H.; Hu, W. Towards development of functional climate-driven early warning systems for climate-sensitive infectious disease: Statistical models and recommendations. Environ. Res. 2024, 249, 118568. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Khan, I.H.; Suman, R. Understanding the potential applications of artificial intelligence in agriculture sector. Adv. Agrochem. 2023, 2, 15–30. [Google Scholar] [CrossRef]
- Urugo, M.M.; Yohannis, E.; Teka, T.A.; Gemede, H.F.; Tola, Y.B.; Forsido, S.F.; Tessema, A.; Suraj, M.; Abdu, J. Addressing post-harvest losses through agro-processing for sustainable development in Ethiopia. J. Agric. Food Res. 2024, 18, 101316. [Google Scholar] [CrossRef]
- John, M.A.; Bankole, I.; Ajayi-Moses, O.; Ijila, T.; Jeje, O.; Lalit, P. Relevance of advanced plant disease detection techniques in disease and pest management for ensuring food security and their implication: A review. Am. J. Plant Sci. 2023, 14, 1260–1295. [Google Scholar] [CrossRef]
- Usama, M. Application of digital technologies & remote sensing in precision agriculture for sustainable crop production. In Geospatial Technology to Support Communities and Policy: Pathways to Resiliency; Springer: Berlin/Heidelberg, Germany, 2024; pp. 203–223. [Google Scholar] [CrossRef]
- Abdullah, H.M.; Mohana, N.T.; Khan, B.M.; Ahmed, S.M.; Hossain, M.; Islam, K.S.; Redoy, M.H.; Ferdush, J.; Bhuiyan, M.; Hossain, M.M. Present and future scopes and challenges of plant pest and disease (P&D) monitoring: Remote sensing, image processing, and artificial intelligence perspectives. Remote Sens. Appl. Soc. Environ. 2023, 32, 100996. [Google Scholar] [CrossRef]
- Finger, R.; Swinton, S.M.; El Benni, N.; Walter, A. Precision farming at the nexus of agricultural production and the environment. Annu. Rev. Resour. Econ. 2019, 11, 313–335. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Heim, R.H.-J.; Brugger, A.; Gold, K.; Li, Y.; Bashir, A.K.; Paulus, S.; Kuska, M.T. Digital plant pathology for precision agriculture. J. Plant Dis. Prot. 2022, 129, 455–456. [Google Scholar] [CrossRef]
- Júnior, M.R.B.; de Almeida Moreira, B.R.; dos Santos Carreira, V.; de Brito Filho, A.L.; Trentin, C.; de Souza, F.L.P.; Tedesco, D.; Setiyono, T.; Flores, J.P.; Ampatzidis, Y. Precision agriculture in the united states: A comprehensive meta-review inspiring further research, innovation, and adoption. Comput. Electron. Agric. 2024, 221, 108993. [Google Scholar] [CrossRef]
- Rozenstein, O.; Cohen, Y.; Alchanatis, V.; Behrendt, K.; Bonfil, D.J.; Eshel, G.; Harari, A.; Harris, W.E.; Klapp, I.; Laor, Y. Data-driven agriculture and sustainable farming: Friends or foes? Precis. Agric. 2024, 25, 520–531. [Google Scholar] [CrossRef]
- Aithal, S.; Aithal, P. Information communication and computation technologies (ICCT) for agricultural and environmental information systems for society 5.0. Int. J. Appl. Eng. Manag. Lett. 2024, 8, 67–100. [Google Scholar] [CrossRef]
- Gebresenbet, G.; Bosona, T.; Patterson, D.; Persson, H.; Fischer, B.; Mandaluniz, N.; Chirici, G.; Zacepins, A.; Komasilovs, V.; Pitulac, T. A concept for application of integrated digital technologies to enhance future smart agricultural systems. Smart Agric. Technol. 2023, 5, 100255. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Arduini, S.; Uyar, H.; Psiroukis, V.; Kasimati, A.; Fountas, S. Economic and environmental benefits of digital agricultural technologies in crop production: A review. Smart Agric. Technol. 2024, 8, 100441. [Google Scholar] [CrossRef]
- Kitole, F.A.; Mkuna, E.; Sesabo, J.K. Digitalization and agricultural transformation in developing countries: Empirical evidence from Tanzania agriculture sector. Smart Agric. Technol. 2024, 7, 100379. [Google Scholar] [CrossRef]
- Richard, B.; Qi, A.; Fitt, B.D. Control of crop diseases through integrated crop management to deliver climate-smart farming systems for low-and high-input crop production. Plant. Pathol. 2022, 71, 187–206. [Google Scholar] [CrossRef]
- Datta, D.; Ghosh, S.; Kumar, S.; Gangola, S.; Majumdar, B.; Saha, R.; Mazumdar, S.P.; Singh, S.V.; Kar, G. Microbial bio surfactants: Multifarious applications in sustainable agriculture. Microbiol. Res. 2024, 279, 127551. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Gandorfer, M. Adoption of digital technologies in agriculture—An inventory in a European small-scale farming region. Precis. Agric. 2023, 24, 68–91. [Google Scholar] [CrossRef]
- Thakur, N.; Nigam, M.; Mann, N.A.; Gupta, S.; Hussain, C.M.; Shukla, S.K.; Shah, A.A.; Casini, R.; Elansary, H.O.; Khan, S.A. Host-mediated gene engineering and microbiome-based technology optimization for sustainable agriculture and environment. Funct. Integr. Genom. 2023, 23, 57. [Google Scholar] [CrossRef]
- Sawhney, R.; Malik, A.; Sharma, S.; Narayan, V. A comparative assessment of artificial intelligence models used for early prediction and evaluation of chronic kidney disease. Decis. Anal. J. 2023, 6, 100169. [Google Scholar] [CrossRef]
- Guja, M.M.; Bedeke, S.B. Smallholders’ climate change adaptation strategies: Exploring effectiveness and opportunities to be capitalized. Environ. Dev. Sustain. 2024, 1–30. [Google Scholar] [CrossRef]
- Blomme, G.; Kearsley, E.; Buta, S.; Chala, A.; Kebede, R.; Addis, T.; Yemataw, Z. Xanthomonas wilt of enset in Ethiopia: Geographical spread, impact on production systems and the effect of training on disease management practices. Afr. J. Agric. Res. 2023, 19, 33–47. [Google Scholar] [CrossRef]
- Piñeiro, V.; Arias, J.; Dürr, J.; Elverdin, P.; Ibáñez, A.M.; Kinengyere, A.; Opazo, C.M.; Owoo, N.; Page, J.R.; Prager, S.D. A scoping review on incentives for adoption of sustainable agricultural practices and their outcomes. Nat. Sustain. 2020, 3, 809–820. [Google Scholar] [CrossRef]
- Nyagadza, B. Sustainable digital transformation for ambidextrous digital firms: Systematic literature review, meta-analysis and agenda for future research directions. Sustain. Technol. Entrep. 2022, 1, 100020. [Google Scholar] [CrossRef]
- Arowoogun, J.O.; Babawarun, O.; Chidi, R.; Adeniyi, A.O.; Okolo, C.A. A comprehensive review of data analytics in healthcare management: Leveraging big data for decision-making. World J. Adv. Res. Rev. 2024, 21, 1810–1821. [Google Scholar] [CrossRef]
- Revathi, A.; Poonguzhali, S. The role of AIoT-based automation systems using UAVs in smart agriculture. In Revolutionizing Industrial Automation Through the Convergence of Artificial Intelligence and the Internet of Things; IGI Global: Pennsylvania, PA, USA, 2023; pp. 100–117. [Google Scholar] [CrossRef]
- Saxena, A.K.; Dwivedi, R.K.; Parygin, D. Field monitoring and automation in agriculture using internet of things (IoT). In Towards the Integration of IoT, Cloud and Big Data: Services, Applications and Standards; Springer: Berlin/Heidelberg, Germany, 2023; pp. 131–154. [Google Scholar] [CrossRef]
- Balaska, V.; Adamidou, Z.; Vryzas, Z.; Gasteratos, A. Sustainable crop protection via robotics and artificial intelligence solutions. Machines 2023, 11, 774. [Google Scholar] [CrossRef]
- Gawande, V.; Saikanth, D.; Sumithra, B.; Aravind, S.A.; Swamy, G.N.; Chowdhury, M.; Singh, B.V. Potential of precision farming technologies for eco-friendly agriculture. Int. J. Plant Soil Sci. 2023, 35, 101–112. [Google Scholar] [CrossRef]
- Kumar, P.; Raghavendran, S.; Silambarasan, K.; Kannan, K.S.; Krishnan, N. Mobile application using DCDM and cloud-based automatic plant disease detection. Environ. Monit. Assess. 2023, 195, 44. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Rahmani, E.; Khouzani, S.J.; Rahmannia, M.; Ghadirzadeh, E.; Bashghareh, P.; Chichagi, F.; Fard, S.S.; Esmaeili, S.; Tavakoli, R. Role of artificial intelligence in the diagnosis and treatment of diseases. Kindle 2023, 3, 1–160. [Google Scholar]
- Ehsan, I.; Irfan Khalid, M.; Ricci, L.; Iqbal, J.; Alabrah, A.; Sajid Ullah, S.; Alfakih, T.M. A conceptual model for blockchain-based agriculture food supply chain system. Sci. Program. 2022, 2022, 7358354. [Google Scholar] [CrossRef]
- Himeur, Y.; Elnour, M.; Fadli, F.; Meskin, N.; Petri, I.; Rezgui, Y.; Bensaali, F.; Amira, A. Ai-big data analytics for building automation and management systems: A survey, actual challenges and future perspectives. Artif. Intell. Rev. 2023, 56, 4929–5021. [Google Scholar] [CrossRef]
- Lone, R.; Hassan, N.; Bashir, B.; Rohela, G.K.; Malla, N.A. Role of growth elicitors and microbes in stress management and sustainable production of sorghum. Plant Stress 2023, 9, 100179. [Google Scholar] [CrossRef]
- Adachi, T.; El-Hattab, A.W.; Jain, R.; Nogales Crespo, K.A.; Quirland Lazo, C.I.; Scarpa, M.; Summar, M.; Wattanasirichaigoon, D. Enhancing equitable access to rare disease diagnosis and treatment around the world: A review of evidence, policies, and challenges. Int. J. Environ. Res. Public Health 2023, 20, 4732. [Google Scholar] [CrossRef]
- Zhang, Z.; Bills, G.F.; An, Z. Advances in the treatment of invasive fungal disease. PLoS Path. 2023, 19, e1011322. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Impalli, I.; Rangarajan, R.; Cohn, J.; Ramjeet, K.; Trainor, B.W.; Strathdee, S.; Sumpradit, N.; Berman, D.; Wertheim, H. Expanding antibiotic, vaccine, and diagnostics development and access to tackle antimicrobial resistance. Lancet 2024, 403, 2534–2550. [Google Scholar] [CrossRef]
- Mwantimwa, K.; Ndege, N. Transferring knowledge and innovations through village knowledge center in Tanzania: Approaches, impact and impediments. VINE J. Inf. Knowl. Manag. Syst. 2024, 54, 379–397. [Google Scholar] [CrossRef]
- Koutridi, E.; Christopoulou, O. The importance of integrating smart farming technologies into rural policies (aiming at sustainable rural development)-stakeholders’ views. Smart Agric. Technol. 2023, 4, 100206. [Google Scholar] [CrossRef]
- Dosso, F.; Gouroubera, M.W.; Idrissou, L.; Moumouni-Mousa, I. The combination of extension approaches strengthens farmers’ innovativeness in sustainable land management. Environ. Dev. Sustain. 2024, 26, 10043–10062. [Google Scholar] [CrossRef]
- Singh, N.; Sunitha, N.; Tripathi, G.; Saikanth, D.; Sharma, A.; Jose, A.E.; Mary, M. Impact of digital technologies in agricultural extension. Asian J. Agric. Ext. Econ. Sociol. 2023, 41, 963–970. [Google Scholar] [CrossRef]
- Shaikh, T.A.; Rasool, T.; Lone, F.R. Towards leveraging the role of machine learning and artificial intelligence in precision agriculture and smart farming. Comput. Electron. Agric. 2022, 198, 107119. [Google Scholar] [CrossRef]
- Lankinen, Å.; Witzell, J.; Aleklett, K.; Furenhed, S.; Karlsson Green, K.; Latz, M.; Liljeroth, E.; Larsson, R.; Löfkvist, K.; Meijer, J. Challenges and opportunities for increasing the use of low-risk plant protection products in sustainable production. A review. Agron. Sustain. Dev. 2024, 44, 21. [Google Scholar] [CrossRef]
- Araya, T.; Ochsner, T.E.; Mnkeni, P.N.; Hounkpatin, K.; Amelung, W. Challenges and constraints of conservation agriculture adoption in smallholder farms in sub-Saharan Africa: A review. Int. Soil Water Conserv. Res. 2024, 12, 828–843. [Google Scholar] [CrossRef]
- Van Asseldonk, M.; Girvetz, E.; Pamuk, H.; Wattel, C.; Ruben, R. Policy incentives for smallholder adoption of climate-smart agricultural practices. Front. Political Sci. 2023, 5, 1112311. [Google Scholar] [CrossRef]
- Wuepper, D.; Wiebecke, I.; Meier, L.; Vogelsanger, S.; Bramato, S.; Fürholz, A.; Finger, R. Agri-environmental policies from 1960 to 2022. Nat. Food 2024, 5, 323–331. [Google Scholar] [CrossRef]
- Khangura, R.; Ferris, D.; Wagg, C.; Bowyer, J. Regenerative agriculture—A literature review on the practices and mechanisms used to improve soil health. Sustainability 2023, 15, 2338. [Google Scholar] [CrossRef]
- Bekee, B.; Segovia, M.S.; Valdivia, C. Adoption of smart farm networks: A translational process to inform digital agricultural technologies. Agric. Hum. Values 2024, 41, 1573–1590. [Google Scholar] [CrossRef]
- Konfo, T.R.C.; Djouhou, F.M.C.; Hounhouigan, M.H.; Dahouenon-Ahoussi, E.; Avlessi, F.; Sohounhloue, C.K.D. Recent advances in the use of digital technologies in agri-food processing: A short review. Appl. Food Res. 2023, 3, 100329. [Google Scholar] [CrossRef]
- Zampati, F. Ethical and legal considerations in smart farming: A farmer’s perspective. In Towards Responsible Plant Data Linkage: Data Challenges for Agricultural Research and Development; Springer: Cham, Switzerland, 2023; Volume 257. [Google Scholar] [CrossRef]
- Rosace, M.; Björklund, N.; Boberg, J.; Bradshaw, C.; Camac, J.; Damus, M.; Kompas, T.; Li, C.; MacLeod, A.; Maggini, R. Including climate change in pest risk assessment: Current practices and perspectives for future implementation. EPPO Bull. 2024, 54, 52–72. [Google Scholar] [CrossRef]
- Ojeyinka, O.T.; Omaghomi, T.T. Climate change and zoonotic diseases: A conceptual framework for predicting and managing health risks in the USA. GSC Biol. Pharm. Sci. 2024, 26, 027–036. [Google Scholar] [CrossRef]
- Adisa, O.; Ilugbusi, B.S.; Adewunmi, O.; Franca, O.; Ndubuisi, L. A comprehensive review of redefining agricultural economics for sustainable development: Overcoming challenges and seizing opportunities in a changing world. World J. Adv. Res. Rev. 2024, 21, 2329–2341. [Google Scholar] [CrossRef]
- Shang, M.; Xie, J. Agricultural sustainable development: Soil, water resources, biodiversity, climate change, and technological innovation. Adv. Resour. Res. 2024, 4, 181–204. [Google Scholar]
- Maiwada, U.D.; Qabasiyu, M.G.; Dauda, M.H.; Aliyu, A.A. Effects of 5g network and climatic fluctuations on sorghum yield in Nigeria. Int. J. Sci. Res. 2024, 4, 18–31. [Google Scholar]
- Yusuph, A.S.; Nzunda, E.F.; Mourice, S.K.; Dalgaard, T. Usage of agro ecological climate-smart agriculture practices among sorghum and maize smallholder farmers in semi-arid areas in Tanzania. East Afr. J. Agric. Biotechnol. 2023, 6, 378–405. [Google Scholar] [CrossRef]
- Nhliziyo, N.; Mushunje, A. Determinants of small-scale farmers’ participation in social capital networks to enhance adoption of climate change adaptation strategies in or Tambo district, south Africa. Agriculture 2024, 14, 441. [Google Scholar] [CrossRef]
- Sylla, A.; Yila, J.O.; Traore, S. The choice and preference of sorghum value chain actors in mali. Int. J. Sociol. Anthropol. 2023, 15, 41–58. [Google Scholar] [CrossRef]
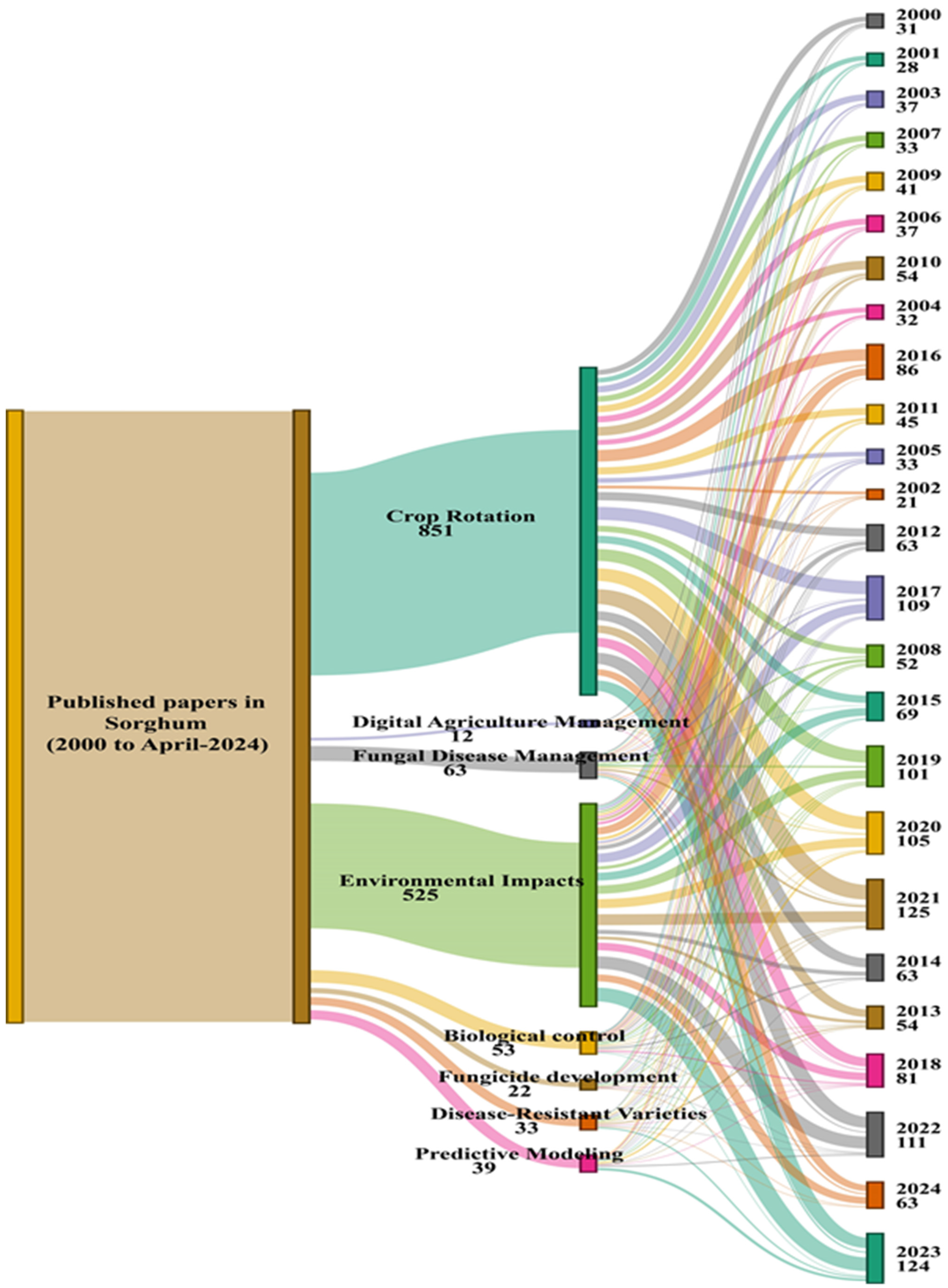
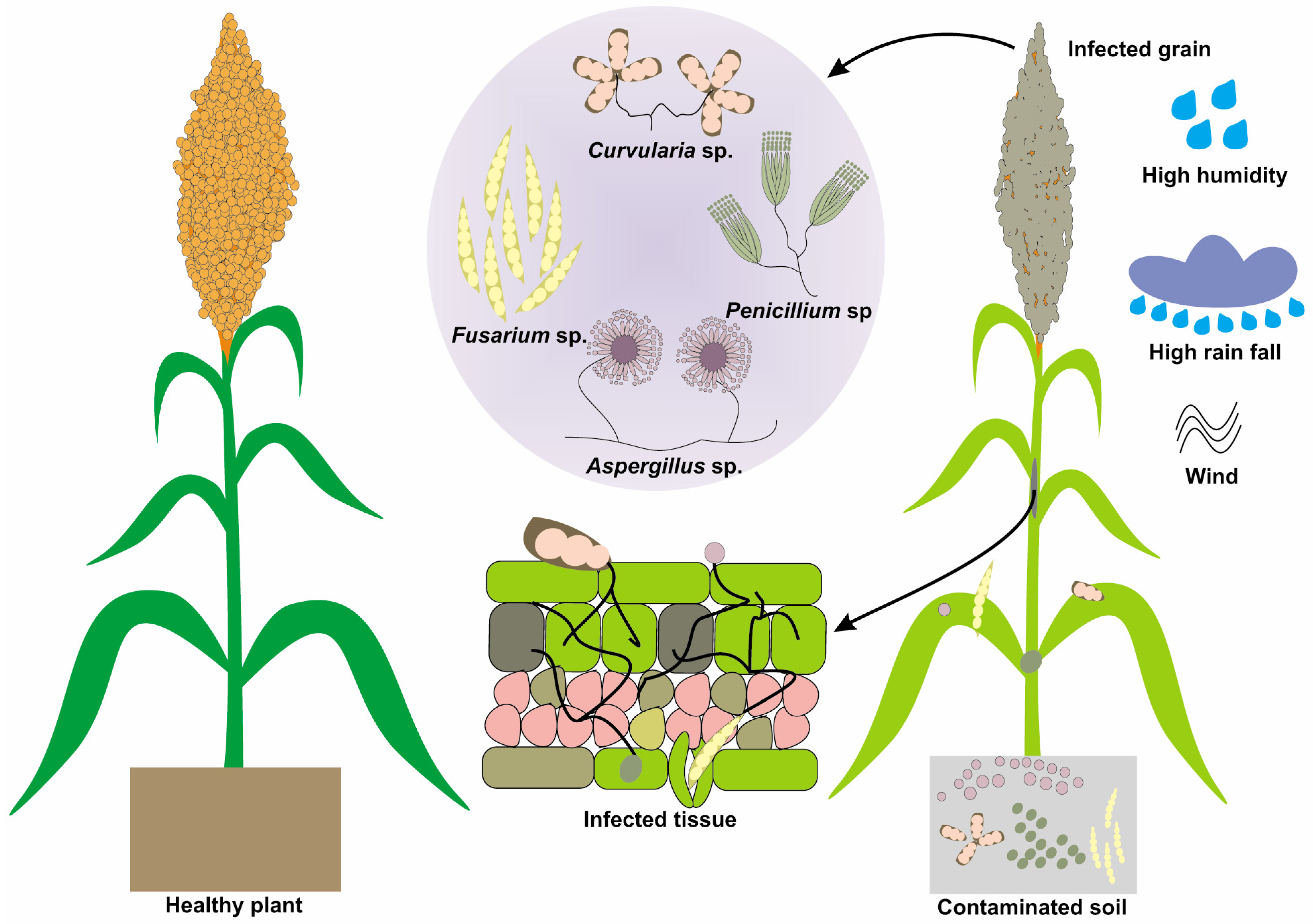

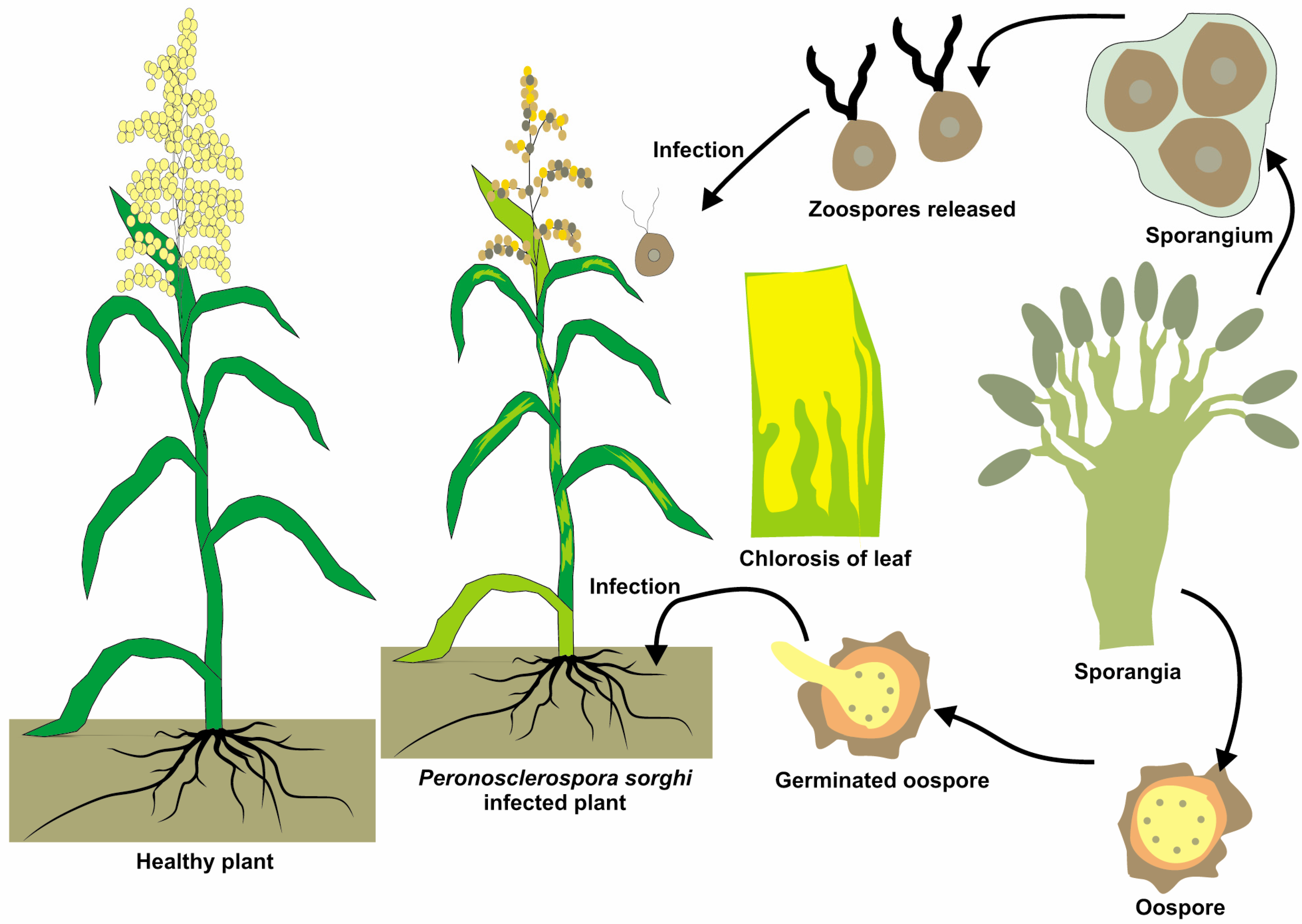
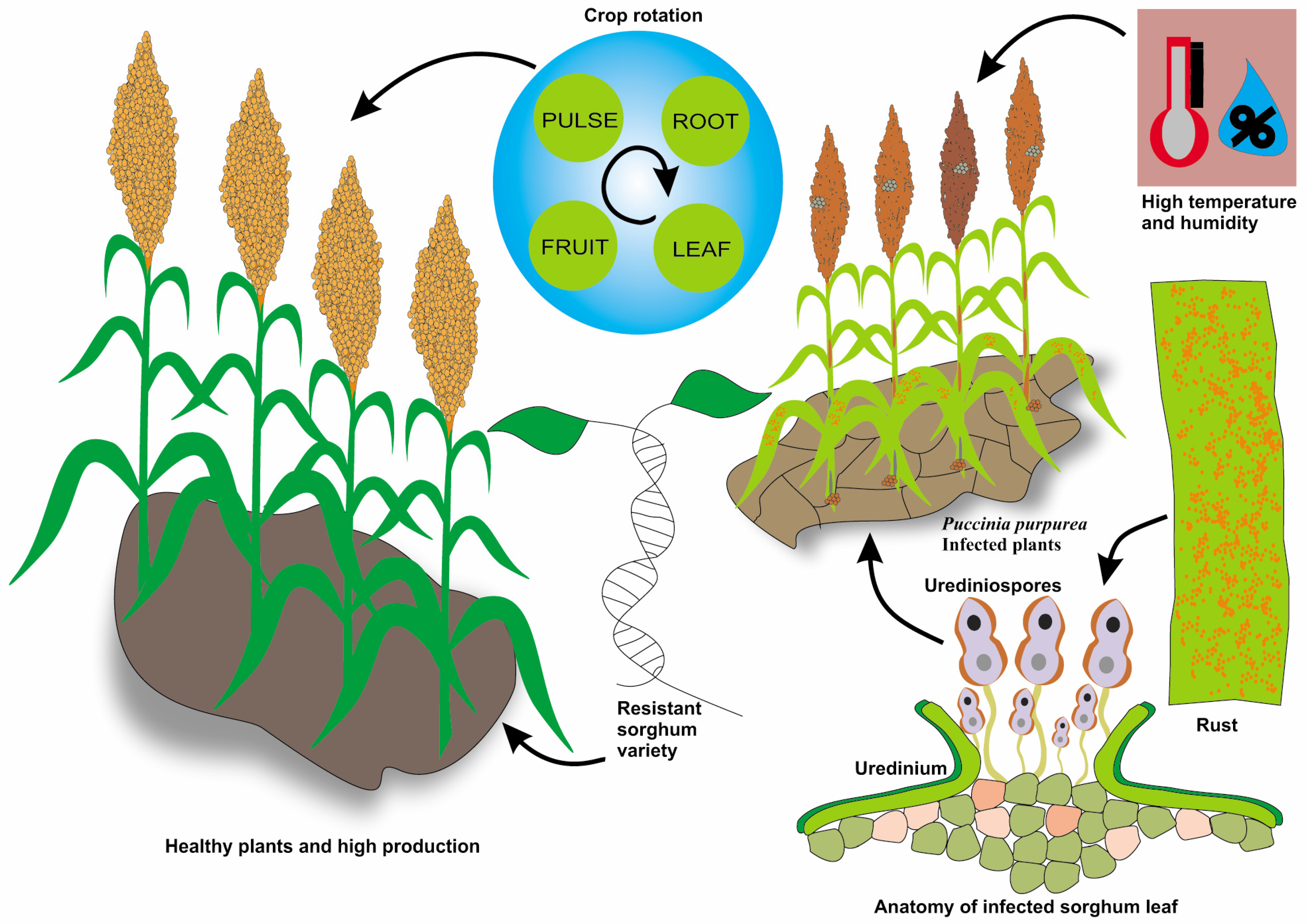
| Practice | Key Points | Implications | References |
|---|---|---|---|
| Planting and Crop Rotation | Tilling or no-till methods, soil health, disease risk, crop rotation benefits | Balancing soil health and disease control, crop diversity | [53,54,55] |
| Irrigation and Drainage | Stable yield vs. fungal pathogen risk, wet condition diseases | Irrigation management, disease prevention in wet conditions | [29,56] |
| Fertilization | Nutrient management, nitrogen levels, plant health vs. fungal growth | Optimizing fertilization, balancing growth and disease resistance | [57,58,59] |
| Pest Management | Chemical, biological, and mechanical methods; insect–fungus interaction | Effective pest control to reduce disease spread | [60,61,62,63] |
| Harvest and Post-Harvest Practices | Harvest timing, pot-harvest handling, grain mold risk, disease inoculum | Reduced disease prevalence, effective crop residue management | [64,65,66] |
| Aspect | Key Points | Implications | References |
|---|---|---|---|
| Yield Protection | Yield losses, Total crop failure | Farmer income protection, crop productivity | [18,67] |
| Grain Quality Maintenance | Reduced grain market value, grain mold effects | Market competitiveness, grain quality | [68,69] |
| Food Security | Staple food, impact on local food security | Community nutrition, dependence on sorghum | [70,71] |
| Feed and Industrial Uses | Usage in animal feed and biofuel, quality and availability impact | Industrial and feed sector reliance | [13,14,72] |
| Environmental Stewardship | Sustainable practices, reduced chemical use | Environmental health, biodiversity promotion | [20,21,73] |
| Economic Stability | Market stability, economic planning impact | Regional/national economic health | [74,75] |
| Fungal Phytopathogens | Key Symptoms | Impact | Prevalence (Percentage Contribution) | Key Management Strategies | References |
|---|---|---|---|---|---|
| Ergot (Claviceps africana) | Honeydew structures, sclerotia formation | Reduced yield and grain quality, toxic sclerotia | 25% | Planting tolerant varieties, crop rotations with legumes | [83,84] |
| Head smut (Sporisorium reilianum) | Grain head replaced with spore mass | Significant yield loss, plants fail to produce grain | 15% | Crop rotation, resistant varieties | [91,92,157] |
| Leaf blight (Exserohilum turcicum) | Rectangular, tan leaf lesions | Reduced yield from impaired photosynthesis, leaf death | 20% | Agronomic cultural practices, the use of resistant or tolerant cultivars, biological control and chemical methods | [119,158] |
| Stalk rot (Fusarium, macrophomina) | Soft, rotten stalk base, plant lodging | Significant yield loss, especially when plants lodge | 10% | Resistant varieties, agronomic practices | [27,120] |
| Sooty stripe (Ramulispora sorghi) | Yellow to tan leaf stripes, sooty spores | Variable yield impact, significant under high disease pressure | 30% | Use protectant fungicides, cultural methods, seed soaking method, rotation of crops, manage proper planting distance | [139,140] |
| Fungal Phytopathogens | Key Symptoms | Impact | Stages of Plant Growth Most Affected | Management Strategies | References |
|---|---|---|---|---|---|
| Anthracnose (Colletotrichum sublineolum) | Water-soaked lesions on leaves, leaf/stem/panicle rot | Yield loss, infected plant debris as fungal source | Vegetative stage affects leaf and stem development, reproductive stage impacts panicle formation and grain development | Crop rotation, cultural practices, use resistant hybrid cultivars, use deep plowing leftover the crop residues from soil, biological control. | [93,159] |
| Grain Mold Complex (Fusarium spp., Curvularia spp., and others) | Grain discoloration, shriveling, quality reduction | Reduced grain quality, problematic in humid conditions | Grain filling stage affects kernel development, maturity stage increases susceptibility to mold formation | Maintain optimal plant population, crop adopting pest management practices, crop rotation, planting sorghum hybrids varieties, harvest panicles timely dry them quickly under natural sunlight, sort out moldy and damaged panicles, prevent insect damage stored grain suitable fumigation, monitor sorghum grain production, process and storage stages for mycotoxin contamination. | [31,33,160] |
| Charcoal rot (Macrophomina phaseolina) | Wilting, yellowing leaves, silver-gray stalk, microsclerotia | Significant yield loss, stalk lodging in drought conditions | Mid to late vegetative stages causes wilting and leaf yellowing, reproductive stage leads to stalk lodging, especially under drought stress | Use fungicides to inhibit mycelial growth, combination of soil solarization and organic amendment, controlling pathogens by soil mulching and large coverings with transparent polyethylene tarp, crop rotation, tillage practices, and reduce soil moisture. | [49,161] |
| Downy Mildew (Peronosclerospora sorghi) | White to purple downy growth on leaves, chlorosis, stunting | Yield loss, sterile heads, or no heads in severe cases | Vegetative stage causes leaf chlorosis and stunting, early reproductive stage reduces head formation and fertility | Use chemical, genetic, and cultural methods, use resistant sorghum varieties, seed treatments with the systemic fungicides metalaxyl and mefenoxam, cultural controls, crop rotation, deep tillage. | [113,121] |
| Rust (Puccinia purpurea) | Small, round pustules on leaves, leaf blight, early senescence | Reduced photosynthesis, impact on yield and grain quality | Vegetative stage affects leaf health and photosynthesis, reproductive stage impacts flowering and grain development | Cultivating resistant varieties, cultivating the slow-rusting sorghum varieties, use cultural practices, destroy infected residues from crop and weed hosts, use healthy seed for planting, treat seeds to prevent urediniospore seed-borne infection. | [97,138,162] |
| Strategy | Key Features | Potential Benefits | References |
|---|---|---|---|
| Bio-based Fungicides | Natural substances, less environmental impact | Eco-friendly, safe for non-target organisms | [184,185] |
| Nano-formulations | Improved absorption, reduced doses, prolonged activity | Increased efficacy, targeted delivery, environmental safety | [186] |
| Fungicide Mixtures | Multiple fungicides, enhanced efficacy, resistance management | Improved disease control, reduced resistance risk | [187,188] |
| New Modes of Action | Novel action modes, controls resistant fungi | Effective against resistant strains, delays resistance development | [185,189] |
| Seed Treatment Fungicides | Protection from seed borne/pathogens, reduced foliar applications | Early protection, reduced chemical use | [36,190] |
| Strategy | Key Features | Potential Benefits | References |
|---|---|---|---|
| Quantitative Trait Loci Mapping | Mapping genes/gene regions, disease resistance | Efficient trait selection, complex trait management | [191,267] |
| Genome-Wide Association Studies | Genetic variant identification, resistance gene location | Understanding gene function and interaction | [157,268] |
| Transcriptomics | Gene expression analysis during infection | Identification of key resistance genes | [269] |
| Proteomics and Metabolomics | Defense response proteins and metabolites analysis | Mechanism revelation, new target identification | [184,270] |
| Genomic Selection | Performance prediction, genomic data utilization | Accelerated breeding process | [254,271] |
| Genome Editing | Precise gene editing, disease resistance enhancement | Direct modification of defense genes | [264,272] |
| Strategy | Key Features | Potential Benefits | Application | References |
|---|---|---|---|---|
| Integrate Disease Management | Genetic resistance, crop management, biological and chemical control | Improved effectiveness, sustainability | Fungal disease management in sorghum | [300,301] |
| Precision Farming | Real-time data, site-specific management, precision in fungicide application | Increased efficacy, cost reduction, Environmental protection | Site-specific sorghum farming practices | [279,302] |
| Breeding Technologies | Genomic selection, genome editing, advanced breeding | Efficient disease-resistant variety development | Breeding of sorghum varieties | [156,258] |
| Biological Control | Beneficial microbes, bio-fungicides, microbiome engineering | Enhanced natural plant defenses | Control of fungal diseases in sorghum | [185,303] |
| Predictive Models | Accuracy, accessibility, early warning | Optimized disease control, preventative measures | Disease management decision-making | [286,304] |
| Climate-Smart Agriculture | Resistant varieties, adapted crop management, climate adaptation | Resilience to climate change, disease resistance | Adapting sorghum farming to climate change | [300,305] |
| Farmer Training and Extension Services | Disease recognition education, localized extension services | Effective implementation, knowledge sharing | Farmer education and support | [306] |
| Policy and Institutional Support | Research support, incentives for sustainable practices, seed system establishment | Promotion of improved practices, policy support | Supporting sorghum disease management | [122,307] |
| Technology | Key Features | Potential Impact | Use Case | References |
|---|---|---|---|---|
| Remote Sensing and Imagery Analysis | Satellite imagery, drone-based sensing, machine learning analysis | Early disease detection | Disease identification | [290,310] |
| IoT and Sensor Technology | Temperature, humidity, rainfall, soil moisture monitoring | Risk prediction, informed decisions | Field condition monitoring | [101,311] |
| Predictive Modeling | Historical data analysis, predictive outbreak modeling | Early warning, preventative actions | Disease outbreak prediction | [49,312] |
| Precision Agriculture | Site-specific disease management, variable rate technology | Optimized fungicide use | Disease management | [294,313] |
| Mobile and Cloud-Based Apps | Real-time data, predictive models, decision support tools | Accessible information, enhanced communication | Farmer decision support | [61,314] |
| Artificial Intelligence and Machine Learning | Pattern detection, predictive insights, improved decision accuracy | Enhanced detection and prediction | Data analysis and insights | [278,315] |
| Block chain Technology | Secure data records, traceability, data integrity | Data security, traceability | Data management and security | [316] |
| Data Analytics and Decision Support Systems | Insight extraction, expert knowledge integration | Evidence-based recommendations | Decision support and analytics | [309,317] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaskheli, M.A.; Nizamani, M.M.; Tarafder, E.; Das, D.; Nosheen, S.; Muhae-Ud-Din, G.; Khaskheli, R.A.; Ren, M.-J.; Wang, Y.; Yang, S.-W. Sustainable Management of Major Fungal Phytopathogens in Sorghum (Sorghum bicolor L.) for Food Security: A Comprehensive Review. J. Fungi 2025, 11, 207. https://doi.org/10.3390/jof11030207
Khaskheli MA, Nizamani MM, Tarafder E, Das D, Nosheen S, Muhae-Ud-Din G, Khaskheli RA, Ren M-J, Wang Y, Yang S-W. Sustainable Management of Major Fungal Phytopathogens in Sorghum (Sorghum bicolor L.) for Food Security: A Comprehensive Review. Journal of Fungi. 2025; 11(3):207. https://doi.org/10.3390/jof11030207
Chicago/Turabian StyleKhaskheli, Maqsood Ahmed, Mir Muhammad Nizamani, Entaj Tarafder, Diptosh Das, Shaista Nosheen, Ghulam Muhae-Ud-Din, Raheel Ahmed Khaskheli, Ming-Jian Ren, Yong Wang, and San-Wei Yang. 2025. "Sustainable Management of Major Fungal Phytopathogens in Sorghum (Sorghum bicolor L.) for Food Security: A Comprehensive Review" Journal of Fungi 11, no. 3: 207. https://doi.org/10.3390/jof11030207
APA StyleKhaskheli, M. A., Nizamani, M. M., Tarafder, E., Das, D., Nosheen, S., Muhae-Ud-Din, G., Khaskheli, R. A., Ren, M.-J., Wang, Y., & Yang, S.-W. (2025). Sustainable Management of Major Fungal Phytopathogens in Sorghum (Sorghum bicolor L.) for Food Security: A Comprehensive Review. Journal of Fungi, 11(3), 207. https://doi.org/10.3390/jof11030207










