Fungal-Infected Weeds: A Potential Source of Leaf Spot Disease in Rubber Trees from Southern Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Fungal Isolation
2.2. Morphology Study
2.3. Molecular Identification
2.4. Pathogenicity Test
3. Results
3.1. Symptom Recognition
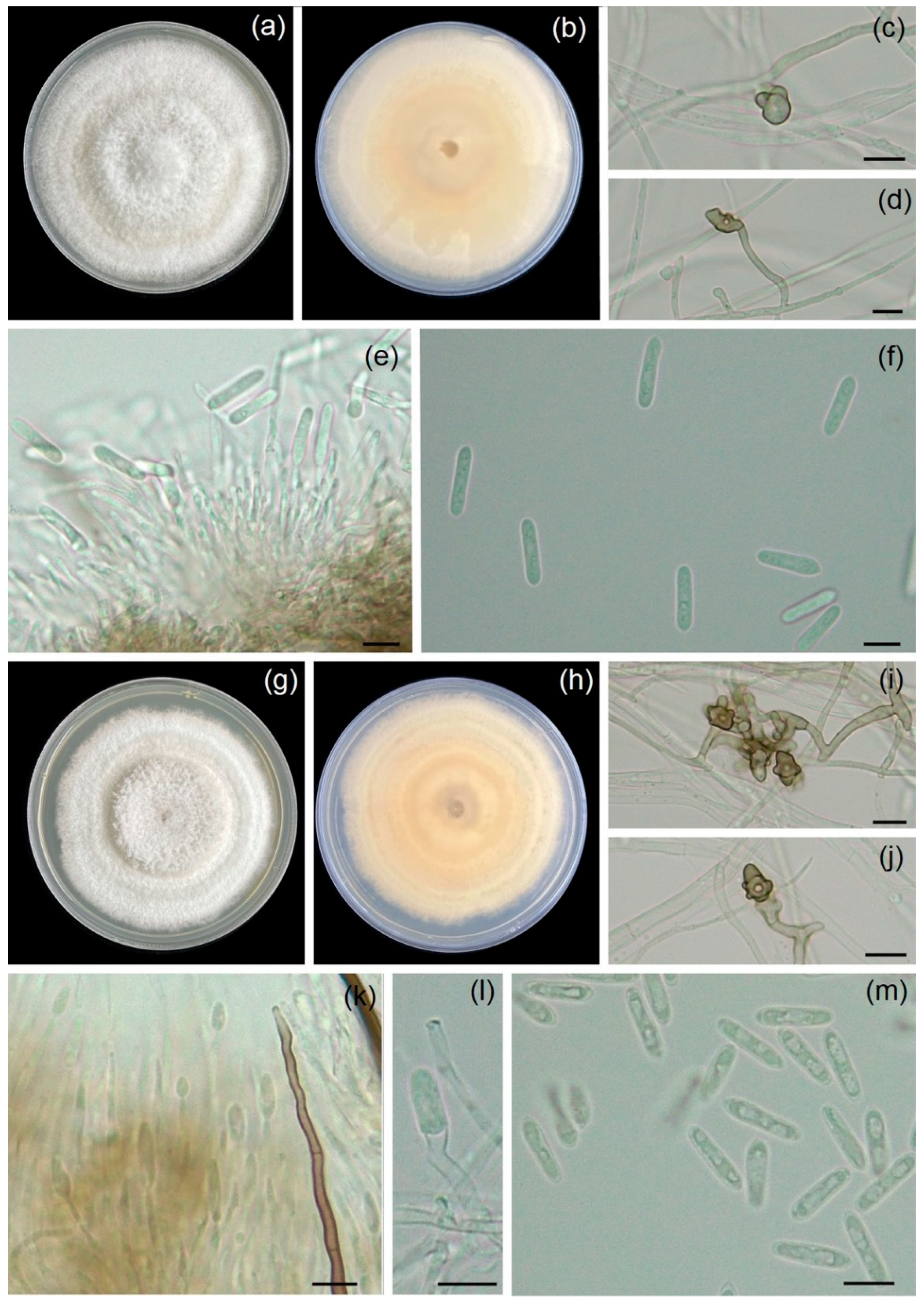
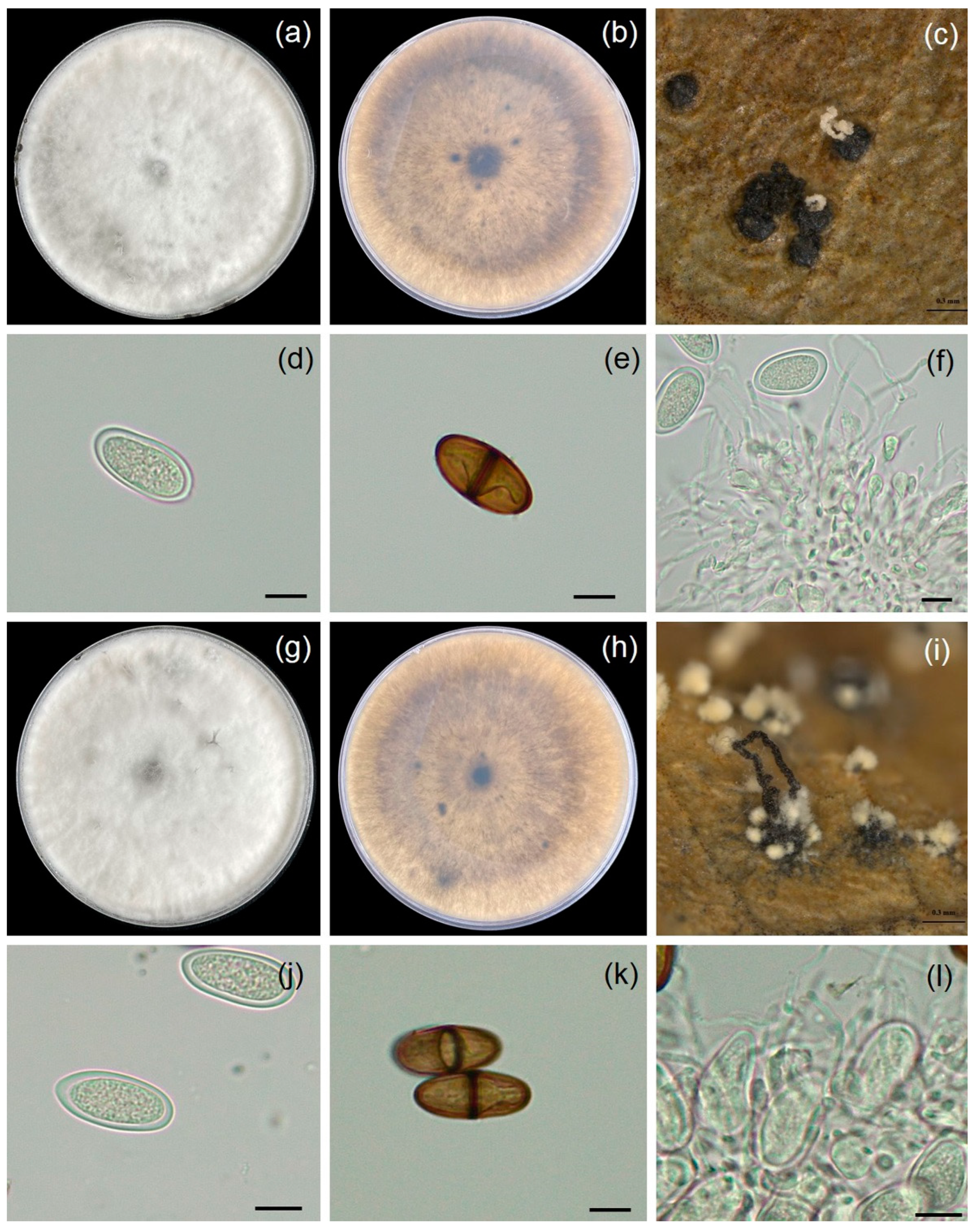
3.2. Morphology Study
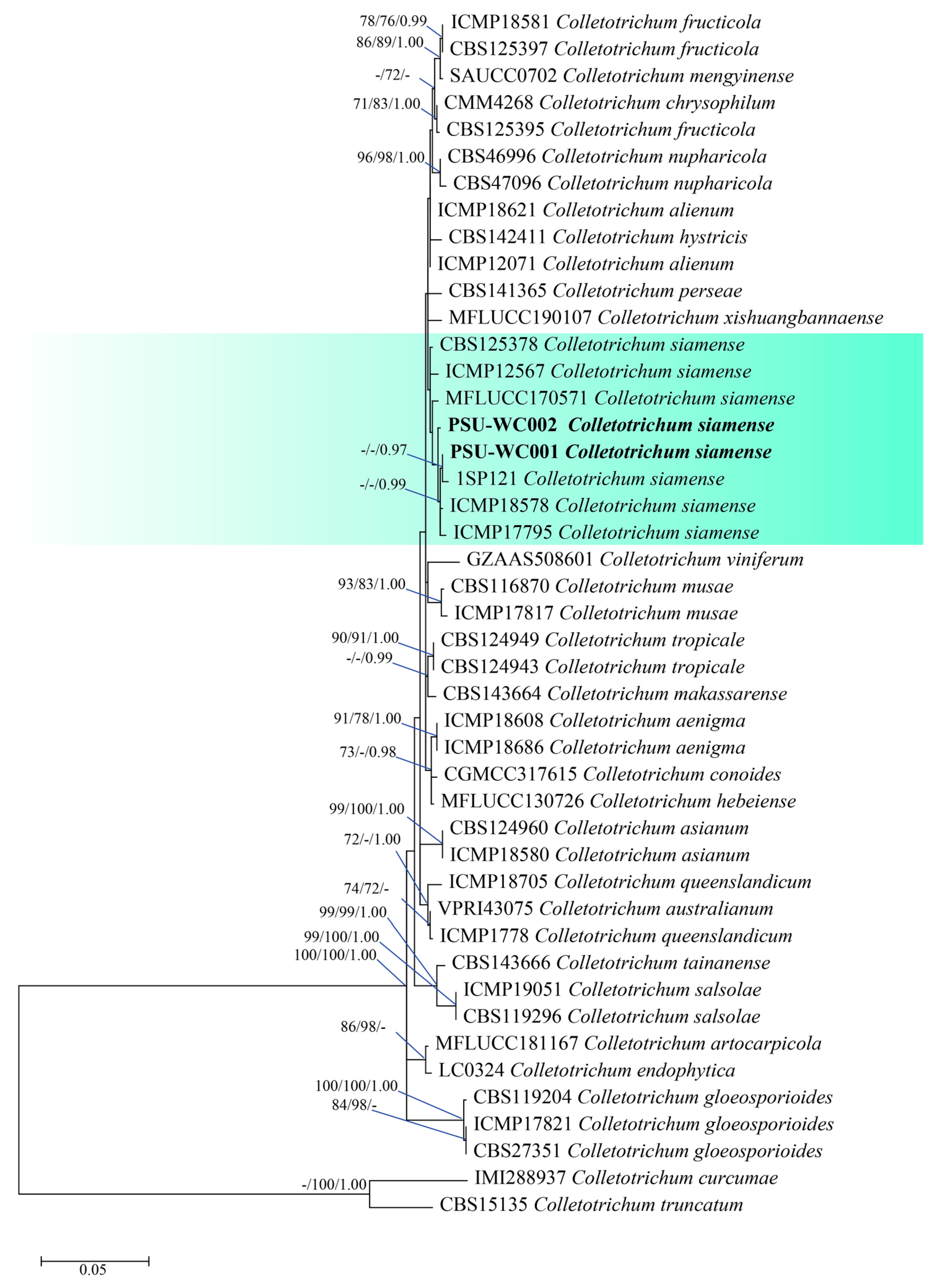
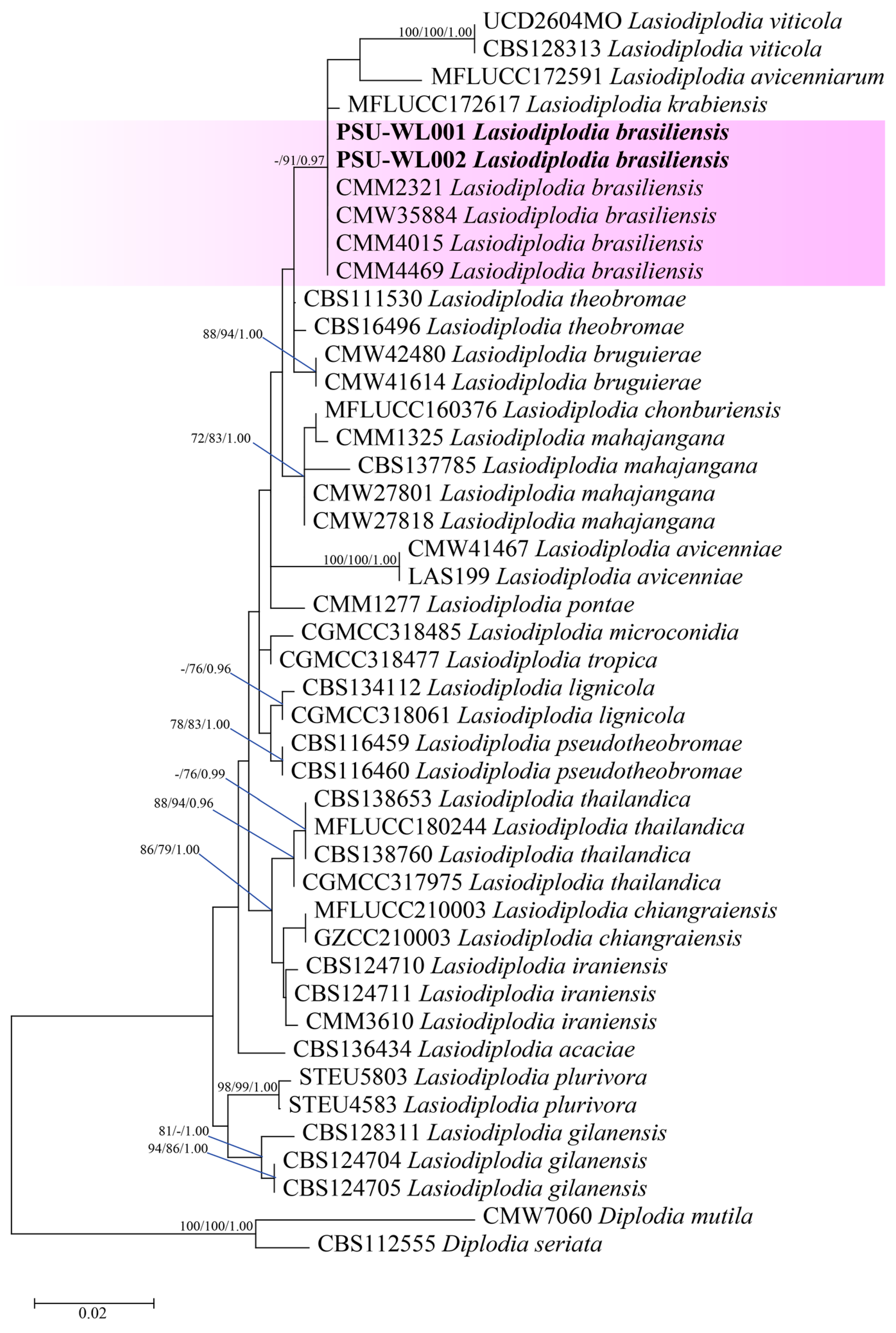
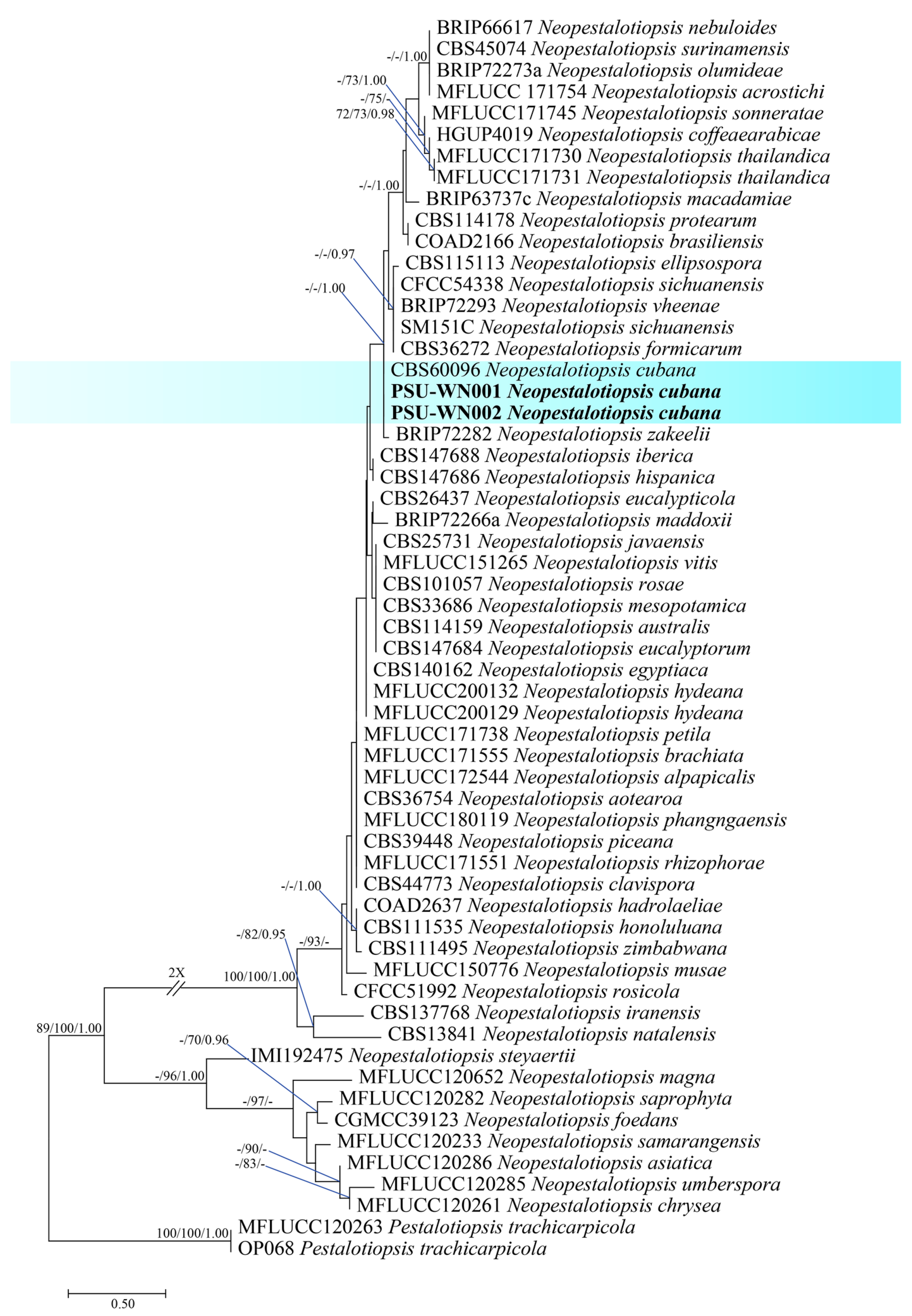
3.3. Molecular Identification
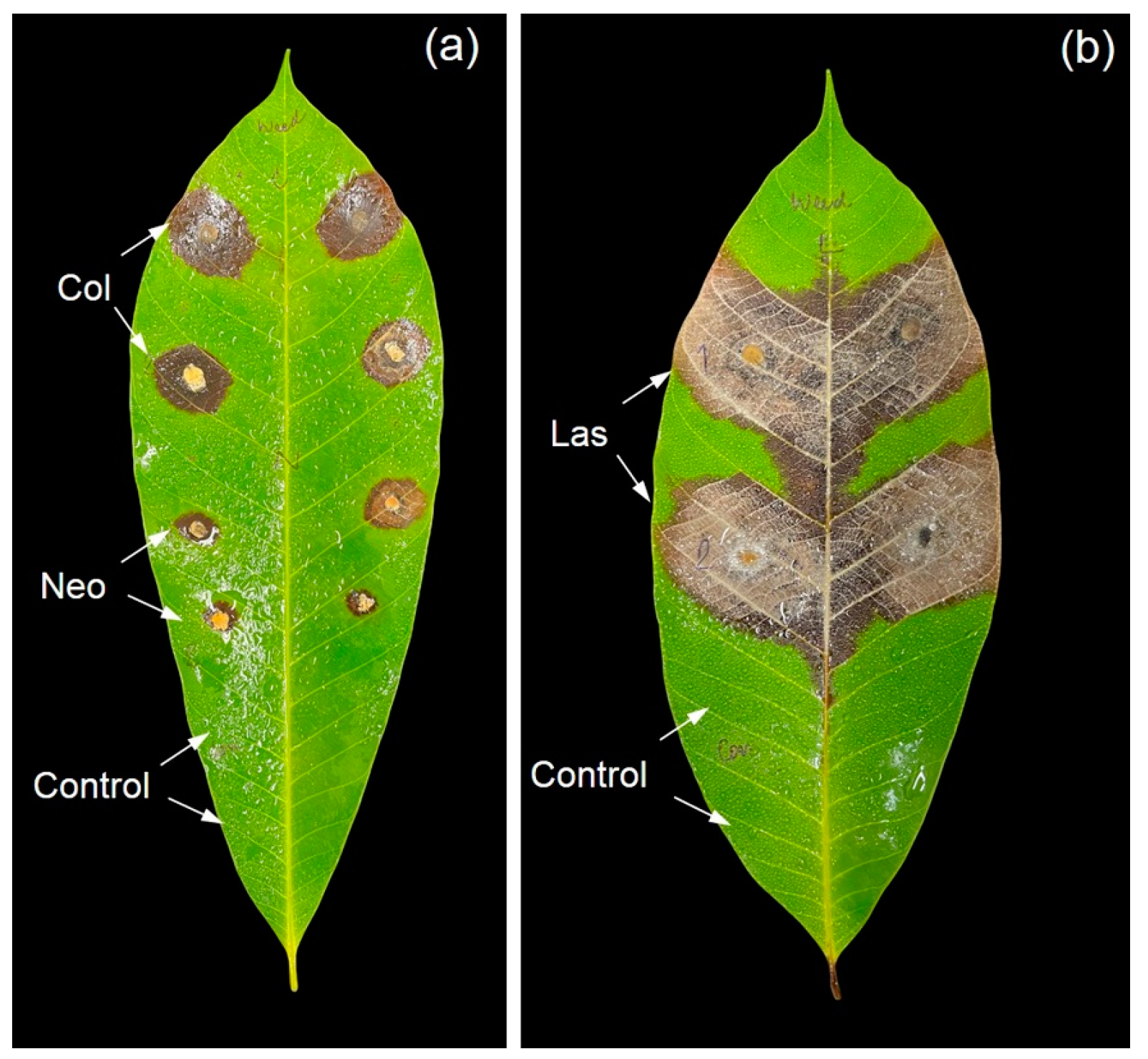
3.4. Pathogenicity Test
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayashi, Y. Production of natural rubber from Para rubber tree. Plant Biotechnol. 2009, 26, 67–70. [Google Scholar] [CrossRef]
- Li, Z.; Fox, J.M. Mapping rubber tree growth in mainland Southeast Asia using time-series MODIS 250 m NDVI and statistical data. Appl. Geogr. 2012, 32, 420–432. [Google Scholar] [CrossRef]
- Ghini, R.; Bettiol, W.; Hamada, E. Diseases in tropical and plantation crops as affected by climate changes: Current knowledge and perspectives. Plant Pathol. 2011, 60, 122–132. [Google Scholar] [CrossRef]
- Ngobisa, A.N.; Abidin, M.Z.; Wong, M.; Noordin, M.W. Neofusicoccum ribis associated with leaf blight on rubber (Hevea brasiliensis) in Peninsular Malaysia. Plant Pathol. J. 2013, 29, 10. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Y.; Li, G.; Wang, Y.; Zhou, M. First report of Alternaria alternata causing black leaf spot of rubber tree in China. Plant Dis. 2015, 99, 290. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Zhang, X.; Qi, Y.; Xie, Y.; Zhang, H.; Zhang, H. First record of Corynespora leaf fall disease of Hevea rubber tree in China. Australas. Plant Dis. Notes 2007, 2, 35–36. [Google Scholar]
- Hashim, I.; Chee, K.H.; Duncan, E.J. Damage assessment and yield losses due to Corynespora leaf fall disease in mature rubber plantations. J. Rubber Res. 2019, 22, 217–226. [Google Scholar]
- Ogbebor, N.O.; Omorusi, V.I.; Orumwense, K. Biochemical mechanisms of rubber tree root degradation by Rigidoporus microporus and implications for control strategies. For. Pathol. 2022, 52, e12715. [Google Scholar]
- Sreelatha, S.; Joseph, K. Anatomical and biochemical alterations in rubber tree bark following Phytophthora infection and their relation to latex yield reduction. Trees 2021, 35, 145–157. [Google Scholar]
- Wong, M.Y.; Zulkifli, Y.; Sunderasan, E. Phytotoxins produced by Pestalotiopsis species in rubber plantations: Detection, characterization and significance. Eur. J. Plant Pathol. 2020, 156, 1151–1163. [Google Scholar]
- Liyanage, K.K.; Khan, S.; Brooks, S. Climate-dependent development of rubber tree pathogens: Implications for disease management under environmental change. Plant Pathol. 2018, 67, 1026–1038. [Google Scholar]
- Liyanage, A.S.; Peries, O.S.; Liyanage, N.I.S. Major diseases of rubber and their management. In Natural Rubber: Biology, Cultivation and Technology; Elsevier Science Publishers: Amsterdam, The Netherlands, 2016; pp. 213–238. [Google Scholar]
- Jayasinghe, C.K. Rubber tree diseases: Economic impact and management strategies. Mycosphere 2020, 11, 795–817. [Google Scholar]
- Norhayati, M.; Nair, H.K.M.; Wong, M.Y. Economic assessment of fungicide application in rubber plantations in Malaysia. J. Plant. Crops 2019, 47, 182–191. [Google Scholar]
- Vongkhamheng, C.; Phimmavong, S.; Koutrakis, E. Economic impacts of white root disease on smallholder rubber plantations in Northern Laos. Forests 2022, 13, 512–526. [Google Scholar]
- European Forest Institute and Rubber Authority of Thailand. Briefing—Thailand’s Natural Rubber Producers Are Preparing for New Market Requirements. Available online: https://efi.int/sites/default/files/files/publication-bank/2024/Briefing%20-%20Thailand’s%20natural%20rubber%20producers%20are%20preparing%20for%20new%20market%20requirements.pdf (accessed on 28 February 2025).
- Puengcharoen, S.; Sirisomboon, P.; Duangpatra, J. Climate change effects on rubber tree diseases in Thailand: A review and future perspectives. Plant Pathol. 2021, 70, 1265–1280. [Google Scholar]
- Chambon, B.; Ruf, F.; Kongmanee, C.; Angthong, S. Can the cocoa industry solve the rubber smallholders’ crisis? A proposal from Thailand. For. Policy Econ. 2018, 85, 132–141. [Google Scholar]
- Thaochan, N.; Pornsuriya, C.; Chairin, T.; Chomnunti, P.; Sunpapao, A. Morphological and molecular characterization of Calonectria foliicola associated with leaf blight on rubber tree (Hevea brasiliensis) in Thailand. J. Fungi 2022, 8, 986. [Google Scholar] [CrossRef]
- Athinuwat, D.; Ruangwong, O.-U.; Harishchandra, D.L.; Latehnuering, F.; Sunpapao, A. Morphology and molecular characterization of Colltetotrichum siamense associated with leaf spot disease of rubber tree (Hevea brasiliensis) in southern Thailand. Physiol. Mol. Plant Pathol. 2024, 130, 102248. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Thaochan, N.; Chairin, T.; Sunpapao, A. Morphological and phylogenetic evidences reveal Lasiodiplodia chonburiensis and L. theobromae associated with leaf blight in Hevea brasiliensis in Southern Thailand. Diversity 2023, 15, 961. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Chairin, T.; Thaochan, N.; Sunpapao, A. Identification and characterization of Neopestalotiopsis fungi associated with a novel leaf fall disease of rubber trees (Hevea brasiliensis) in Thailand. J. Phytopathol. 2020, 168, 416–427. [Google Scholar] [CrossRef]
- Anderson, J.M.; Aitken, E.A.B.; Dann, E.K.; Coates, L.M. Morphological and molecular diversity of Colletotrichum spp. causing pepper spot and anthracnose of lychee (Litchi chinensis) in Australia. Plant Pathol. 2013, 62, 279–288. [Google Scholar] [CrossRef]
- Coutinho, I.B.L.; Freire, F.C.O.; Lima, C.S.; Lima, J.S.; Gonçalves, F.J.T.; Machado, A.R.; Silva, A.M.S.; Cardoso, J.E. Diversity of genus Lasiodiplodia associated with perennial tropical fruit plants in northeastern Brazil. Plant Pathol. 2017, 66, 90–104. [Google Scholar] [CrossRef]
- Darapanit, A.; Boonyuen, N.; Leesutthiphonchai, W.; Nuankaew, S.; Piasai, O. Identification, pathogenicity and effects of plant extracts on Neopestalotiopsis and Pseudopestalotiopsis causing fruit diseases. Sci. Rep. 2021, 11, 22606. [Google Scholar] [CrossRef]
- Saitoh, K.-i.; Togashi, K.; Arie, T.; Teraoka, T. A simple method for a mini-preparation of fungal DNA. J. Gen. Plant Pathol. 2006, 72, 348–350. [Google Scholar] [CrossRef]
- White, T. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press, Inc.: Cambridge, MA, USA, 1990. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Guerber, J.C.; Liu, B.; Correll, J.C.; Johnston, P.R. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 2003, 95, 872–895. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Ito, S.I.; Sunpapao, A. First report of leaf spot on lettuce caused by Curvularia aeria. J. Gen. Plant Pathol. 2018, 84, 296–299. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Li, M.; Feng, W.; Yang, J.; Gao, Z.; Zhang, Z.; Zhang, W.; Wang, S.; Wang, W.; Gong, D.; Hu, M. First report of anthracnose caused by Colletotrichum siamense on avocado fruits in China. Crop Prot. 2022, 155, 105922. [Google Scholar] [CrossRef]
- Pacheco-Esteva, M.C.; Soto-Castro, D.; Vásquez-López, A.; Tovar-Pedraza, J.M. First report of Colletotrichum siamense causing papaya anthracnose in Mexico. J. Plant Pathol. 2022, 104, 1175. [Google Scholar] [CrossRef]
- Li, H.; Liao, Y.-C.-Z.; Wan, Y.; Li, D.-W.; Zhu, L.-H. Colletotrichum siamense, a novel causal agent of Viburnum odoratissimum leaf blotch and its sensitivity to fungicides. J. Fungi 2023, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.P.S.S.; Negreiros, A.M.P.; Barros, A.P.O.; Souza, D.M.S.; Michereff, S.J.; Sales, R., Jr.; Correia, K.C. First report of Lasiodiplodia brasiliensis causing root rot on watermelon in Brazil. Plant Dis. 2023, 107, 573. [Google Scholar] [CrossRef] [PubMed]
- Maia da Silva, R.; Ambrósio, M.M.d.Q.; Neto, J.A.d.S.; Silva, J.L.d.S.; da Costa, T.E.; Figueiredo, F.R.A.; Barroso, K.A.; da Silva, W.; Holanda, I.S.A. First report of Lasiodiplodia brasiliensis causing root rot in melon plants in northeastern Brazil. Plant Dis. 2024, 108, 219. [Google Scholar] [CrossRef]
- Khoo, Y.W.; Tan, H.T.; Khaw, Y.S.; Li, S.-F.; Chong, K.P. First report of Neopestalotiopsis cubana causing leaf blight on Ixora chinensis in Malaysia. Plant Dis. 2022, 106, 2747. [Google Scholar] [CrossRef]
- Johanson, D.; Wyse, D.; Janes, K. Controlling weeds phytopathogen. Weed Tech. 2003, 10, 621–624. [Google Scholar] [CrossRef]
- Iffat Siddiqui, I.S.; Rukhsana Bajwa, R.B.; Arshad Javaid, A.J. Field evaluation of Alternaria alternata as mycoherbicide for the management of Rumex dentatus L. Philipp. Agric. Sci. 2010, 93, 116–120. [Google Scholar]
- Ngobisa, A.I.C.N.; Zainal Abidin, M.A.; Wong, M.Y.; Noordin, W.D. Characterisation and pathogenicity of Pestalotiopsis species associated with leaf disease of rubber (Hevea brasiliensis). Arch. Phytopathol. Plant Protect. 2015, 48, 448–457. [Google Scholar]
- Zhao, F.; Wang, D.; Tao, Z.; Fan, Z.; Guo, H.; Zhang, H.; Huang, L. First report of Neopestalotiopsis rosae causing leaf spot of rubber tree in China. Plant Dis. 2019, 103, 2947. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thaochan, N.; Pornsuriya, C.; Chairin, T.; Thoawan, K.; Chomnunti, P.; Sunpapao, A. Fungal-Infected Weeds: A Potential Source of Leaf Spot Disease in Rubber Trees from Southern Thailand. J. Fungi 2025, 11, 220. https://doi.org/10.3390/jof11030220
Thaochan N, Pornsuriya C, Chairin T, Thoawan K, Chomnunti P, Sunpapao A. Fungal-Infected Weeds: A Potential Source of Leaf Spot Disease in Rubber Trees from Southern Thailand. Journal of Fungi. 2025; 11(3):220. https://doi.org/10.3390/jof11030220
Chicago/Turabian StyleThaochan, Narit, Chaninun Pornsuriya, Thanunchanok Chairin, Kodeeyah Thoawan, Putarak Chomnunti, and Anurag Sunpapao. 2025. "Fungal-Infected Weeds: A Potential Source of Leaf Spot Disease in Rubber Trees from Southern Thailand" Journal of Fungi 11, no. 3: 220. https://doi.org/10.3390/jof11030220
APA StyleThaochan, N., Pornsuriya, C., Chairin, T., Thoawan, K., Chomnunti, P., & Sunpapao, A. (2025). Fungal-Infected Weeds: A Potential Source of Leaf Spot Disease in Rubber Trees from Southern Thailand. Journal of Fungi, 11(3), 220. https://doi.org/10.3390/jof11030220








