Abstract
Biofilms are structurally organized communities of microorganisms that adhere to a variety of surfaces. These communities produce protective matrices consisting of polymeric polysaccharides, proteins, nucleic acids, and/or lipids that promote shared resistance to various environmental threats, including chemical, antibiotic, and immune insults. While algal and bacterial biofilms are more apparent in the scientific zeitgeist, many fungal pathogens also form biofilms. These surprisingly common biofilms are morphologically distinct from the multicellular molds and mushrooms normally associated with fungi and are instead an assemblage of single-celled organisms. As a collection of yeast and filamentous cells cloaked in an extracellular matrix, fungal biofilms are an extreme threat to public health, especially in conjunction with surgical implants. The encapsulated yeast, Cryptococcus neoformans, is an opportunistic pathogen that causes both pulmonary and disseminated infections, particularly in immunocompromised individuals. However, there is an emerging trend of cryptococcosis among otherwise healthy individuals. C. neoformans forms biofilms in diverse environments, including within human hosts. Notably, biofilm association correlates with increased expression of multiple virulence factors and increased resistance to both host defenses and antifungal treatments. Thus, it is crucial to develop novel strategies to combat fungal biofilms. In this review, we discuss the development and treatment of fungal biofilms, with a particular focus on C. neoformans.
1. Introduction
The term “biofilm” has been used for nearly 40 years to describe communities of microorganisms encased in extracellular matrices—a concept first observed by van Leeuwenhoek and Pasteur [1,2,3]. Biofilm-associated organisms enhance immune evasion, antimicrobial resistance, and physical resilience, driving research into their mechanisms and pathogenicity. Biofilms are structured microbial communities attached to surfaces and embedded within an extracellular matrix (ECM) [4,5]. Each organism produces a unique ECM composed of a combination of polysaccharides, proteins, nucleic acids, and/or lipids, creating a protective and adaptive environment [4]. Water channels within mature biofilms facilitate nutrient distribution and waste removal, supporting embedded microbes [6,7,8]. Biofilm-associated organisms exhibit distinct transcriptional profiles, altered growth rates, quorum sensing, and polymer secretion [7,9,10,11,12]. Together, this complex structure shields the densely packed microbes from chemical disruption, antimicrobial damage, and phagocytosis [9,13].
More than 65% of human microbial infections involve biofilms [14], with bacterial biofilms primarily studied due to their more readily observable role in nature [10,15,16]. However, interest in fungal biofilms has expanded in tandem with the rise in clinical cases involving these networks. Various fungal pathogens possess the ability to generate biofilms with unique properties impacting public health [11,17,18,19,20,21,22]. A foundational study by Murillo et al. on the fungal pathogen Candida albicans revealed differences in gene expression between planktonic and biofilm states, with clear differences in metabolism, stress response, and cell wall biosynthesis—key factors in pathogenicity and antimicrobial resistance [13,16]. Fungal biofilms exhibit significantly higher resistance to antifungal treatments than their planktonic counterparts, contributing to persistent infections [23,24,25,26].
These principle characteristics of fungal biofilms have also been investigated in pathogenic Cryptococcus spp., where cells within biofilms demonstrate elevated levels of proteins associated with oxidative reduction, proteolysis, and stress responses while showing reduced levels of proteins involved in metabolism, transport, and translation [27]. In addition, C. neoformans readily adheres to various surfaces, including living tissues and medical devices [10]. Therefore, as the prevalence of implanted medical objects like, shunts and catheters, has increased, so too has the frequency of medically-relevant cryptococcal biofilms. This surge in cases has raised significant concerns within the scientific and medical communities, as there remains a great deal of uncertainty surrounding biofilms in general, particularly those related to fungi.
Several other fungal species also form biofilms or organize into communities that demonstrate biofilm-like characteristics. These biofilms are highly prevalent in natural environments; however, certain species can also act as opportunistic pathogens in clinical settings [28,29,30]. Examples of these pathogenic fungi include species within the genera Candida, Aspergillus, Malassezia, Trichosporon, Fusarium, and Coccidioides [17,18,22,23,25,31,32]. While some of these species are considered part of the normal mammalian flora, all can become pathogenic under certain conditions [33,34,35,36]. The biofilms from each of these pathogens display similar general architecture and components. However, the characteristics of biofilms vary significantly across species and environmental conditions [4,37,38]. Infections caused by biofilm-associated fungal pathogens, particularly Cryptococcus, Candida, and Aspergillus species, are estimated to cause over one million deaths annually, emphasizing the urgent need for a deeper understanding of biofilm-associated pathogenesis [39]. In this review, our primary focus is on C. neoformans pathogenesis and biofilm formation. However, we also highlight the key characteristics of medically relevant fungal biofilms and summarize current therapeutic approaches and ongoing research efforts designed to combat fungal biofilms.
2. Cryptococcus Biology and Its Role in Biofilm Development
Cryptococcus infection, or cryptococcosis, is a fungal infection that most commonly affects immunocompromised individuals. While the fungus initially infects the lung it can occasionally disseminate to cause life-threatening conditions such as meningitis. The two Cryptococcus species most associated with clinically relevant human infections are C. neoformans and C. gattii. C. neoformans is the most widely studied Cryptococcus species and, therefore, will be the primary topic of this review. C. neoformans was first characterized as a mammalian pathogen in the late 19th century and was recognized as a cause of human disease early in the 20th century [40,41]. Its significance as an opportunistic pathogen, particularly in immunocompromised individuals, became more evident with the advent of immunosuppressive therapies and later during the human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) epidemic [41].
Metabolically active Cryptococcus spp. exist as basidiomycete yeasts that commonly divide by budding but can perform mating in conjunction with short hyphae formation and spore generation [41]. In certain environments, Cryptococcus can also expand to form polyploid titan cells and assemble into biofilms [11,42,43,44,45,46]. C. neoformans cells are commonly divided into several serotypes based on agglutination reactions of the capsular components, including serotypes A, D, and hybrid AD. C. neoformans serotype A strain is the most prevalent serotype, accounting for approximately 95% of all cryptococcal infections [41]. With the increase in susceptible human populations, changes to the distribution of infectious agents, and the significant public health burden, scientists are now placing greater emphasis on understanding the basic biology and pathobiology of infectious Cryptococcus spp.
Cryptococcus species form biofilms both in natural environments and within host tissues, contributing to survival and pathogenicity [47,48]. In nature, these biofilms develop on surfaces such as plant matter and soil, where they provide protection against environmental stresses and/or predation [47,49]. Biofilms also aid in nutrient acquisition by concentrating metabolic activity and enabling cooperative interactions among fungal cells [15,50]. In vivo, cryptococcal biofilms form on medical devices and within tissues, enhancing resistance to antifungal treatments and facilitating persistence within the host [42,51]. Given its role in both environmental survival and host persistence, understanding the formation and regulation of biofilms in Cryptococcus species is crucial for improving our management of cryptococcosis.
2.1. Dynamics of Cryptococcal Infection
2.1.1. Cryptococcosis: Infection and Latency
C. neoformans yeasts and spores are ubiquitous in soil, bird excreta, decaying organic material, and certain types of trees [52,53]. Fungal spores are released into the air and are readily inhaled by humans and animals because the 1.5–3.5 µm Cryptococcus spores are small enough to easily travel to the lungs, whereas particles larger than 5 µm are efficiently cleared by the mucociliary airway [53,54]. Therefore, cryptococcosis invariably begins as a pulmonary infection. In healthy hosts, this infection is most commonly asymptomatic or presents as cold-like symptoms that subside with or without antifungal therapy [55,56]. However, individuals with weakened immune systems are susceptible to persistent lung infection or dissemination from the lungs to the central nervous system. This disseminated infection is known as cryptococcal meningoencephalitis/meningitis [57]. This condition is of particular interest, as Cryptococcus is a leading cause of adult meningitis in numerous regions across the globe [57].
As a spore-forming fungus, C. neoformans can become dormant within the body, resulting in latent infection [58]. This is evidenced by the capacity of fungal spores to survive within phagocytes without inducing an effective intracellular innate immune response [59]. In the absence of intracellular killing of spores, the immune system often forms granulomas in an attempt to cordon off the infected myeloid cells [60]. These granulomas are highly structured, with a core of either actively or latently infected macrophages surrounded by layers of additional immune cells, including lymphocytes and fibroblasts [61]. Latent C. neoformans can reemerge from these granulomas to reinitiate fulminant infection when the host experiences an immunosuppressed environment, such as the development of chronic diseases, age-related issues, AIDS, and immunosuppressive medications [62,63,64]. Thus, there are two sources of severe cryptococcosis: primary infection and latent infection. Each of these sources of infection can result in systemic fungal infection in an immunocompromised host.
2.1.2. Cryptococcosis: Epidemiology, Clinical Presentation, and Treatment
Cryptococcosis has a worldwide environmental distribution and is associated with a wide variety of clinical symptoms that depend on both the pathogenicity of the fungus and the immune status of the host. Serological investigations demonstrate that exposure to Cryptococcus in humans is extremely prevalent, but fulminant disease development is comparatively rare [65]. Until the early 1980s, cryptococcosis was considered a rare disease that primarily affected immunocompromised individuals. However, the HIV/AIDS epidemic dramatically shifted this perspective, as cryptococcosis became a leading cause of death among patients suffering from this condition. By the mid-1980s, the prevalence of cryptococcosis had increased significantly, where 80% of Cryptococcus infections involved patients with HIV/AIDS [63,66]. It was soon discovered that the acute susceptibility of AIDS patients to cryptococcosis was a result of severely impaired CD4+ T-cell immunity [67].
Fortunately, the successful and widespread implementation of anti-retroviral therapy (ART) has greatly decreased the incidence of AIDS andconsequently decreased the burden of HIV-associated cryptococcosis. However, these positive developments have mainly occurred in developed nations with access to the necessary medications. As such, HIV-associated cryptococcosis remains prevalent in developing regions, specifically in sub-Saharan Africa, where there is a higher number of at-risk populations [68]. While ART has been a groundbreaking advancement for preventing the rapid progression of AIDS, another somewhat surprising Cryptococcus-associated condition has recently been identified that is a direct consequence of the re-emergence of immunocompetence after ART therapy. Immune reconstitution inflammatory syndrome (IRIS), occurs in 10% to 45% of AIDS patients who are actively or latently infected with C. neoformans. In these cases, the rapid reemergence of C. neoformans-specific CD4+ T-cells in conjunction with existing infection induces widespread disseminated inflammation, with severe consequences for the patient [69].
Although cryptococcosis initially manifests as a pulmonary infection, its progression to the central nervous system (CNS) represents the most severe and clinically significant form of the disease [57]. Dissemination of C. neoformans into the CNS results in an infection called cryptococcal meningitis (CM), and this infection is associated with a rapid escalation in clinical signs and symptoms. CM is a severe and often fatal condition, with approximately 152,000 annual cases and a 74% case fatality rate [70]. CM patients present with diverse neurological symptoms, such as headache, altered mental status, stiff neck, lethargy, fever, nausea, and vomiting [71,72]. More rare manifestations include dementia, seizures, hearing loss, and vision disturbances [71]. Due to the similarity of CM symptoms to various other conditions and low clinical suspicion, diagnosis of CM is frequently missed or delayed. Therefore, the World Health Organization and the Infectious Diseases Society of America recommend conducting a lumbar puncture followed by a rapid cryptococcal antigen assay of the cerebrospinal fluid in patients presenting with certain pulmonary and neurological symptoms. If this assay is unavailable, an India ink microscopy test is advised [73,74]. Screening for plasma, serum, or whole-blood cryptococcal antigen is the optimal approach for guiding resources in a public health approach. This is especially imperative when considering those who are living with HIV [73].
The United States Centers for Disease Control and Prevention recommends that all individuals diagnosed with cryptococcosis should take antifungal medications for at least six months. Fluconazole is recommended for mild to moderate lung infections as well as for asymptomatic infections in those who are HIV/AIDS positive [75]. Initial treatment of severe lung infections and/or CM requires liposomal amphotericin B (AmB) and flucytosine, followed by fluconazole [75]. The regimen for AIDS-positive patients is significantly different from that for immunocompetent patients. In these cases, CM requires a multistep management plan that begins with an intensive antifungal regimen, known as the induction phase. To prevent IRIS, antiretroviral therapy is only started after this aggressive antifungal therapy.. The clinical treatment regimen then progresses through additional consolidation and maintenance phases, with a gradual adjustment in antifungal therapy [75]. Effective management of cryptococcosis requires timely diagnosis, appropriate antifungal therapy, and careful coordination with ART initiation, underscoring the importance of an integrated approach to reducing the global burden of this devastating disease.
2.2. Virulence Factors and Immune Evasion Strategies of C. neoformans
2.2.1. Virulence-Associated Structural Components of Yeast Cell
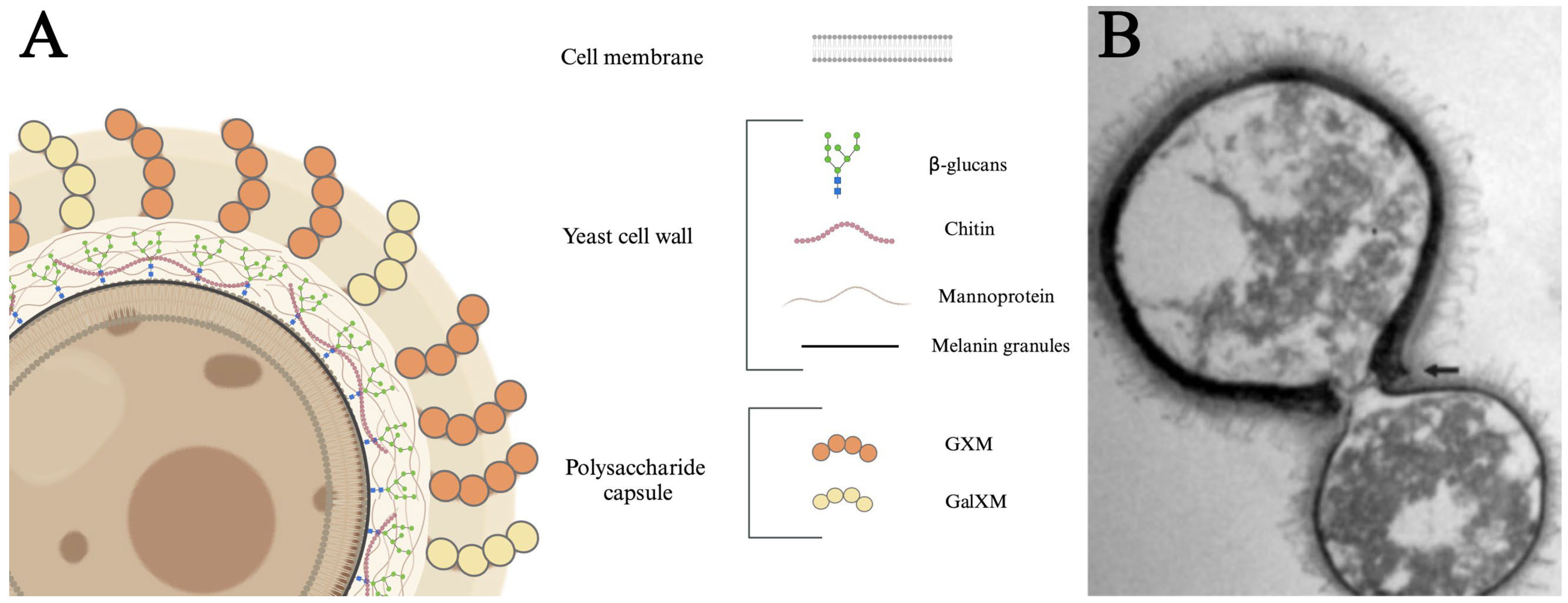
The C. neoformans yeast cell is organized in distinct layers, each contributing to its functionality and pathogenicity (Figure 1). The innermost layer is the cell membrane that divides the cytoplasm from the extracellular milieu [76,77]. The cell membrane is surrounded by a cell wall consisting of glucans, chitin, and proteins, with distinct layers of polymerized melanin. Cell wall melanin protects the fungus from a variety of stresses [78,79]. β-glucans are crucial for maintaining cell viability and organizing the capsule [80,81]. Mannoproteins are highly immunogenic, stimulating T-cell responses, promoting cytokine production, and facilitating the adhesion of C. neoformans to host cells [82,83]. Chitin has been linked to ineffective Th2 immune responses, which can exacerbate disease progression [84]. Distal to the cell wall is the outermost layer, referred to as the polysaccharide capsule. This diffuse layer consists primarily of polymerized glucuronoxylomannan (GXM) and galactoxylomannan (GalXM) sugars that provide structural support, aid in adhesion to host tissues, suppress pro-inflammatory cytokines, block phagocytosis, and induce immune cell death [85,86,87,88,89,90]. Finally, the capsule is associated with antigenic variability [91,92,93,94,95]. Together, these structural components work in concert to enhance fungus survival and pathogenic potential.
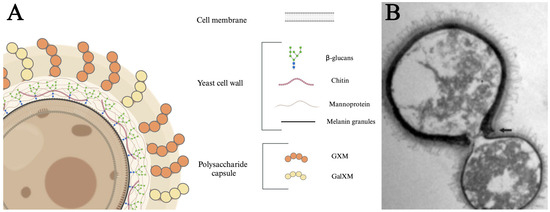
Figure 1.
Structure and components of C. neoformans. (A) The proximal cell membrane consists of a phospholipid bilayer composed of various proteins and lipids. The central cell wall consists of a dense conglomeration of β-glucans, mannoproteins, chitin, and melanin. The distal layer consists of a 5–10 µm capsule primarily made of glucuronoxylomannan (GXM) and galactoxylomannan (GalXM). (B) Transmission electron micrograph of a mother cell with a clear dark melanin-containing cell wall and a budding daughter cell. The arrow indicates the disrupted edges of the mother cell. The dark pigmentation is the melanin granules and the fibrillar structures on the cell surface are polysaccharide components of the capsule. Adapted from Mandal et al. [96] under license https://creativecommons.org/licenses/by-nc/4.0/ (accessed on 22 January 2025).
2.2.2. Extramembranous Virulence Factors: Polysaccharide Capsule and Cell Wall Melanin
The most well-understood C. neoformans virulence factor is the previously mentioned polysaccharide capsule [97]. This approximately 5 to 10 µm structure resides outside the cell wall and serves to shield the yeast from desiccation and phagocytic predation [98]. The C. neoformans capsule is a bona fide virulence factor, given that the absence of the capsule results in defective mammalian pathogenesis [99,100]. However, the capsule is not necessary for the yeast to live, as capsule-free C. neoformans has been shown to survive and replicate in vitro [101]. Early work revealed that challenge with capsular polysaccharides results in immunological unresponsiveness, mainly caused by an inhibition of antibody production [102,103]. Thus, a leading hypothesis is that the capsule shields the various pathogen-associated molecular patterns from innate immune recognition [104,105]. In addition, C. neoformans readily sheds its capsular polysaccharides, which possess immunomodulatory properties, including inhibiting neutrophil migration, altering cytokine production, modifying dendritic cell maturation, inducing apoptosis, and disrupting antigen presentation [89,106,107,108,109,110,111,112,113,114,115,116,117,118]. Finally, C. neoformans has the unique ability to alter the size and composition of its capsule in response to environmental or host-related conditions, a phenomenon known as capsule phase variation [119]. Environmental factors such as nutrient scarcity or stress and host-specific triggers like elevated CO2 levels, limited iron availability, and body temperature can drive these changes [119,120]. Capsule phase variation enables C. neoformans to thrive in the host environment and enhance its pathogenic potential [121]. For instance, a smaller capsule may strengthen initial tissue evasion or traversal of the blood–brain barrier (BBB), while a larger capsule provides robust protection against immune defenses during systemic infection [44,122]. This dynamic capsule regulation plays a central role in the organism’s virulence and its capacity to cause disease [97].
The second extramembranous C. neoformans virulence factor is the accumulation of cell wall melanin. Melanin is a dark pigment commonly found in animals and fungi that has demonstrated roles in many different infections and diseases [123]. Cryptococcal melanin is produced and polymerized within intracellular vesicles, known as melanosomes, before being transported to the cell wall [78,124]. Previous research suggests that strains of C. neoformans with higher melanin production are more capable of causing illness, whereas melanin-deficient mutant strains show a diminished ability to induce disease [125]. Melanin contributes to mammalian virulence by mediating resistance from free radicals, ionizing radiation, heat, and antifungal drugs [89,117,118,125,126,127,128,129]. Melanin has also been implicated in several key pathological processes, including facilitating fungal dissemination, altering host cytokine responses, and offering protection against macrophage-mediated defenses [130,131,132]. Interestingly, C. neoformans melanin is directly immunoreactive, as injection of melanin induces production of granulomas in the spleen, lungs, and liver, indicating that this pigment can induce pro-inflammatory responses [123]. These findings underscore the critical role of melanin in enhancing the pathogenic potential of C. neoformans and its ability to modulate host defenses, making it a key target for future therapeutic interventions. Together, the polysaccharide capsule and cell wall melanin are two primary structural components that serve as virulence mechanisms by disrupting the host immune response and providing protection against specific attacks orchestrated by the immune system [133].
2.2.3. Phagocytosis Avoidance and Intracellular Pathogenesis
In addition to surface-associated virulence factors, C. neoformans also employs various strategies to evade host immune recognition and attack. These tactics enable the pathogen to persist within tissues and organs [133]. One such strategy was intimated above: prevention of phagocytosis. This phenotype is partially mediated by the fungal capsule but is aided by other antiphagocytic factors like antiphagocytic protein 1 (App1) [134]. However, instances where macrophages successfully engulf C. neoformans are not inevitably detrimental to the yeast [133,135]. An interesting characteristic of C. neoformans is that it is a facultative intracellular pathogen, meaning the yeast can resist phagolysosomal destruction and proliferate within myeloid cells [101,136]. This phenotype is linked to environmental survival of the yeast when subjected to engulfment by amoeba [137]. Fungal cells can continue replicating within both environmental and mammalian phagocytic cells [138]. In vivo, the intracellular macrophage–yeast interplay can lead to multiple different outcomes, including death of the yeast [101], C. neoformans proliferation and inflammatory lysis of the host cell [139], persistent co-existence [140], or a compromise where the fungus escapes from the host cell through non-lytic exocytosis called vomocytosis [141,142]. Vomocytosis is a unique mechanism by which C. neoformans escapes from macrophages while the macrophage remains alive [143,144]. This non-inflammatory process enables C. neoformans to persist within phagocytes without triggering strong inflammatory responses [145].
2.2.4. Titan Cells
C. neoformans typically exists in its asexual (haploid) form as spherical budding yeasts but can also form short hyphae and spores during sexual reproduction [53,146]. In the absence of classical multicellular fungal differentiation phenotypes like molds and mushrooms, C. neoformans instead possesses the ability to retain its blastoconidial structure but grow into astoundingly large polyploid titan cells [44,147]. Approximately 20% of 5 to 10 μm C. neoformans yeast cells within the lungs differentiate into much larger ~100 μm titan cells [148,149]. Titan cells have been observed in human and animal models of cryptococcosis [148,149,150,151,152]. These giant cells evade macrophage phagocytosis, display a thickened cell wall, possess a denser capsule, and undergo significant alterations to the content and morphology of cytoplasmic organelles [43,45]. An important characteristic of these cells is that they are regulated via the protein kinase A (PKA) pathway, the same pathway that regulates the production of many other cryptococcal virulence factors, including the polysaccharide capsule and melanin [104,105,126,127,148,149,153,154].
While the existence of titan cells and genetically conserved signaling systems for cell differentiation is well established, their precise role during lung infection has not been elucidated. A leading hypothesis suggests that titan cells may directly facilitate the dissemination of typical-sized cryptococcal cells or that the normal-sized progeny of titan cells serves as a consistent source of replicative yeast during latent infection [43,44]. Numerous studies have consistently demonstrated that titan cells are a virulence factor for C. neoformans and are critical for the survival of the fungus [43,44,45,149,152]. However, further research is needed to clarify the exact role of these giant cells in Cryptococcus pathogenesis.
2.3. Cryptococcus Biofilm Formation and Contents
C. neoformans is commonly found in the environment. Consequently, cryptococcal cells have been exposed to various environmental insults and predation throughout the ecological history of the species [155,156,157]. This demanding environment has provided the necessary evolutionary pressure for the development of diverse and multi-situational defense mechanisms, specifically the ability to exist in protected biofilms [158]. Microorganisms often form biofilms as a strategy to endure challenging conditions, establish themselves in new habitats, and shield themselves from predation [159,160]. While cryptococcal biofilm formation likely evolved as a competitive advantage in stressful environmental settings, the ability to form biofilms also appears to be advantageous during human pathogenesis.
The discovery of medically relevant C. neoformans biofilms stemmed from a failure of a ventriculoatrial shunt that should have been draining cerebrospinal fluid into vascular circulation. Upon removal of the implanted medical device, ultrastructural studies revealed a biofilm consisting of yeast-like Cryptococcus [161]. Since this initial identification in 1980, a plethora of C. neoformans biofilms have been identified on various implanted medical devices, within the lungs, and in brain lesions [42,162,163,164,165,166]. These occurrences underscore the significance of biofilms in contributing to persistent Cryptococcus infections and highlight current and future treatment challenges. To address the rising incidence of Cryptococcus biofilms, scientists are increasingly focused on unraveling the complexities of these structures to better equip the medical community to combat them. In this section, we aim to highlight both the current knowledge and gaps in understanding surrounding C. neoformans biofilms.
2.3.1. Structure and Maturation of Biofilms
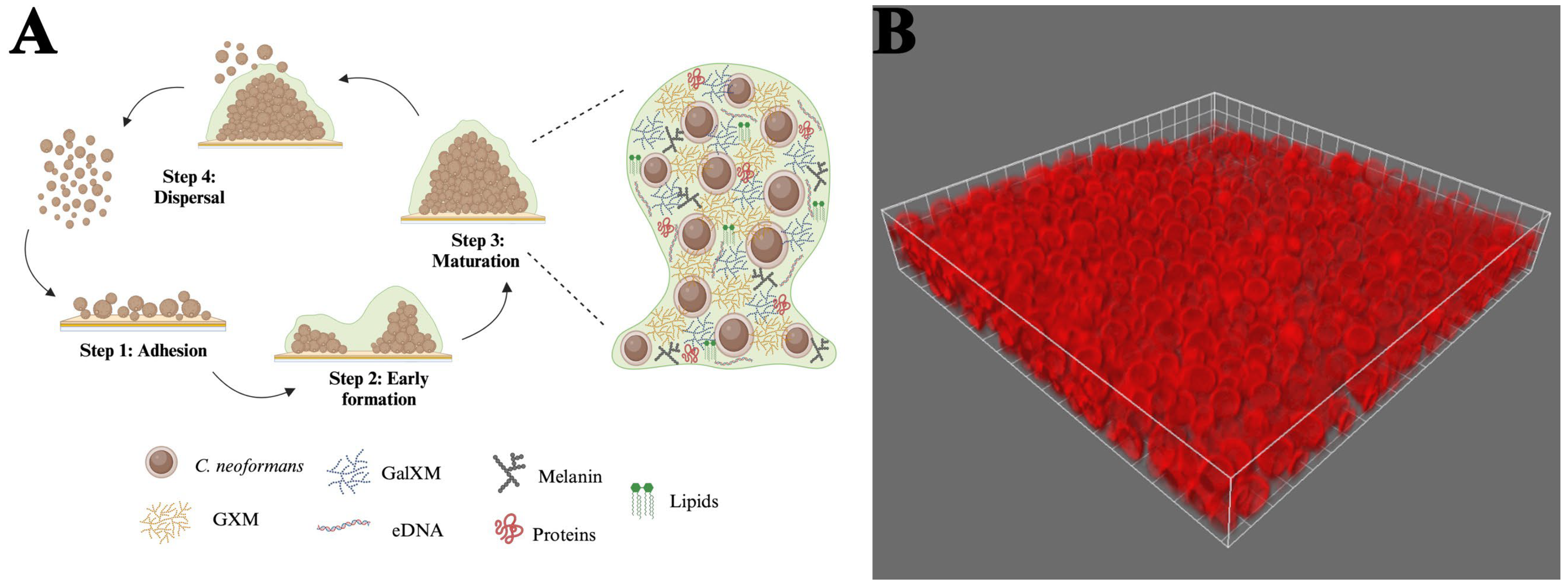
Cryptococcus biofilms consist of yeast cells and somewhat elongated pseudohyphal cells embedded in an extensive ECM [167,168]. The characteristics of C. neoformans biofilm development were initially recorded using fungi that readily adhered to polystyrene microtiter plates. The authors described three coordinated phases of early biofilm development, including surface attachment, microcolony formation, and matrix production [11,26] (Figure 2). During the adhesion phase, metabolically active cryptococcal cells attach to a surface, forming a monolayer [11]. C. neoformans expresses several surface proteins, such as Cfl1, which contribute to its ability to adhere to surfaces [169,170]. The adhesion rate of the fungal cells can be enhanced by a conditioning layer composed of compounds released by the host [171]. This phenomenon has been observed in the brain, where cerebrospinal fluid surrounding a ventriculoatrial shunt contains increased amounts of cations. These positively charged ions may promote microbial adhesion to biomaterials, influencing the degree of fungal attachment [172]. During the intermediate stage, the yeasts divide, but the majority of the progeny remains associated with the microcolony. This process generates microcolonies with three-dimensional structures consisting of evenly dispersed yeast cells. The close proximity between the cells in these microcolonies assists in creating an environment conducive to establishing nutrient gradients, genetic exchange, and quorum sensing [11]. Throughout the final maturation stage, the architecture of these communities becomes more complex as the concentration of the ECM around the cells increases [9,11]. As the structures condense, they become more cohesive and adhesive [9,172,173]. Biofilm structural arrangement, along with the presence of flowing water channels, facilitates the exchange of nutrients and gases [8]. This mature structure offers fungal cells a protected environment, shielding them from environmental threats, immune responses, physical stress, and antimicrobial treatments [11].
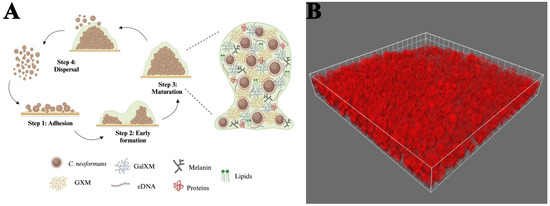
Figure 2.
Stages of cryptococcal biofilm maturation and components. (A) (1) Adhesion: C. neoformans yeast cells adhere to a surface with the help of cell wall components. (2) Early formation: cells proliferate to form microcolonies; polysaccharide capsule production increases, providing a protective barrier; and extracellular matrix (ECM) deposition begins, comprising polysaccharides, proteins, DNA, and lipids. (3) Maturation: the biofilm structure becomes three-dimensional, with densely packed cells embedded in an extensive ECM. This ECM comprises key components, including GXM, GalXM, and melanin. (4) Dispersal: some cells revert to a planktonic state to colonize new areas. (B) A 63x immunofluorescent micrograph of C. neoformans 52D adhered to a glass coverslip and stained with Alexa Fluor 647-conjugated 18B7 antibody. Biofilm formation was performed according to Martinez and Casadevall with limited modifications [9].
An important factor involved in controlling biofilm development is the cell-to-cell communication known as quorum sensing, which affects biofilm formation by regulating cell growth, the release of GXM, and melanin synthesis [174]. C. neoformans employs different molecules that act as quorum sensing signals, including pantothenic acid (vitamin B5) and extracellular vesicles [174,175]. Alternatively, quorum quenching results in the disruption of quorum sensing pathways, preventing cells from coordinating biofilm formation and virulence [176,177,178]. Thus, developing solutions to target these pathways could weaken C. neoformans biofilms, making them more susceptible to antifungal drugs [179].
In addition to providing a physical barrier to antifungal agents, mature biofilms employ several maintenance and defense mechanisms. For example, C. neoformans can upregulate efflux pumps, which actively pump antifungal drugs out of the cells [180]. Furthermore, cells within the mature biofilm are generally less metabolically active, making them more resistant to drugs that target dividing cells [26]. Following biofilm maturation, some cells become planktonic and disperse into the surrounding environment, enabling them to colonize new areas [181]. Interestingly, these dispersed cells often exhibit transcriptional differences compared to their sessile counterparts within the biofilm. Specifically, genes involved in virulence, stress resistance, and metabolic pathways are upregulated in planktonic cells, priming them for survival and colonization in new niches [15,182]. Upon attaching to a new surface, the biofilm formation process reinitiates, and as the cells adapt to their surroundings, the biofilm structure evolves and reorganizes accordingly [183,184]. These stages highlight the dynamic and adaptive nature of C. neoformans biofilm development, emphasizing its role in persistence, environmental resilience, and potential for colonization of new surfaces.
In addition to transcriptional differences, C. neoformans biofilms harbor distinct populations of metabolically inert or dormant cells, sometimes referred to as persister cells [185]. These cells can survive extreme conditions, including nutrient deprivation and antifungal treatments, ensuring the survival of the biofilm even under adverse conditions [186,187,188]. These dormant cells may be critical for biofilm longevity, as biofilms can persist for weeks to months under suitable conditions, even in hostile environments such as those surrounding medical devices [50,189]. Moreover, the biofilm environment promotes increased opportunities for sexual reproduction and spore formation, particularly under nutrient-limiting conditions. The dense cellular proximity and genetic exchange within biofilms can enhance the likelihood of meiotic events, resulting in the production of stress-resistant spores capable of dispersal and colonization [190].
2.3.2. In Vivo C. neoformans Biofilms
C. neoformans primarily forms biofilms on abiotic surfaces like medical devices. Demonstrated biofilm substrates include prosthetic cardiac valves, prosthetic dialysis fistulas, and prosthetic hip joints [162,164,165]. In addition, it is believed that C. neoformans may produce biofilm-like clusters within the lungs and brain [163,191]. Cranial C. neoformans infections include biofilm-like structures known as cryptococcomas [192,193,194]. The development of these structures occurs as a result of uncontrolled infection and plays a vital role in fungal neurotropism [42]. The fungal communities within cryptococcomas are characterized by substantial amounts of capsular polysaccharides surrounding yeast cells and can appear as cystic lesions in the brain [42,195]. Further research into biofilm formation on lung tissue and the role of cryptococcomas in neurotropism may provide valuable insights into the pathogenesis of C. neoformans and its impact on both systemic and localized infections.
2.3.3. Extracellular Polysaccharides in Cryptococcal Biofilm Development
Many of the factors that contribute to C. neoformans pathogenesis also aid in biofilm formation. A major virulence factor discussed earlier that supports biofilm development is the polysaccharide capsule. Substantial amounts of capsular polysaccharide are shed during biofilm formation and become a main component of the extracellular matrix (ECM). This release creates the three-dimensional framework of the ECM that encases and protects the embedded cells to ensure preservation and stability [9,172]. This is evidenced by the finding that the acapsular cap59 mutant strain does not form biofilms, while the encapsulated wild-type and complement strains exhibited similar biofilm-forming abilities. Since the primary component of the C. neoformans capsule is a key element of the extracellular biofilm matrix, the authors concluded that the mutant strain’s failure to form biofilms is due to its inability to shed polysaccharides [9].
The cryptococcal ECM is composed primarily of the capsular polysaccharides (GXM and GalXM) but also contains various proteins, including laccases and phospholipases, extracellular DNA (eDNA), lipids, and melanin. Other molecules, including metabolites and ions, can also be sequestered within the ECM [196]. Glycoproteins like laccase, mannoproteins, and phospholipase B1 (PLB1) play vital roles in biofilm formation, structural integrity, antifungal resistance, and interactions with the host immune system. Laccase contributes to melanin production, mannoproteins mediate adhesion to surfaces and trigger immune modulation, and PLB1 facilitates biofilm integrity and virulence [48,82,83,197,198,199,200]. eDNA is also a critical component of the fungal ECM [201,202,203], and its essentiality was established when Whitchurch et al. demonstrated that enzymatic degradation of eDNA decreased biofilm biomass, increased susceptibility to antifungal treatments, and resulted in structural disintegration of the biofilm matrix [204]. Since then, eDNA has been recognized in the importance of biofilm formation, structural stability, and adhesion and signaling between cells [205]. While there is still significant debate as to whether eDNA is actively secreted or derived from lysed cells, this extracellular nucleic acid contributes to the resilience of C. neoformans in both environmental and host settings [202,206,207]. Together, capsular polysaccharides, proteins, and eDNA are the major components of the C. neoformans biofilm.
2.3.4. Connection Between Intracellular Survival of C. neoformans in Macrophages and Biofilm Development
Many of the virulence factors associated with C. neoformans survival within macrophages likely also contribute to biofilm formation. First, mobile mononuclear host cells likely act as a vehicle for C. neoformans dissemination via a mechanism named the “Trojan horse” [208]. Here, intracellular yeasts can exit macrophages via lytic or non-lytic exocytosis, and these released cells are primed to establish biofilms at new sites [139,141,142]. Second, intracellular challenges like limited nutrients and oxidative stress induce C. neoformans to adapt by initiating biofilm formation [27,42]. Finally, intracellular C. neoformans produces capsule polysaccharides, enabling those yeasts that escape the phagocyte to immediately contribute to biofilm formation [121,209]. These released cells can aggregate and form biofilms on host tissues or medical devices, using quorum-sensing signals to coordinate biofilm development [11]. Taken together, these factors suggest that intra-macrophage survival modulates the yeast to elaborate many of the virulence mechanisms that result in biofilm initiation. This dichotomy underscores the ability of C. neoformans to transition between different survival strategies, contributing to its pathogenicity, persistence in the host, and resistance to treatment. Understanding this connection offers potential targets for disrupting these processes to improve therapeutic outcomes.
Interestingly, the binding of antibodies to the outer layers of C. neoformans can contribute to biofilm formation by agglutinating cryptococcal cells into biofilm-like microcolonies [210]. While this cell clumping is notable, anti-capsule antibodies are not the primary contributors to biofilm formation and, therefore, are not particularly effective at inducing it. Microcolonies play a crucial role as foundational units of biofilms, facilitating the spread of C. neoformans to new environments. As discussed in a previous review paper, the fungus may establish biofilms within tissues in these areas, enter a dormant state, and evade clearance by macrophages, antimicrobial therapies, or immune cell molecules [42]. This phenomenon is particularly pronounced in the brains of individuals with cryptococcosis, where cryptococcomas are formed and play a vital role in the pathogen’s neurotropic success [42,211].
2.3.5. Involvement of Titan Cells in the Biofilm Generation
As integral parts of lung biofilms, the thickened cell wall and capsule of titan cells aid in restricting host immune responses and contribute to overall structural integrity [45]. Additionally, studies suggest that these large cells can confer protective benefits not only to themselves but also to normal-sized cryptococcal cells [45]. This is supported by the fact that C. neoformans biofilm formation protects densely-packed cryptococci from antimicrobial damage and macrophage phagocytosis in tissues, enhancing fungal resistance, quorum sensing, and survival [9]. Therefore, titan cells may contribute to the initial stages of biofilm formation by adhering to surfaces and facilitating the aggregation of other cryptococcal cells.
In C. neoformans, titan cell formation is triggered by the same environmental conditions—such as high CO2, low oxygen, and nutrient limitation—and genetic factors, including the cAMP signaling pathway, that drive biofilm development [45,212,213,214,215,216,217,218]. These processes serve as adaptive responses to stress, which enhance fungal survival in hostile environments. Titan cells, being larger and more resistant to immune responses, contribute to biofilm formation by enriching the extracellular matrix with polysaccharides [42]. This overlap in conditions and pathways illustrates a coordinated survival strategy, allowing the fungus to persist in the host and establish chronic infections.
2.4. C. neoformans Biofilms in Human Infections
2.4.1. Contribution of C. neoformans Biofilms to Implanted Medical Device Infections
C. neoformans forms biofilms on a wide range of synthetic materials, including catheters, prosthetic valves, and other indwelling medical devices. These biofilms are facilitated by the ability of the organism to produce a polysaccharide capsule and secrete exopolysaccharides that contribute to the ECM [15,28,219]. Biofilms provide an ideal niche for C. neoformans to evade antifungal treatments through ECM-mediated drug sequestration, metabolic heterogeneity, and reduced drug penetration [220,221]. Infections involving C. neoformans biofilms on medical devices are difficult to eradicate and often require removal of the infected device [221,222,223]. These infections are associated with high morbidity and, in some cases, mortality [15,220,221]. This increasing prevalence of biofilm formation on indwelling medical devices demonstrates the urgent need for antifungal strategies targeting biofilm-associated infections.
2.4.2. Contribution of Biofilms to C. neoformans Brain Infections
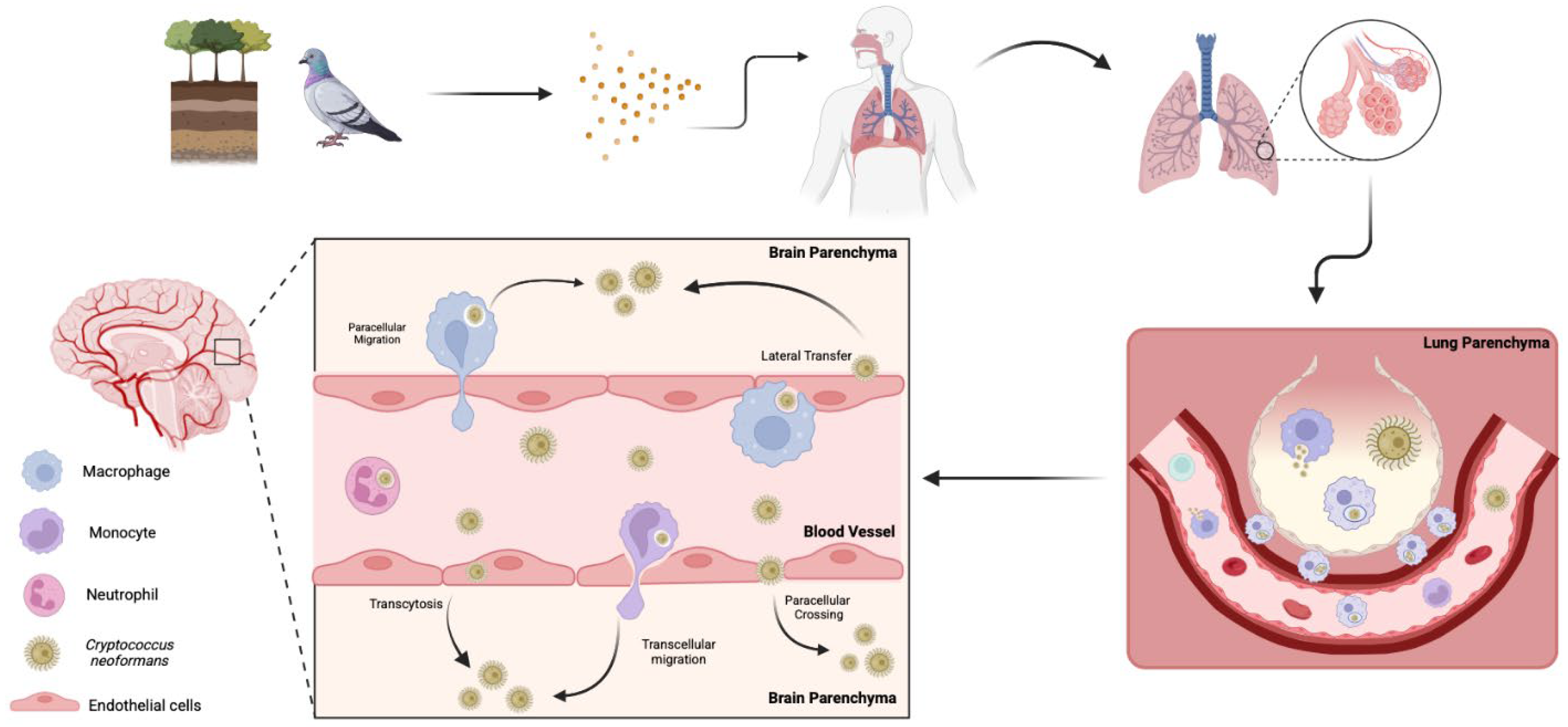
Infiltration of C. neoformans into the central nervous system (CNS) can result in fatal meningoencephalitis. Thus, it is imperative to understand the factors that contribute to the mechanisms by which this fungus disrupts the blood–brain barrier (BBB). There are three main theories for the mechanism(s) of cryptococcal brain invasion; the fungus can cross transcellularly through endothelial cells [224], paracellularly between endothelial cells [224], or through lateral transfer, where phagocytes in the bloodstream release fungal cells at the BBB, allowing them to cross. It also has the unique capability to employ what is known as the “Trojan horse” mechanism, where infected macrophages carry the fungus across the BBB [208,225] (Figure 3). From the fungal perspective, the lung, BBB, and brain are all different environments, as exemplified by the oxygen-rich lung environment compared to the low-oxygen brain parenchyma. Cryptococcal cells may prefer the oxygen-rich environment, as the fungi tend to occupy areas near capillary-rich endothelial cells within the brain [226]. However, the oxygen-depleted environment within the brain likely induces similar signaling events to those occurring in biofilm formation in other host environments, ultimately leading to morphological adaptations that enhance persistence [224].
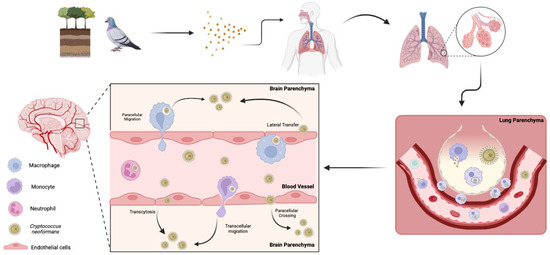
Figure 3.
Pathways of C. neoformans invasion into the brain. Spores from soil or bird excreta are inhaled into the lungs, the primary site of infection. If the infection is not cleared, C. neoformans yeasts may transverse the lungs and enter the bloodstream. This occurs either through direct fungal migration into the blood or via mobile phagocytes that engulf the fungus and transport it across barriers. Once in circulation, the fungus disseminates throughout the body, with a predilection for the central nervous system. C. neoformans crosses the blood–brain barrier via multiple mechanisms, including transcytosis, paracellular crossing, and lateral transfer. Additionally, the fungus employs “Trojan horse” strategies in which phagocytes carry fungal cells crossing the blood–brain barrier via transcellular and paracellular migration.
Within the CNS, cryptococcal biofilm-like structures may form along endothelial surfaces or in association with immune cells, contributing to persistent infection and recurrent disease [227]. Additionally, these biofilm structures can serve as a reservoir for episodic dispersal of fungal cells, potentially allowing for re-infection of the brain parenchyma [228]. The hypoxic and nutrient-limited environment of the CNS may further promote the transition to biofilm-associated states, as observed in other pathogenic fungi [226,229]. These diverse mechanisms, combined with the critical role of biofilm formation in protecting the fungus and promoting persistent infection, highlight the adaptability of C. neoformans and underscore the challenges in effectively treating cryptococcal infections in the CNS. Ultimately, the intricate interplay between biofilm formation, immune evasion, and the ability to disseminate undetected presents significant obstacles in managing cryptococcosis.
3. Other Pathogenic Fungi That Form Biofilms
3.1. Candida spp.
Candida albicans is undoubtedly the most conspicuous example of a pathogenic fungus that forms biofilms during infection. As a natural component of the human microbiota, C. albicans is typically found in the gastrointestinal tract, female reproductive tract, oral cavity, and skin [31,230]. However, when the host/fungus equilibrium is disrupted by factors such as environmental changes (e.g., pH or nutrient shifts), antibiotic use, or immune system alterations (from infections or immunosuppressive treatments), the fungus can rapidly proliferate, leading to candidiasis [231]. The symptoms of candidiasis range from mild conditions like oral skin rashes and genital infections to severe cases with fever, chills, and, potentially, organ failure [16,232]. Candidiasis often leads to an infection known as thrush, characterized by a white biofilm on the tongue, throat, or surrounding areas of the mouth [233]. Extensive research on oral thrush has greatly advanced the general understanding of biofilm resilience and the development of therapeutic strategies for biofilm-related diseases [234].
Candida species can also form biofilms in other circumstances, particularly in hospital settings [21]. C. albicans and other closely related species are the predominant fungi isolated from infected medical devices and are among the leading causes of bloodstream infections [235]. Among patients with invasive candidiasis, mortality rates remain high, with approximately 40% succumbing to the infection despite antifungal treatment [236]. Thus, secondary candidiasis has become a critical focus in hospital settings, as C. albicans biofilm formation accounts for roughly 15% of all sepsis cases and 40% of bloodstream infections [235]. Quorum sensing plays a crucial role in C. albicans by regulating its transition between yeast and hyphal morphologies, a key factor in its virulence and biofilm formation [237]. Candida biofilms are notoriously difficult to treat, often requiring device removal and high doses of antifungal drugs, which carry risks such as organ damage [16,222,223]. The resilience of Candida biofilms highlights the urgent need for new therapeutic strategies to combat these persistent infections, especially in critically ill and immunocompromised patients.
3.2. Aspergillus spp.
Aspergillus species such as A. fumigatus, A. flavus, A. niger, and A. terreus are known to form harmful biofilms, which contribute to various diseases in humans, birds, and mammals [17]. A. fumigatus is among the most common airborne fungal pathogens in humans and is primarily associated with pulmonary disease [5]. However, A. fumigatus infections can result in various conditions ranging from rhinitis to fatal invasive aspergillosis in immunocompromised patients [238,239,240]. Aflatoxin-producing A. flavus can cause severe respiratory issues and allergic reactions [241,242]. The black mold A. niger can lead to aspergillosis in immunocompromised individuals [243]. Finally, A. terreus is notable for its growing prevalence and resistance to certain antifungal agents [244]. The incidence of aspergillosis has risen, with some strains showing reduced susceptibility to antifungal treatments, likely due to biofilm-associated drug resistance [242,245,246,247].
The formation of biofilm by Aspergillus species is a key factor in their virulence and plays a significant role in antifungal resistance [248,249]. Aspergillus biofilms differ somewhat from classical biofilms due to the presence of differentiated hyphae, but these communities share many characteristics of all biofilms, including ECM production, surface adherence, and enhanced antifungal resistance [17]. Therefore, the term “Aspergillus biofilm” has become widely accepted because these communities share similar clinical and industrial challenges with classical biofilms [250]. Biofilms provide a sheltered niche for the fungi, protecting them from immune cells, environmental predators, and shear forces [251]. Despite their importance in Aspergillus infections, the biofilm trait remains underexplored, with most research focusing on the conidial form of the organism [17]. A deeper understanding of Aspergillus biofilms is crucial to address their ability to evade immune responses and resist antifungal therapies.
3.3. Malassezia spp.
Many different lipophilic yeasts in the genus Malassezia can form biofilms. Malassezia spp. are commonly associated with the skin of humans and various mammals, where the fungi live in close association with fat-producing sebaceous glands [158,252,253]. Normally, Malassezia spp. are considered part of the normal skin microbiota, as most interactions are commensal. However, changes to this host/fungus equilibrium can lead to minor disorders like dandruff, seborrheic dermatitis, otitis externa, and dermatitis [254,255,256] or more severe infections like fungemia, catheter infections, and severe nosocomial infections [18,253,257]. This change from commensal to opportunistic pathogen correlates strongly with the formation of biofilms with nearly 2000 times higher resistance to antimicrobial therapeutics [257,258,259].
Malassezia biofilms are commonly found on medical devices, particularly catheters, and are associated with serious infections in immunocompromised patients [18,253]. The catheter-related disease is classified as a nosocomial infection, affecting many intensive care unit patients, and is often associated with high mortality rates [257]. It is important to note that a significant proportion of nosocomial infections are due to biofilm formation [18,257]. Given the rising incidence of biofilm-associated infections and the unique challenges posed by Malassezia species, further research is essential to better understand their pathogenic mechanisms and improve treatment strategies for affected patients.
3.4. Trichosporon spp.
Trichosporon spp. are comparatively less commonly pathogenic biofilm-forming fungi. While long known to be a member of the skin microbiota, Trichosporon fungi have emerged as a significant public health concern in immunosuppressed patients [260,261]. In rare circumstances, Trichosporon can cause a life-threatening condition known as disseminated trichosporonosis, where the fungus spreads throughout the body, damaging multiple organs and systems. It most commonly affects those with compromised immune systems but can also affect healthy people under certain conditions [23,260]. It was found that most of the reported cases of T. asahii infections correlated with the presence of indwelling devices such as intravenous or urinary catheters [262], endoscopic forceps [263], and arteriovenous grafts [264]. These findings corroborate the notion that prosthetic devices can act as an ideal substance for the adhesion and growth of biofilms.
Similar to the other fungal species mentioned thus far, Trichosporon biofilms are often associated with persistent infection and high mortality [23]. Several Trichosporon species form biofilms [25], the most common one being T. asahii. Biofilms comprising this fungus have been seen to form on various medical devices [265]. T. ashaii and T. inkin both form biofilms that are resistant to classic antifungals [19,266]. A unique characteristic of the biofilms formed by these species is their ability to form persister cells [267]. These cells can enter a dormant state or express certain genes for inactivation, enabling them to exhibit antimicrobial tolerance [268]. Trichosporon-specific biofilms result in the same consequences seen in other fungal biofilms, marked by enhanced resistance to antimicrobial agents and protection against host immune defenses.
3.5. Fusarium spp.
Other fungi that can form biofilms are members of the Fusarium genus, including F. oxysporum and F. solani [20]. F. solani is one of the most commonly isolated filamentous fungi from soil and plant debris [269]. While these species are primarily saprophytic, they also serve as host-specific pathogens for many agricultural plants and have been sporadically associated with severe and invasive mycoses in immunocompromised human patients [269,270,271,272,273]. F. oxysporum is distinguished by its capacity to form biofilms on various unique surfaces, including contact lenses and water systems [274,275]. However, it is most notable for its formation of biofilms on human nails, leading to an infection known as onychomycosis [276].
Instances of Fusarium infections have increased in recent decades. This rise may be attributed to several factors, such as a growing population of immunocompromised patients [272], rising medical tourism [277,278], climate change [279], and resistance to antifungal treatments [280]. Interestingly, the recent 2023 outbreak of F. solani revealed a trend that diverges from previous observations, whereby immunocompetent patients developed meningitis [277]. This outbreak differed significantly from past occurrences involving Fusarium, mainly because this fungus usually targets immunocompromised individuals and because cases of fusariosis in the CNS are seldom documented [278]. Due to their ability to form biofilms, Fusarium species are resistant to current antifungal interventions, making infections all the more difficult to treat [280]. As the complexities of Fusarium infections continue to evolve, advancing our understanding of their biofilm formation is essential for developing more effective diagnostic and therapeutic approaches.
3.6. Coccidioides spp.
Coccidioides spp. also possess the ability to form disease-causing biofilms. C. immitis and C. posadasii both cause a lung infection called valley fever and pulmonary coccidioidomycosis [281,282]. Coccidioides is endemic to arid and semi-arid regions of the southwestern United States, Mexico, Central America, and South America [283]. The majority of coccidioidomycosis cases resolve without significant complications, but rare chronic infections can persist for a lifetime, occasionally causing additional health issues [284]. Coccidioidomycosis most commonly presents as an upper respiratory infection; however, it may also lead to disseminated disease, which has been proven to be treatment-resistant and thus potentially fatal [285]. As with the previously discussed fungal infections, this illness can infect immunocompetent individuals but is considerably more common in immunocompromised patients [283]. Due to its similar presentation of pneumonia, outbreaks of coccidioidomycosis are often misdiagnosed and are, therefore, a common cause of community-acquired pneumonia [283].
Coccidioides spp. can adhere to and form biofilms on medical devices [22]. A case study documented recurrent coccidioidal meningitis caused by a fungal biofilm on the tip of a ventriculoperitoneal shunt tubing despite the patient receiving an adequate dosage of fluconazole, highlighting the role of biofilms in treatment failure [22]. The authors described how the biofilm structure likely shielded the fungal cells from the antifungal drug and host immune response, complicating treatment outcomes. Biofilm formation in Coccidioides shares similarities with other pathogenic fungi, such as their ability to attach to biomaterials and resist antifungal treatments [286]. Due to the specific region that this fungus resides in, along with the fact that it is so challenging to diagnose, there is limited information available regarding Coccidioides biofilms [221]. Given the challenges in diagnosis and treatment associated with Coccidioides infections, further research into its biofilm formation and pathogenic mechanisms is crucial for improving management strategies and patient outcomes.
4. Current and Future Treatment Strategies for Fungal Infections
4.1. Current Fungal Infection Treatment Tools
There are four primary classes of antifungal drugs available to treat fungal infections [287] (Table 1). Polyenes, such as amphotericin B (AmB), target ergosterol in fungal membranes, causing a leakage of cellular components [288,289,290,291]. While AmB remains the principal drug of choice for systemic infections, a major limiting factor in its efficacy is its negative side effects. The extensive use of AmB is correlated with increased nephrotoxicity, though its liposomal formulation can reduce adverse reactions [292,293]. Azoles inhibit lanosterol 14-alpha-demethylase, disrupting ergosterol biosynthesis, and are commonly used for cutaneous C. albicans infections due to their accessibility and low dermal toxicity [294,295]. Although less toxic than AmB, azoles are associated with hepatotoxicity by affecting key enzymes involved in regulating liver activity [296]. Echinocandins block β-1,3-D-glucan synthase, an enzyme necessary for the synthesis of β-1,3-D-glucan, a major constituent of the fungal cell wall. While effective against Candida and Aspergillus, they are not effective against C. neoformans [297,298,299]. Despite having a more favorable tolerability profile compared to polyenes and azoles, echinocandins are not without drawbacks. Studies have linked their use to potential cardiotoxicity [300]. Furthermore, the high cost of echinocandins and the absence of oral formulations significantly limit their widespread application in clinical practice [301]. Allylamines, exemplified by terbinafine, disrupt ergosterol biosynthesis by targeting the enzyme squalene epoxidase [302,303]. This class of antifungals boasts a favorable safety profile and is primarily employed in treating superficial dermatophyte infections. However, their effectiveness against systemic Candida and Cryptococcus infections remains limited, constraining their use in such cases [304,305]. While conventional antifungal drugs are effective against planktonic fungi, only echinocandins and liposomal AmB demonstrate efficacy in combating biofilm-associated infections and may be used in combination with other therapies to elicit a greater effect [306]. However, even these treatments may result in suboptimal outcomes, underscoring the urgent need for more advanced therapeutic strategies to address invasive fungal infections [306,307,308].

Table 1.
Table summarizing the characteristics of each fungal group.
As it pertains to Cryptococcus infections, the WHO has recently revised its recommended induction therapy for cryptococcal meningitis to include a single high dose (10 mg/kg) of liposomal AmB, administered alongside 14 days of flucytosine (100 mg/kg per day, divided into four doses) and fluconazole (1200 mg daily for adults, 12 mg/kg per day for children and adolescents, up to a maximum of 800 mg daily) [73]. Unfortunately, certain elements of this treatment remain inaccessible in some regions, particularly in areas with high disease prevalence, such as sub-Saharan Africa [309]. The WHO has published alternative guidelines for when certain medications are not available [73]. Its guidelines for the consolidation phase include fluconazole (800 mg daily for adults, 6–12 mg/kg per day for children and adolescents, up to a maximum of 800 mg daily) for eight weeks following the induction phase [73]. For the maintenance therapy phase of treatment, they recommend fluconazole (200 mg daily for adults, 6 mg/kg per day for adolescents and children) until immune reconstitution (CD4 > 200 cells/mm3) and suppression of viral loads on ART [73].
4.2. Investigational and Proposed Fungal Biofilm Therapeutics
Fungal biofilms are an emerging public health threat, prompting significant efforts to identify effective treatments for biofilm-associated infections [310]. The current challenges can be summarized in six major themes: (1) Well-structured biofilms cause drastic changes in the genetic and metabolic states of cells, with yeast cells deep within the biofilm being less metabolically active and thus more resistant to antifungal treatment [11,50]. (2) Biofilms on medical implants are inherently resistant to phagocytosis and immune clearance [311]. (3) C. neoformans can reproduce both sexually and asexually, leading to genetic, morphological, and phenotypic variations that enhance its adaptability [146]. (4) Biofilms adhere strongly to surfaces, making them resistant to chemical or physical inactivation [312,313]. (5) Fungal biofilms serve as persistent sources of infection, shedding yeast cells that can spread to other areas within the host [26,314]. (6) Rapid disruption of biofilms may release an excess of immunostimulatory molecules, potentially triggering autoimmune diseases [107,315]. These characteristics of fungal biofilms present an immediate and noteworthy challenge for physicians and scientists alike. In response, researchers have begun exploring various strategies to tackle the challenge of fungal biofilms, with the primary goal of preventing biofilm formation, as mature biofilms are notably more resistant to treatment [316].
4.2.1. Antifungal Lock Therapy
A promising development is the use of catheter lock therapy. The extensive use of indwelling catheters, particularly intravenous catheters, has greatly contributed to the increase in bloodstream infections resulting from both bacterial and fungal biofilms [317]. Infections resulting from biofilm formation on catheters usually require removal of the tubing and systemic antifungal therapy to treat the disease effectively [318]. However, an alternative approach, known as antimicrobial lock therapy (ALT), has gained traction in recent years. The premise behind ALT is that it utilizes extended delivery of a solution that contains high concentrations of antimicrobial or antiseptic agents. The mixture is then introduced into an infected intravascular catheter, often alongside systemic antifungal therapy, to disinfect the catheter [318]. Previous reviews have outlined some of the most promising antifungal lock strategies involving the use of conventional antifungal drugs like AmB and echinocandins, antibiotics with antifungal properties such as doxycycline, antimicrobial peptides (AMPs), and antiseptics, particularly ethanol [318,319,320]. ALT has shown success in both in vitro and in vivo studies with Candida biofilms [321,322,323]; however, clinical studies for C. albicans, specifically, remain limited, with even less research available for C. neoformans biofilms. Therefore, further research on ALT as a means of treating biofilm-associated infections must be conducted to ensure the safe implementation of this treatment into clinical practice.
4.2.2. Nanoformulations
A novel approach involving nanocomposites has been emerging as a promising treatment for fungal biofilms due to their ability to disrupt biofilm structure, enhance drug delivery, and exhibit antimicrobial properties [324]. One of the key advantages of nanomedicine is the use of nanocomposite-coated or tethered biomaterials, which can prevent fungal attachment and colonization, thereby reducing infection risks. Nanotherapeutics involve newly developed antifungal agents and enhance the effectiveness of existing drugs by improving their bioavailability, targeted delivery, solubility, and stability [325,326]. Notably, recent work has shown that newly engineered nanocomposites can directly target fungal biofilms, a capability that many conventional antifungal therapies lack due to their inability to penetrate these protective structures [327]. Nanocomposites are constructed by embedding nanomaterials into a host material to create an intricate three-dimensional structure [328]. By taking advantage of the protective biofilm structure, nanocomposites encapsulate antifungal drugs, bypass biofilm defenses, and facilitate drug delivery to the cell surface or within the cell, thereby enhancing the effectiveness of traditional treatments [329]. Nanocomposite therapies are advantageous, as they are structurally diverse and can be administered through nearly all routes. However, implementing this in clinical practice is challenging due to its inherent instability and the complexities involved in achieving precise, large-scale production [324]. Furthermore, even minor alterations in their spatial configuration can significantly alter their safety and effectiveness, often resulting in clinical trial failure [330]. While nanomedicine has shown potential in commercialization, its use for wide-scale implementation is still quite limited. The primary reason for this is the absence of clear guidelines and regulations for producers, medical personnel, and international public health agencies [331]. Thus, while nanocomposites show great promise in overcoming the challenges posed by fungal biofilms, their clinical implementation faces significant hurdles, including stability issues and the lack of comprehensive regulatory frameworks. Addressing these obstacles will be crucial for the successful commercialization and integration of nanomedicine into standard antifungal treatments.
4.2.3. Modified Surfaces and Antimicrobial Coatings
Various biomaterials commonly utilized in medical devices have been found to facilitate fungal attachment, proliferation, and biofilm production. [316,332]. These materials include silicone, polyurethane, polyethylene, polypropylene, polymethylmethacrylate, titanium, titanium alloys, stainless steel, and polytetrafluoroethylene [333,334]. Over time, scientists have identified three major factors influencing biofilm formation: (1) surface roughness, (2) hydrophobicity, and (3) protein adsorption [333]. Rough surfaces offer increased surface area for microbial attachment, hydrophobic materials attract fungi due to their affinity for similar properties, and many implants adsorb proteins from bodily fluids, creating a conditioning layer that promotes fungal adhesion [335]. Although fewer studies focus exclusively on antifungal biomaterials compared to antibacterial ones, some significant discoveries have been made in this arena. Specifically, surface coatings that incorporate antifungal agents, such as cationic compounds, low-molecular-weight antiseptics, antimicrobial peptides, and polyenes, bind to the surface [336]. When these agents are covalently bound to the biomaterial surface, they are neither released nor damaged and, in theory, should persist at high concentrations to continuously kill or inhibit microorganisms upon contact [336]. This concept has also been explored with bacterial biofilms. Numerous studies have proposed using natural or modified polysaccharide polymers as surface treatments to inhibit biofilm formation [337,338,339]. The use of modified surfaces and antimicrobial coatings represents a promising approach to preventing fungal biofilm formation on medical implants, and ongoing research into antifungal surface treatments continues to uncover innovative strategies for enhancing the effectiveness of these materials.
4.2.4. Natural Remedies
Natural products have also been explored as possible treatment options for fungal biofilms. The development of microtiter plate-based models of fungal biofilm formation and antifungal susceptibility testing has led to an increase in research investigating the antifungal properties of natural products on biofilms. One study showed that a plant-derived decapeptide, known as OSIP108, interferes with C. albicans biofilm formation without affecting the viability or growth of the individual cells [340]. While research has predominantly focused on C. albicans, recent studies have highlighted several promising organic strategies for combating Cryptococcus biofilms. Notably, ethanolic extract propolis has been shown to effectively reduce biofilm formation by C. neoformans [200]. Additionally, researchers have identified natural compounds derived from freshwater mussels that disrupt key factors of fungal virulence. These compounds not only reduce polysaccharide capsule production and inhibit biofilm formation but also enhance the fluconazole susceptibility of C. neoformans in the presence of macrophages [341]. Another noteworthy area of interest is chitosan, a polymer derived from chitin found in crustacean exoskeletons. Chitosan has demonstrated promising results in disrupting biofilms formed by both C. albicans and C. neoformans, both in vitro and in vivo [173,342]. Interestingly, this compound also exhibits antibiotic properties and has been observed in layered formations with other polysaccharides that create coatings that inhibit binding and are bactericidal [337]. Moreover, certain natural products have the potential to enhance the efficacy of conventional antifungal drugs. For instance, a study revealed that shearinines, a group of structurally complex molecules produced by fungi in the Penicillium genus, significantly enhanced the activity of AmB against C. albicans biofilms in clinical isolates [343]. This multifaceted approach highlights natural remedies as promising tools for combating fungal biofilms, with studies showing their potential to inhibit biofilm formation, enhance antifungal efficacy, and reduce virulence. Further exploration into natural products is essential for developing complementary or alternative treatments.
4.2.5. Advances in Biofilm Detection and Doctor Education
Implant-associated infections pose a significant problem for the healthcare industry, as they are exacerbated by the formation of microbial biofilms and are often difficult to diagnose [344,345]. Advancements in detecting fungal biofilms on implants have revolutionized diagnostic capabilities [15]. The agar encasement culturing method combined with the candle dip method is a notable innovation, allowing for the visualization and precise localization of biofilm formation on endoprostheses [346]. This technique identifies susceptible implant areas and facilitates further analysis of isolated colonies. High-resolution scanning electron microscopy has become a leading tool for detailed microscopic analysis, successfully visualizing bacterial biofilms on infected cochlear implants and offering insights into biofilm structure and composition [347,348]. Additionally, sensor-based approaches are emerging as accurate and user-friendly methods for detecting and monitoring biofilms at implant sites. These portable technologies are particularly valuable in resource-limited settings. Machine learning and AI-driven deep learning models also hold great potential for identifying polymicrobial biofilms, surpassing the 50% accuracy of human specialists [349]. These innovations provide efficient alternatives to traditional, time-intensive biochemical procedures, significantly enhancing biofilm management in clinical environments worldwide.
Efforts to educate healthcare providers in underserved areas about fungal biofilm infections are increasingly recognized as vital, given the impact of social determinants of health on fungal disease prevalence. While specific initiatives remain limited, broader frameworks offer opportunities for integration. For instance, the WHO has advocated for incorporating fungal diseases and priority pathogens into medical and public health curricula [350]. Such initiatives could be expanded to address fungal biofilm infections, equipping doctors in resource-constrained settings with the knowledge needed to manage these challenging infections effectively.
5. Concluding Remarks
Fungal biofilms have emerged as a significant concern in both clinical and environmental settings, as these structured communities exhibit increased resistance to antifungal therapies and the immune system. These biofilms are formed by various fungal species, such as Cryptococcus, Candida, and Aspergillus, and contribute to chronic infections that are difficult to treat. The biofilm matrix provides physical protection, limits drug penetration, and promotes genetic exchange, which can further drive antifungal resistance.
Fungal species that can form biofilms complicate treatment strategies, as established biofilms often require higher doses of antifungal drugs and are more resistant to the host immune response. This is particularly relevant in healthcare-associated infections, where biofilms can lead to severe complications. The ability of fungal biofilms to persist on a diverse range of surfaces, such as medical devices, tissues, and environmental areas, poses a significant challenge for researchers. This lack of effective treatments to fully eradicate biofilms highlights the need for novel therapeutic approaches, such as targeting biofilm-specific pathways or utilizing combination therapies.
Cryptococcal biofilms are especially important because they are associated with increased resistance to antifungal treatments, including commonly used drugs like azoles and polyenes, leading to poor therapeutic outcomes. This resistance, combined with a global increase in cases of cryptococcosis and the high mortality rate of cryptococcal meningoencephalitis, underscores the urgency of developing effective treatments that can target these resilient biofilms.
It is important to note that there is still much that is unknown regarding fungal biofilms and their interaction with the host immune system. Future research must focus on better understanding the molecular mechanisms underlying biofilm formation, as well as identifying novel therapeutic strategies capable of disrupting or preventing biofilm development. The growing prevalence of fungal diseases in both healthy and immunocompromised individuals, particularly as a result of biofilm formation, has resulted in a surge in research investigating these infections. With ongoing advancements, there is promise that more effective treatments can be developed to reduce the morbidity and mortality associated with fungal biofilm infections.
Author Contributions
Conceptualization, H.M.P.; writing—original draft preparation, H.M.P.; imaging and figure preparation, H.M.P. and J.C.Z.; writing—review and editing, S.P.R. and M.S.; funding acquisition, M.S. and S.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Institutes of Health (NIH) grants to M.S. (AI173611 and AI177099) and S.P.R. (AI171817). Some figures were created with BioRender.com.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- McCoy, W.F.; Bryers, J.D.; Robbins, J.; Costerton, J.W. Observations of fouling biofilm formation. Can. J. Microbiol. 1981, 27, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Williams, P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu. Rev. Microbiol. 1985, 39, 527–556. [Google Scholar] [CrossRef]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Mitchell, A.P. Fungal Biofilms. PLoS Pathog. 2012, 8, e1002585. [Google Scholar] [CrossRef]
- Liu, S.; Le Mauff, F.; Sheppard, D.C.; Zhang, S. Filamentous fungal biofilms: Conserved and unique aspects of extracellular matrix composition, mechanisms of drug resistance and regulatory networks in Aspergillus fumigatus. NPJ Biofilms Microbiomes 2022, 8, 83. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Yan, D.; Yang, P.; Rowan, M.; Ren, S.; Pitts, D. Biofilm Accumulation and Structure in the Flow Path of Drip Emitters Using Reclaimed Wastewater. Trans. ASABE 2010, 53, 751–758. [Google Scholar] [CrossRef]
- de Beer, D.; Stoodley, P.; Lewandowski, Z. Liquid flow in heterogeneous biofilms. Biotechnol. Bioeng. 1994, 44, 636–641. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect. Immun. 2005, 73, 6350–6362. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Biofilm Formation by Cryptococcus neoformans. Microbiol. Spectr. 2015, 3, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.; Bangera, M.G.; Bumgarner, R.E.; Parsek, M.R.; Teitzel, G.M.; Lory, S.; Greenberg, E.P. Gene expression in Pseudomonas aeruginosa biofilms. Nature 2001, 413, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Murillo, L.A.; Newport, G.; Lan, C.-Y.; Habelitz, S.; Dungan, J.; Agabian, N.M. Genome-wide transcription profiling of the early phase of biofilm formation by Candida albicans. Eukaryot. Cell 2005, 4, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Martinez, L.R.; Fries, B.C. Fungal Biofilms: Relevance in the Setting of Human Disease. Curr. Fungal Infect. Rep. 2010, 4, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Morelli, K.A.; Kerkaert, J.D.; Cramer, R.A. Aspergillus fumigatus biofilms: Toward understanding how growth as a multicellular network increases antifungal resistance and disease progression. PLoS Pathog. 2021, 17, e1009794. [Google Scholar] [CrossRef]
- Cannizzo, F.T.; Eraso, E.; Ezkurra, P.A.; Villar-Vidal, M.; Bollo, E.; Castellá, G.; Cabañes, F.J.; Vidotto, V.; Quindós, G. Biofilm development by clinical isolates of Malassezia pachydermatis. Med. Mycol. 2007, 45, 357–361. [Google Scholar] [CrossRef]
- Montoya, A.M.; Elizondo-Zertuche, M.; Treviño-Rangel, R.d.J.; Becerril-García, M.; González, G.M. Biofilm formation and antifungal susceptibility of Trichosporon asahii isolates from Mexican patients. Rev. Iberoam. Micol. 2018, 35, 22–26. [Google Scholar] [CrossRef]
- Sav, H.; Rafati, H.; Öz, Y.; Dalyan-Cilo, B.; Ener, B.; Mohammadi, F.; Ilkit, M.; van Diepeningen, A.D.; Seyedmousavi, S. Biofilm Formation and Resistance to Fungicides in Clinically Relevant Members of the Fungal Genus Fusarium. J. Fungi 2018, 4, 16. [Google Scholar] [CrossRef]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm Formation in Medically Important Candida Species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef]
- Davis, L.E.; Cook, G.; Costerton, J.W. Biofilm on ventriculo-peritoneal shunt tubing as a cause of treatment failure in coccidioidal meningitis. Emerg. Infect. Dis. 2002, 8, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Di Bonaventura, G.; Pompilio, A.; Picciani, C.; Iezzi, M.; D’Antonio, D.; Piccolomini, R. Biofilm formation by the emerging fungal pathogen Trichosporon asahii: Development, architecture, and antifungal resistance. Antimicrob. Agents Chemother. 2006, 50, 3269–3276. [Google Scholar] [CrossRef]
- Ramage, G.; Vande Walle, K.; Wickes, B.L.; López-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Iturrieta-González, I.A.; Padovan, A.C.B.; Bizerra, F.C.; Hahn, R.C.; Colombo, A.L. Multiple species of Trichosporon produce biofilms highly resistant to triazoles and amphotericin B. PLoS ONE 2014, 9, e109553. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Casadevall, A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 2006, 50, 1021–1033. [Google Scholar] [CrossRef]
- Santi, L.; Beys-da-Silva, W.O.; Berger, M.; Calzolari, D.; Guimarães, J.A.; Moresco, J.J.; Yates, J.R. Proteomic profile of Cryptococcus neoformans biofilm reveals changes in metabolic processes. J. Proteome Res. 2014, 13, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.d.S.; Prado, A.; Bagon, N.P.; Negri, M.; Svidzinski, T.I.E. Mixed Fungal Biofilms: From Mycobiota to Devices, a New Challenge on Clinical Practice. Microorganisms 2022, 10, 1721. [Google Scholar] [CrossRef]
- Sentenac, H.; Loyau, A.; Leflaive, J.; Schmeller, D.S. The significance of biofilms to human, animal, plant and ecosystem health. Funct. Ecol. 2022, 36, 294–313. [Google Scholar] [CrossRef]
- Corte, L.; Roscini, L.; Colabella, C.; Tascini, C.; Leonildi, A.; Sozio, E.; Menichetti, F.; Merelli, M.; Scarparo, C.; Meyer, W.; et al. Exploring ecological modelling to investigate factors governing the colonization success in nosocomial environment of Candida albicans and other pathogenic yeasts. Sci. Rep. 2016, 6, 26860. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wiederhold, N.; Calderone, R.; Li, D. Biofilm Formation in Clinical Isolates of Fusarium. J. Fungi 2024, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Yiallouris, A.; Pana, Z.D.; Marangos, G.; Tzyrka, I.; Karanasios, S.; Georgiou, I.; Kontopyrgia, K.; Triantafyllou, E.; Seidel, D.; Cornely, O.A.; et al. Fungal diversity in the soil Mycobiome: Implications for ONE health. One Health 2024, 18, 100720. [Google Scholar] [CrossRef]
- Hall, R.A.; Noverr, M.C. Fungal interactions with the human host: Exploring the spectrum of symbiosis. Curr. Opin. Microbiol. 2017, 40, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, K. Dimorphic fungi, molds, and mold-like agents of medical importance. In Microbes of Medical Importance; Prajapati, A.K., Ed.; Iterative International Publishers, Selfypage Developers Pvt Ltd.: Novi, MI, USA, 2024; pp. 354–438. [Google Scholar]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi that Infect Humans. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.P.; Singh-Babak, S.D.; Hartooni, N.; Nobile, C.J. Biofilms and Antifungal Resistance. In Antifungals: From Genomics to Resistance and the Development of Novel Agents; Caister Academic Press: Poole, UK, 2015; pp. 71–90. [Google Scholar]
- Aguilar-Marcelino, L.; Al-Ani, L.K.T.; Freitas Soares, F.E.D.; Moreira, A.L.E.; Téllez-Téllez, M.; Castañeda-Ramírez, G.S.; Lourdes Acosta-Urdapilleta, M.D.; Díaz-Godínez, G.; Pineda-Alegría, J.A. Formation, Resistance, and Pathogenicity of Fungal Biofilms: Current Trends and Future Challenges. In Recent Trends in Mycological Research; Yadav, A.N., Ed.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 411–438. [Google Scholar]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Knoke, M.; Schwesinger, G. One hundred years ago: The history of cryptococcosis in Greifswald. Medical mycology in the nineteenth century. Mycoses 1994, 37, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- Aslanyan, L.; Sanchez, D.A.; Valdebenito, S.; Eugenin, E.A.; Ramos, R.L.; Martinez, L.R. The Crucial Role of Biofilms in Cryptococcus neoformans Survival within Macrophages and Colonization of the Central Nervous System. J. Fungi 2017, 3, 10. [Google Scholar] [CrossRef]
- Crabtree, J.N.; Okagaki, L.H.; Wiesner, D.L.; Strain, A.K.; Nielsen, J.N.; Nielsen, K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect. Immun. 2012, 80, 3776–3785. [Google Scholar] [CrossRef]
- Okagaki, L.H.; Nielsen, K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot. Cell 2012, 11, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B.; Mukaremera, L.; Cordero, R.J.B.; Coelho, C.; Desjardins, C.A.; Sturny-Leclère, A.; Janbon, G.; Perfect, J.R.; Fraser, J.A.; Casadevall, A.; et al. Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog. 2018, 14, e1006982. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans Cells in Biofilms Are Less Susceptible than Planktonic Cells to Antimicrobial Molecules Produced by the Innate Immune System. Infect. Immun. 2006, 74, 6118–6123. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Pierce, C.; Witt, C.; Wormley, F.L. Biofilm Formation by Cryptococcus neoformans Under Distinct Environmental Conditions. Mycopathologia 2009, 167, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Benaducci, T.; Sardi, J.d.C.O.; Lourencetti, N.M.S.; Scorzoni, L.; Gullo, F.P.; Rossi, S.A.; Derissi, J.B.; de Azevedo Prata, M.C.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Virulence of Cryptococcus sp. Biofilms In Vitro and In Vivo using Galleria mellonella as an Alternative Model. Front. Microbiol. 2016, 7, 290. [Google Scholar] [CrossRef] [PubMed]
- Joubert, L.-M.; Wolfaardt, G.M.; Botha, A. Microbial exopolymers link predator and prey in a model yeast biofilm system. Microb. Ecol. 2006, 52, 187–197. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Falkler, W.A.; Meiller, T.F. Fungal Biofilms and Drug Resistance. Emerg. Infect. Dis. 2004, 10, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Desai, G.M.; Frases, S.; Cordero, R.J.B.; DeLeon-Rodriguez, C.M.; Eugenin, E.A.; Nosanchuk, J.D.; Martinez, L.R. Methamphetamine Enhances Cryptococcus neoformans Pulmonary Infection and Dissemination to the Brain. mBio 2013, 4, e00400-13. [Google Scholar] [CrossRef]
- Ellis, D.H.; Pfeiffer, T.J. Natural habitat of Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 1990, 28, 1642–1644. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.; Janbon, G.; Idnurm, A.; Bahn, Y.-S. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb. Perspect. Med. 2014, 4, a019760. [Google Scholar] [CrossRef]
- Velagapudi, R.; Hsueh, Y.-P.; Geunes-Boyer, S.; Wright, J.R.; Heitman, J. Spores as infectious propagules of Cryptococcus neoformans. Infect. Immun. 2009, 77, 4345–4355. [Google Scholar] [CrossRef] [PubMed]
- Elsegeiny, W.; Marr, K.A.; Williamson, P.R. Immunology of Cryptococcal Infections: Developing a Rational Approach to Patient Therapy. Front. Immunol. 2018, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Graybill, R.J.; Larsen, R.A.; Pappas, P.G.; Perfect, J.R.; Powderly, W.G.; Sobel, J.D.; Dismukes, W.E. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin. Infect. Dis. 2000, 30, 710–718. [Google Scholar] [CrossRef]
- Williamson, P.R.; Jarvis, J.N.; Panackal, A.A.; Fisher, M.C.; Molloy, S.F.; Loyse, A.; Harrison, T.S. Cryptococcal meningitis: Epidemiology, immunology, diagnosis and therapy. Nat. Rev. Neurol. 2017, 13, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Dromer, F.; Ronin, O.; Dupont, B. Isolation of Cryptococcus neoformans var. gattii from an Asian patient in France: Evidence for dormant infection in healthy subjects. J. Med. Vet. Mycol. 1992, 30, 395–397. [Google Scholar] [CrossRef]
- Normile, T.G.; Bryan, A.M.; Del Poeta, M. Animal Models of Cryptococcus neoformans in Identifying Immune Parameters Associated with Primary Infection and Reactivation of Latent Infection. Front. Immunol. 2020, 11, 581750. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Hirata, A.; Omuta, J.; Sugamata, M.; Katori, S.; Saito, N.; Murata, N.; Morita, A.; Takahashi, K.; Hasegawa, C.; et al. Granuloma and cryptococcosis. J. Infect. Chemother. 2005, 11, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ristow, L.C.; Davis, J.M. The granuloma in cryptococcal disease. PLoS Pathog. 2021, 17, e1009342. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2004, 4, 11–24. [Google Scholar] [CrossRef]
- Perfect, J.R.; Casadevall, A. Cryptococcosis. Infect. Dis. Clin. N. Am. 2002, 16, 837–874. [Google Scholar] [CrossRef]
- Okata-Nwali, O.D.; Ikechukwu, O. Factors exacerbating immunodeficiency and antifungal resistance in the treatment of fungal infections. Biomed. Diagn. J. 2023, 7, 250–276. [Google Scholar]
- Goldman, D.L.; Khine, H.; Abadi, J.; Lindenberg, D.J.; Pirofski, L.; Niang, R.; Casadevall, A. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 2001, 107, E66. [Google Scholar] [CrossRef] [PubMed]
- Hajjeh, R.A.; Conn, L.A.; Stephens, D.S.; Baughman, W.; Hamill, R.; Graviss, E.; Pappas, P.G.; Thomas, C.; Reingold, A.; Rothrock, G.; et al. Cryptococcosis: Population-based multistate active surveillance and risk factors in human immunodeficiency virus-infected persons. Cryptococcal Active Surveillance Group. J. Infect. Dis. 1999, 179, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Tugume, L.; Rhein, J.; Hullsiek, K.H.; Mpoza, E.; Kiggundu, R.; Ssebambulidde, K.; Schutz, C.; Taseera, K.; Williams, D.A.; Abassi, M.; et al. HIV-Associated Cryptococcal Meningitis Occurring at Relatively Higher CD4 Counts. J. Infect. Dis. 2019, 219, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Bratton, E.W.; El Husseini, N.; Chastain, C.A.; Lee, M.S.; Poole, C.; Stürmer, T.; Juliano, J.J.; Weber, D.J.; Perfect, J.R. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS ONE 2012, 7, e43582. [Google Scholar] [CrossRef]
- Shi, Z.W.; Chen, Y.; Ogoke, K.M.; Strickland, A.B.; Shi, M. Cryptococcal Immune Reconstitution Inflammatory Syndrome: From Clinical Studies to Animal Experiments. Microorganisms 2022, 10, 2419. [Google Scholar] [CrossRef]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef]
- Pescador Ruschel, M.A.; Thapa, B. Cryptococcal Meningitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Freitas, I.; Salazar, T.; Rodrigues, P.; Vilela, M.; Duarte, A. An Uncommon Presentation of Cryptococcal Meningoencephalitis. Cureus 2022, 14, e21984. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease Among Adults, Adolescents and Children Living with HIV. 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK581832/ (accessed on 15 December 2024).
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef]
- CDC. Cryptococcosis. Treatment of Cryptococcosis. 2024. Available online: https://www.cdc.gov/cryptococcosis/treatment/index.html (accessed on 13 January 2025).
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nakayasu, E.S.; Almeida, I.C.; Nimrichter, L. The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J. Proteom. 2014, 97, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B.; Camacho, E.; Casadevall, A. Melanization in Cryptococcus neoformans Requires Complex Regulation. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Mattoon, E.R.; Cordero, R.J.B.; Casadevall, A. Melaninization Reduces Cryptococcus neoformans Susceptibility to Mechanical Stress. mSphere 2023, 8, e0059122. [Google Scholar] [CrossRef] [PubMed]
- Mukaremera, L. The Cryptococcus wall: A different wall for a unique lifestyle. PLoS Pathog. 2023, 19, e1011141. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.R.; Douglas, C.M.; Li, W.; Jue, C.K.; Pramanik, B.; Yuan, X.; Rude, T.H.; Toffaletti, D.L.; Perfect, J.R.; Kurtz, M. A glucan synthase FKS1 homolog in Cryptococcus neoformans is single copy and encodes an essential function. J. Bacteriol. 1999, 181, 444–453. [Google Scholar] [CrossRef]
- Levitz, S.M.; Nong, S.; Mansour, M.K.; Huang, C.; Specht, C.A. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc. Natl. Acad. Sci. USA 2001, 98, 10422–10427. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C.; Messina, L.; Bombaci, M.; Mancuso, G.; Midiri, A.; Beninati, C.; Cusumano, V.; Gerace, E.; Papasergi, S.; Teti, G. Characterization of two novel cryptococcal mannoproteins recognized by immune sera. Infect. Immun. 2005, 73, 7348–7355. [Google Scholar] [CrossRef]
- Wiesner, D.L.; Specht, C.A.; Lee, C.K.; Smith, K.D.; Mukaremera, L.; Lee, S.T.; Lee, C.G.; Elias, J.A.; Nielsen, J.N.; Boulware, D.R.; et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015, 11, e1004701. [Google Scholar] [CrossRef] [PubMed]
- Bose, I.; Reese, A.J.; Ory, J.J.; Janbon, G.; Doering, T.L. A Yeast under Cover: The Capsule of Cryptococcus neoformans. Eukaryot. Cell 2003, 2, 655–663. [Google Scholar] [CrossRef]
- Pericolini, E.; Cenci, E.; Monari, C.; De Jesus, M.; Bistoni, F.; Casadevall, A.; Vecchiarelli, A. Cryptococcus neoformans capsular polysaccharide component galactoxylomannan induces apoptosis of human T-cells through activation of caspase-8. Cell Microbiol. 2006, 8, 267–275. [Google Scholar] [CrossRef]
- De Jesus, M.; Nicola, A.M.; Frases, S.; Lee, I.R.; Mieses, S.; Casadevall, A. Galactoxylomannan-Mediated Immunological Paralysis Results from Specific B Cell Depletion in the Context of Widespread Immune System Damage. J. Immunol. 2009, 183, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nunes, M.P.; Oliveira, P.A.V.; Nascimento, D.d.O.; Freire-de-Lima, L.; Takiya, C.M.; Morrot, A.; Decote-Ricardo, D.; Previato, J.O.; et al. Involvement of the capsular GalXM-induced IL-17 cytokine in the control of Cryptococcus neoformans infection. Sci. Rep. 2018, 8, 16378. [Google Scholar] [CrossRef]
- Villena, S.N.; Pinheiro, R.O.; Pinheiro, C.S.; Nunes, M.P.; Takiya, C.M.; DosReis, G.A.; Previato, J.O.; Mendonça-Previato, L.; Freire-de-Lima, C.G. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol. 2008, 10, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Rodrigues, M.L.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar] [PubMed]
- Cherniak, R.; Jones, R.G.; Reiss, E. Structure determination of Cryptococcus neoformans serotype A-variant glucuronoxylomannan by 13C-n.m.r. spectroscopy. Carbohydr. Res. 1988, 172, 113–138. [Google Scholar] [CrossRef]
- Heiss, C.; Klutts, J.S.; Wang, Z.; Doering, T.L.; Azadi, P. The structure of Cryptococcus neoformans galactoxylomannan contains beta-D-glucuronic acid. Carbohydr. Res. 2009, 344, 915–920. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.C.; Fries, B.C.; Wang, F.; Casadevall, A. Capsule Structural Heterogeneity and Antigenic Variation in Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Spiropulu, C.; Eppard, R.A.; Otteson, E.; Kozel, T.R. Antigenic variation within serotypes of Cryptococcus neoformans detected by monoclonal antibodies specific for the capsular polysaccharide. Infect. Immun. 1989, 57, 3240–3242. [Google Scholar] [CrossRef] [PubMed]
- Fries, B.C.; Goldman, D.L.; Casadevall, A. Phenotypic switching in Cryptococcus neoformans. Microbes Infect. 2002, 4, 1345–1352. [Google Scholar] [CrossRef]
- Mandal, P.; Roy, T.S.; Das, T.K.; Banerjee, U.; Xess, I.; Nosanchuk, J.D. Differences in the cell wall architecture of melanin lacking and melanin producing Cryptococcus neoformans clinical isolates from India: An electron microscopic study. Braz. J. Microbiol. 2007, 38, 662–666. [Google Scholar] [CrossRef]
- Casadevall, A.; Coelho, C.; Cordero, R.J.B.; Dragotakes, Q.; Jung, E.; Vij, R.; Wear, M.P. The capsule of Cryptococcus neoformans. Virulence 2018, 10, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Gates, M.A.; Thorkildson, P.; Kozel, T.R. Molecular architecture of the Cryptococcus neoformans capsule. Mol. Microbiol. 2004, 52, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Kwon-Chung, K.J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell Biol. 1994, 14, 4912–4919. [Google Scholar] [PubMed]
- Fromtling, R.A.; Shadomy, H.J.; Jacobson, E.S. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia 1982, 79, 23–29. [Google Scholar] [CrossRef]
- Tucker, S.C.; Casadevall, A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 2002, 99, 3165–3170. [Google Scholar] [CrossRef] [PubMed]
- Blackstock, R.; Hall, N.K. Non-specific immunosuppression by Cryptococcus neoformans infection. Mycopathologia 1984, 86, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.W.; Cozad, G.C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect. Immun. 1972, 5, 896–901. [Google Scholar] [CrossRef]
- Kozel, T.R.; Gotschlich, E.C. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 1982, 129, 1675–1680. [Google Scholar] [CrossRef]
- Kozel, T.R.; Mastroianni, R.P. Inhibition of phagocytosis by cryptococcal polysaccharide: Dissociation of the attachment and ingestion phases of phagocytosis. Infect. Immun. 1976, 14, 62–67. [Google Scholar] [CrossRef]
- Vecchiarelli, A.; Pericolini, E.; Gabrielli, E.; Kenno, S.; Perito, S.; Cenci, E.; Monari, C. Elucidating the Immunological Function of the Cryptococcus neoformans Capsule. Future Microbiol. 2013, 8, 1107–1116. [Google Scholar] [CrossRef]
- Vecchiarelli, A. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 2000, 38, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.M.; Murphy, J.W. Mobility of human neutrophils in response to Cryptococcus neoformans cells, culture filtrate antigen, and individual components of the antigen. Infect. Immun. 1993, 61, 5067–5077. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.M.; Murphy, J.W. Cryptococcal polysaccharides induce L-selectin shedding and tumor necrosis factor receptor loss from the surface of human neutrophils. J. Clin. Investig. 1996, 97, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Ellerbroek, P.M.; Hoepelman, A.I.M.; Wolbers, F.; Zwaginga, J.J.; Coenjaerts, F.E.J. Cryptococcal glucuronoxylomannan inhibits adhesion of neutrophils to stimulated endothelium in vitro by affecting both neutrophils and endothelial cells. Infect. Immun. 2002, 70, 4762–4771. [Google Scholar] [CrossRef] [PubMed]
- Ellerbroek, P.M.; Ulfman, L.H.; Hoepelman, A.I.; Coenjaerts, F.E.J. Cryptococcal glucuronoxylomannan interferes with neutrophil rolling on the endothelium. Cell Microbiol. 2004, 6, 581–592. [Google Scholar] [CrossRef]
- Dong, Z.M.; Murphy, J.W. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect. Immun. 1997, 65, 557–563. [Google Scholar] [CrossRef]
- Vecchiarelli, A.; Pericolini, E.; Gabrielli, E.; Chow, S.-K.; Bistoni, F.; Cenci, E.; Casadevall, A. Cryptococcus neoformans galactoxylomannan is a potent negative immunomodulator, inspiring new approaches in anti-inflammatory immunotherapy. Immunotherapy 2011, 3, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Blackstock, R. Cryptococcal capsular polysaccharide utilizes an antigen-presenting cell to induce a T-suppressor cell to secrete TsF. J. Med. Vet. Mycol. 1996, 34, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Blackstock, R.; Casadevall, A. Presentation of cryptococcal capsular polysaccharide (GXM) on activated antigen-presenting cells inhibits the T-suppressor response and enhances delayed-type hypersensitivity and survival. Immunology 1997, 92, 334–339. [Google Scholar] [CrossRef]
- Vecchiarelli, A. The cellular responses induced by the capsular polysaccharide of Cryptococcus neoformans differ depending on the presence or absence of specific protective antibodies. Curr. Mol. Med. 2005, 5, 413–420. [Google Scholar] [CrossRef]
- Chiapello, L.S.; Baronetti, J.L.; Aoki, M.P.; Gea, S.; Rubinstein, H.; Masih, D.T. Immunosuppression, interleukin-10 synthesis and apoptosis are induced in rats inoculated with Cryptococcus neoformans glucuronoxylomannan. Immunology 2004, 113, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Chiapello, L.S.; Baronetti, J.L.; Garro, A.P.; Spesso, M.F.; Masih, D.T. Cryptococcus neoformans glucuronoxylomannan induces macrophage apoptosis mediated by nitric oxide in a caspase-independent pathway. Int. Immunol. 2008, 20, 1527–1541. [Google Scholar] [CrossRef]
- Zaragoza, O.; Casadevall, A. Experimental modulation of capsule size in Cryptococcus neoformans. Biol. Proced. Online 2004, 6, 10–15. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, T.R.; Alspaugh, J.A. The Cryptococcus neoformans Capsule: A Sword and a Shield. Clin. Microbiol. Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef]
- Zaragoza, O.; Chrisman, C.J.; Castelli, M.V.; Frases, S.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L.; Casadevall, A. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008, 10, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.J.; Najjuka, G.; Rolfes, M.A.; Akampurira, A.; Jain, N.; Anantharanjit, J.; von Hohenberg, M.; Tassieri, M.; Carlsson, A.; Meya, D.B.; et al. Cryptococcus neoformans Ex Vivo Capsule Size Is Associated with Intracranial Pressure and Host Immune Response in HIV-associated Cryptococcal Meningitis. J. Infect. Dis. 2014, 209, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Rosas, A.L.; MacGill, R.S.; Nosanchuk, J.D.; Kozel, T.R.; Casadevall, A. Activation of the alternative complement pathway by fungal melanins. Clin. Diagn. Lab. Immunol. 2002, 9, 144–148. [Google Scholar] [CrossRef]
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Polacheck, I.; Popkin, T.J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 1982, 150, 1414–1421. [Google Scholar] [CrossRef]
- Rosas, A.L.; Casadevall, A. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol. Lett. 1997, 153, 265–272. [Google Scholar] [CrossRef]
- Wang, Y.; Casadevall, A. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl. Environ. Microbiol. 1994, 60, 3864–3866. [Google Scholar] [CrossRef]
- Wang, Y.; Casadevall, A. Growth of Cryptococcus neoformans in presence of L-dopa decreases its susceptibility to amphotericin B. Antimicrob. Agents Chemother. 1994, 38, 2648–2650. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Casadevall, A.; Nosanchuk, J.D. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 2002, 46, 3394–3400. [Google Scholar] [CrossRef]
- Noverr, M.C.; Williamson, P.R.; Fajardo, R.S.; Huffnagle, G.B. CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Infect. Immun. 2004, 72, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Mednick, A.J.; Nosanchuk, J.D.; Casadevall, A. Melanization of Cryptococcus neoformans affects lung inflammatory responses during cryptococcal infection. Infect. Immun. 2005, 73, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tewari, R.P.; Williamson, P.R. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect. Immun. 1999, 67, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O. Basic principles of the virulence of Cryptococcus. Virulence 2019, 10, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.; Del Poeta, M. Role of Glucose in the Expression of Cryptococcus neoformans Antiphagocytic Protein 1, App1. Eukaryot. Cell 2011, 10, 293–301. [Google Scholar] [CrossRef]
- Rohatgi, S.; Pirofski, L. Host Immunity to Cryptococcus neoformans. Future Microbiol. 2015, 10, 565–581. [Google Scholar] [CrossRef]
- Diamond, R.D.; Bennett, J.E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect. Immun. 1973, 7, 231–236. [Google Scholar] [CrossRef]
- Watkins, R.A.; Andrews, A.; Wynn, C.; Barisch, C.; King, J.S.; Johnston, S.A. Cryptococcus neoformans Escape from Dictyostelium Amoeba by Both WASH-Mediated Constitutive Exocytosis and Vomocytosis. Front. Cell. Infect. Microbiol. 2018, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, J.N.; Shuman, H.A.; Casadevall, A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 2001, 98, 15245–15250. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Casadevall, A. In Fungal Intracellular Pathogenesis, Form Determines Fate. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kassaza, K.; Wasswa, F.; Nielsen, K.; Bazira, J. Cryptococcus neoformans Genotypic Diversity and Disease Outcome among HIV Patients in Africa. J. Fungi 2022, 8, 734. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Veri, A.O.; Ketela, T.; Jiang, B.; Roemer, T.; Cowen, L.E. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat. Commun. 2015, 6, 6741. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Coelho, C.; Alanio, A. Mechanisms of Cryptococcus neoformans-Mediated Host Damage. Front. Immunol. 2018, 9, 855. [Google Scholar] [CrossRef]
- Alvarez, M.; Casadevall, A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 2006, 16, 2161–2165. [Google Scholar] [CrossRef]
- Ma, H.; Croudace, J.E.; Lammas, D.A.; May, R.C. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 2006, 16, 2156–2160. [Google Scholar] [CrossRef]
- Johnston, S.A.; May, R.C. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 2010, 6, e1001041. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, X. Cryptococcus neoformans: Sex, morphogenesis, and virulence. Infect. Genet. Evol. 2021, 89, 104731. [Google Scholar] [CrossRef]
- Zaragoza, O. Multiple Disguises for the Same Party: The Concepts of Morphogenesis and Phenotypic Variations in Cryptococcus neoformans. Front. Microbiol. 2011, 2, 181. [Google Scholar] [CrossRef] [PubMed]
- Okagaki, L.H.; Strain, A.K.; Nielsen, J.N.; Charlier, C.; Baltes, N.J.; Chrétien, F.; Heitman, J.; Dromer, F.; Nielsen, K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010, 6, e1000953. [Google Scholar] [CrossRef]
- Zaragoza, O.; García-Rodas, R.; Nosanchuk, J.D.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L.; Casadevall, A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010, 6, e1000945. [Google Scholar] [CrossRef]
- Love, G.L.; Boyd, G.D.; Greer, D.L. Large Cryptococcus neoformans isolated from brain abscess. J. Clin. Microbiol. 1985, 22, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, J.G.; Cavill, R.; Jelbert, M. Cryptococcus neoformans of unusual morphology. Appl. Microbiol. 1973, 25, 309–312. [Google Scholar] [CrossRef] [PubMed]
- García-Rodas, R.; Casadevall, A.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS ONE 2011, 6, e24485. [Google Scholar] [CrossRef]
- D’Souza, C.A.; Alspaugh, J.A.; Yue, C.; Harashima, T.; Cox, G.M.; Perfect, J.R.; Heitman, J. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell Biol. 2001, 21, 3179–3191. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Casadevall, A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 2006, 50, 3519–3528. [Google Scholar] [CrossRef]
- Hubálek, Z.; Príkazský, Z. Growth of Cryptococcus neoformans in UV-irradiated excreta of pigeons. Folia Microbiol. 1975, 20, 231–235. [Google Scholar] [CrossRef]
- Bunting, L.A.; Neilson, J.B.; Bulmer, G.S. Cryptococcus neoformans: Gastronomic delight of a soil ameba. Sabouraudia 1979, 17, 225–232. [Google Scholar] [CrossRef]
- Ruiz, A.; Neilson, J.B.; Bulmer, G.S. Control of Cryptococcus neoformans in nature by biotic factors. Sabouraudia 1982, 20, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Prohic, A.; Jovovic Sadikovic, T.; Krupalija-Fazlic, M.; Kuskunovic-Vlahovljak, S. Malassezia species in healthy skin and in dermatological conditions. Int. J. Dermatol. 2016, 55, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Walsh, T.J.; Schlegel, R.; Moody, M.M.; Costerton, J.W.; Salcman, M. Ventriculoatrial shunt infection due to Cryptococcus neoformans: An ultrastructural and quantitative microbiological study. Neurosurgery 1986, 18, 373–375. [Google Scholar] [CrossRef]
- Banerjee, U.; Gupta, K.; Venugopal, P. A case of prosthetic valve endocarditis caused by Cryptococcus neoformans var. neoformans. J. Med. Vet. Mycol. 1997, 35, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Lopes, W.; Vainstein, M.H.; De Sousa Araujo, G.R.; Frases, S.; Staats, C.C.; de Almeida, R.M.C.; Schrank, A.; Kmetzsch, L.; Vainstein, M.H. Geometrical Distribution of Cryptococcus neoformans Mediates Flower-Like Biofilm Development. Front. Microbiol. 2017, 8, 2534. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.K.; Janssen, D.A.; Marcus, J.R.; Kauffman, C.A. Cryptococcal infection of a prosthetic dialysis fistula. Am. J. Kidney Dis. 1994, 24, 864–867. [Google Scholar] [CrossRef]
- Johannsson, B.; Callaghan, J.J. Prosthetic hip infection due to Cryptococcus neoformans: Case report. Diagn. Microbiol. Infect. Dis. 2009, 64, 76–79. [Google Scholar] [CrossRef]
- Shah, N.B.; Shoham, S.; Nayak, S. Cryptococcus neoformans prosthetic joint infection: Case report and review of the literature. Mycopathologia 2015, 179, 275–278. [Google Scholar] [CrossRef]
- Lee, S.C.; Phadke, S.; Sun, S.; Heitman, J. Pseudohyphal growth of Cryptococcus neoformans is a reversible dimorphic transition in response to ammonium that requires Amt1 and Amt2 ammonium permeases. Eukaryot. Cell 2012, 11, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, X. The morphotype heterogeneity in Cryptococcus neoformans. Curr. Opin. Microbiol. 2015, 26, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhai, B.; Lin, X. The Link between Morphotype Transition and Virulence in Cryptococcus neoformans. PLoS Pathog. 2012, 8, e1002765. [Google Scholar] [CrossRef]
- Wang, L.; Tian, X.; Gyawali, R.; Lin, X. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc. Natl. Acad. Sci. USA 2013, 110, 11571–11576. [Google Scholar] [CrossRef]
- Mittelman, M.W. Adhesion to Biomaterials. In Bacterial Adhesion: Molecular and Ecological Diversity; Wiley-Liss: New York, NY, USA, 1996. [Google Scholar]
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 2007, 73, 4592–4601. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Mihu, M.R.; Han, G.; Frases, S.; Cordero, R.J.B.; Casadevall, A.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. The use of chitosan to damage Cryptococcus neoformans biofilms. Biomaterials 2010, 31, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.; Nicola, A.M.; Nieves, E.; Paes, H.C.; Williamson, P.R.; Silva-Pereira, I.; Casadevall, A. Quorum Sensing-Mediated, Cell Density-Dependent Regulation of Growth and Virulence in Cryptococcus neoformans. mBio 2014, 5, e00986-13. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.M.; Espadas-Moreno, J.; Luque-Garcia, J.L.; Casadevall, A. Interaction of Cryptococcus neoformans Extracellular Vesicles with the Cell Wall. Eukaryot. Cell 2014, 13, 1484–1493. [Google Scholar] [CrossRef]
- Barriuso, J.; Hogan, D.A.; Keshavarz, T.; Martínez, M.J. Role of quorum sensing and chemical communication in fungal biotechnology and pathogenesis. FEMS Microbiol. Rev. 2018, 42, 627–638. [Google Scholar] [CrossRef]
- Zhu, J.; Kaufmann, G.F. Quo vadis quorum quenching? Curr. Opin. Pharmacol. 2013, 13, 688–698. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N.; Malek, I.; Schmitz, R.A. Metagenomic quorum quenching enzymes affect biofilm formation of Candida albicans and Staphylococcus epidermidis. PLoS ONE 2019, 14, e0211366. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, H.; Jang, J.-U.; Kim, H.; Park, H.; Iqbal, T.; Oh, H.-S.; Choo, K.-H.; Lee, K. Benefits of fungal-to-bacterial quorum quenching as anti-biofouling strategy in membrane bioreactors for wastewater treatment and water reuse. Bioresour. Technol. 2024, 403, 130848. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Sionov, E.; Khanal Lamichhane, A.; Kwon-Chung, K.J.; Chang, Y.C. Roles of Three Cryptococcus neoformans and Cryptococcus gattii Efflux Pump-Coding Genes in Response to Drug Treatment. Antimicrob. Agents Chemother. 2018, 62, e01751-17. [Google Scholar] [CrossRef]
- Mack, W.N.; Mack, J.P.; Ackerson, A.O. Microbial film development in a trickling filter. Microb. Ecol. 1975, 2, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Factories 2016, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.; Montelongo-Jauregui, D.; Vidal Bonifacio, B.; Lopez-Ribot, J.L.; Uppuluri, P. Candida albicans biofilm growth and dispersal: Contributions to pathogenesis. Curr. Opin. Microbiol. 2019, 52, 1–6. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, T.; Du, M.; Soteyome, T.; Lan, H.; Hong, W.; Peng, F.; Fu, X.; Peng, G.; Liu, J.; et al. Regulatory network controls microbial biofilm development, with Candida albicans as a representative: From adhesion to dispersal. Bioengineered 2022, 13, 253–267. [Google Scholar] [CrossRef]
- Ke, W.; Xie, Y.; Chen, Y.; Ding, H.; Ye, L.; Qiu, H.; Li, H.; Zhang, L.; Chen, L.; Tian, X.; et al. Fungicide-tolerant persister formation during cryptococcal pulmonary infection. Cell Host Microbe 2024, 32, 276–289.e7. [Google Scholar] [CrossRef]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of Biofilm Resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Ramage, G.; Robertson, S.N.; Williams, C. Strength in numbers: Antifungal strategies against fungal biofilms. Int. J. Antimicrob. Agents 2014, 43, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Alspaugh, J.A.; Liu, H.; Harris, S. Fungal Morphogenesis. Cold Spring Harb. Perspect. Med. 2015, 5, a019679. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, A.F.; Lee, H.H.; Ahmadi, M.; Martinez, L.R. Fungal serotype-specific differences in bacterial-yeast interactions. Virulence 2015, 6, 652–657. [Google Scholar] [CrossRef]
- Velamakanni, S.S.; Bahr, N.C.; Musubire, A.K.; Boulware, D.R.; Rhein, J.; Nabeta, H.W. Central nervous system cryptococcoma in a Ugandan patient with Human Immunodeficiency Virus. Med. Mycol. Case Rep. 2014, 6, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Ulett, K.B.; Cockburn, J.W.J.; Jeffree, R.; Woods, M.L. Cerebral cryptococcoma mimicking glioblastoma. BMJ Case Rep. 2017, 2017, bcr2016218824. [Google Scholar] [CrossRef] [PubMed]
- Chastain, D.B.; Rao, A.; Yaseyyedi, A.; Henao-Martínez, A.F.; Borges, T.; Franco-Paredes, C. Cerebral Cryptococcomas: A Systematic Scoping Review of Available Evidence to Facilitate Diagnosis and Treatment. Pathogens 2022, 11, 205. [Google Scholar] [CrossRef]
- Corti, M.; Florencia Villafañe, M.; Negroni, R.; Arechavala, A.; Maiolo, E. Hallazgos en las imágenes por resonancia magnética en pacientes con sida y neurocriptococosis. Rev. Iberoam. Micol. 2008, 25, 211–214. [Google Scholar] [CrossRef]
- Koo, H.; Yamada, K.M. Dynamic cell-matrix interactions modulate microbial biofilm and tissue 3D microenvironments. Curr. Opin. Cell Biol. 2016, 42, 102–112. [Google Scholar] [CrossRef]
- Albuquerque, P.; de Sousa, H.R.; de Oliveira Frazão, S.; do Nascimento Miranda, L.V.; Paes, H.C.; Pereira, I.S.; Nicola, A.M. Measuring Laccase Activity and Melanin Production in Cryptococcus neoformans. In Cryptococcus neoformans: Methods and Protocols; McClelland, E.E., Ed.; Springer US: New York, NY, USA, 2024; pp. 257–268. [Google Scholar]
- Kronstad, J.W.; Attarian, R.; Cadieux, B.; Choi, J.; D’Souza, C.A.; Griffiths, E.J.; Geddes, J.M.H.; Hu, G.; Jung, W.H.; Kretschmer, M.; et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol. 2011, 9, 193–203. [Google Scholar] [CrossRef]
- Hamed, M.F.; Araújo, G.R.d.S.; Munzen, M.E.; Reguera-Gomez, M.; Epstein, C.; Lee, H.H.; Frases, S.; Martinez, L.R. Phospholipase B Is Critical for Cryptococcus neoformans Survival in the Central Nervous System. mBio 2023, 14, e0264022. [Google Scholar] [CrossRef]
- Kietrungruang, K.; Sookkree, S.; Sangboonruang, S.; Semakul, N.; Poomanee, W.; Kitidee, K.; Tragoolpua, Y.; Tragoolpua, K. Ethanolic Extract Propolis-Loaded Niosomes Diminish Phospholipase B1, Biofilm Formation, and Intracellular Replication of Cryptococcus neoformans in Macrophages. Molecules 2023, 28, 6224. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Williams, C.; Lappin, D.F.; Millington, O.; Martins, M.; Ramage, G. Extracellular DNA Release Acts as an Antifungal Resistance Mechanism in Mature Aspergillus fumigatus Biofilms. Eukaryot. Cell 2013, 12, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Zarnowski, R.; Andes, D.R. The Extracellular Matrix of Fungal Biofilms. In Fungal Biofilms and Related Infections: Advances in Microbiology, Infectious Diseases and Public Health; Imbert, C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 3, pp. 21–35. [Google Scholar]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Panlilio, H.; Rice, C.V. The role of extracellular DNA in the formation, architecture, stability, and treatment of bacterial biofilms. Biotechnol. Bioeng. 2021, 118, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Extracellular DNA (eDNA). A Major Ubiquitous Element of the Bacterial Biofilm Architecture. Int. J. Mol. Sci. 2021, 22, 9100. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez de Aldecoa, A.L.; Zafra, O.; González-Pastor, J.E. Mechanisms and Regulation of Extracellular DNA Release and Its Biological Roles in Microbial Communities. Front. Microbiol. 2017, 8, 1390. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Nielsen, K.; Daou, S.; Brigitte, M.; Chretien, F.; Dromer, F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 2009, 77, 120–127. [Google Scholar] [CrossRef]
- Decote-Ricardo, D.; LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nascimento, D.O.; Nunes, M.P.; Morrot, A.; Freire-de-Lima, L.; Previato, J.O.; Mendonça-Previato, L.; Freire-de-Lima, C.G. Immunomodulatory Role of Capsular Polysaccharides Constituents of Cryptococcus neoformans. Front. Med. 2019, 6, 129. [Google Scholar] [CrossRef]
- Alvarez, M.; Saylor, C.; Casadevall, A. Antibody action after phagocytosis promotes Cryptococcus neoformans and Cryptococcus gattii macrophage exocytosis with biofilm-like microcolony formation. Cell Microbiol. 2008, 10, 1622–1633. [Google Scholar] [CrossRef]
- Gültaşli, N.Z.; Ercan, K.; Orhun, S.; Albayrak, S. MRI findings of intramedullary spinal cryptococcoma. Diagn. Interv. Radiol. 2007, 13, 64–67. [Google Scholar] [PubMed]
- Trevijano-Contador, N.; de Oliveira, H.C.; García-Rodas, R.; Rossi, S.A.; Llorente, I.; Zaballos, Á.; Janbon, G.; Ariño, J.; Zaragoza, Ó. Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog. 2018, 14, e1007007. [Google Scholar] [CrossRef]
- Dambuza, I.M.; Drake, T.; Chapuis, A.; Zhou, X.; Correia, J.; Taylor-Smith, L.; LeGrave, N.; Rasmussen, T.; Fisher, M.C.; Bicanic, T.; et al. The Cryptococcus neoformans Titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog. 2018, 14, e1006978. [Google Scholar] [CrossRef]
- García-Barbazán, I.; Torres-Cano, A.; García-Rodas, R.; Sachse, M.; Luque, D.; Megías, D.; Zaragoza, O. Accumulation of endogenous free radicals is required to induce titan-like cell formation in Cryptococcus neoformans. mBio 2024, 15, e02549-23. [Google Scholar] [CrossRef]
- Caza, M.; Kronstad, J.W. The cAMP/Protein Kinase A Pathway Regulates Virulence and Adaptation to Host Conditions in Cryptococcus neoformans. Front. Cell. Infect. Microbiol. 2019, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Kronstad, J.W. Disarming Fungal Pathogens: Bacillus safensis Inhibits Virulence Factor Production and Biofilm Formation by Cryptococcus neoformans and Candida albicans. mBio 2017, 8, e01537-17. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory Circuitry Governing Fungal Development, Drug Resistance, and Disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Zhou, Y.; Zang, X.; Deng, H.; Liu, Y.; Shen, D.; Xue, X. Cryptococcus escapes host immunity: What do we know? Front. Cell Infect. Microbiol. 2022, 12, 1041036. [Google Scholar] [CrossRef]
- Robertson, E.J.; Casadevall, A. Antibody-mediated immobilization of Cryptococcus neoformans promotes biofilm formation. Appl. Environ. Microbiol. 2009, 75, 2528–2533. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D. Fungal Biofilms: In vivo models for discovery of anti-biofilm drugs. Microbiol. Spectr. 2015, 3, E30. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.; Rowan, N.J. Pathogenic Drug Resistant Fungi: A Review of Mitigation Strategies. Int. J. Mol. Sci. 2023, 24, 1584. [Google Scholar] [CrossRef]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J.; Mycoses Study Group. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Stins, M.F.; McCaffery, M.J.; Miller, G.F.; Pare, D.R.; Dam, T.; Paul-Satyaseela, M.; Kim, K.S.; Kwon-Chung, K.J. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 2004, 72, 4985–4995. [Google Scholar] [CrossRef]
- Eugenin, E.A.; Greco, J.M.; Frases, S.; Nosanchuk, J.D.; Martinez, L.R. Methamphetamine alters blood brain barrier protein expression in mice, facilitating central nervous system infection by neurotropic Cryptococcus neoformans. J. Infect. Dis. 2013, 208, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Moranova, Z.; Kawamoto, S.; Raclavsky, V. Hypoxia sensing in Cryptococcus neoformans: Biofilm-like adaptation for dormancy? Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2009, 153, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Karkowska-Kuleta, J.; Rapala-Kozik, M.; Kozik, A. Fungi pathogenic to humans: Molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochim. Pol. 2009, 56, 211–224. [Google Scholar] [CrossRef]
- Qian, W.; Li, X.; Liu, Q.; Lu, J.; Wang, T.; Zhang, Q. Antifungal and Antibiofilm Efficacy of Paeonol Treatment Against Biofilms Comprising Candida albicans and/or Cryptococcus neoformans. Front. Cell. Infect. Microbiol. 2022, 12, 884793. [Google Scholar] [CrossRef]
- Mayer, F.L.; Sánchez-León, E.; Kronstad, J.W. A chemical genetic screen reveals a role for proteostasis in capsule and biofilm formation by Cryptococcus neoformans. Microb. Cell 2018, 5, 495–510. [Google Scholar] [CrossRef]
- Ganguly, S.; Mitchell, A.P. Mucosal biofilms of Candida albicans. Curr. Opin. Microbiol. 2011, 14, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans-The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- R, A.N.; Rafiq, N.B. Candidiasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Benabderrahmane, A.; Atmani, M.; Hamriri, K.; Laghrari, Z.; Belmalha, S. A comprehensive literature review on the oral microbiota: Key concepts and implications for dental and systemic health. Nov. Res. Microbiol. J. 2024, 8, 2790–2808. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Wey, S.B.; Mori, M.; Pfaller, M.A.; Woolson, R.F.; Wenzel, R.P. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch. Intern. Med. 1988, 148, 2642–2645. [Google Scholar] [CrossRef]
- Lu, Y.; Su, C.; Unoje, O.; Liu, H. Quorum sensing controls hyphal initiation in Candida albicans through Ubr1-mediated protein degradation. Proc. Natl. Acad. Sci. USA 2014, 111, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.-P.; Steinbach, W.J. A Perspective on Aspergillus fumigatus Research for the Next Ten Years. In Aspergillus fumigatus and Aspergillosis; Latgé, J.-P., Steinbach, W.J., Eds.; ASM Press: Washington, DC, USA, 2014; pp. 547–558. [Google Scholar]
- Taylor, P.R.; Leal, S.M.; Sun, Y.; Pearlman, E. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J. Immunol. 2014, 192, 3319–3327. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, C.; Li, X.; He, Y.; Zhou, L.; Pang, G.; Sun, S. In vitro antifungal activity of silver nanoparticles against ocular pathogenic filamentous fungi. J. Ocul. Pharmacol. Ther. 2013, 29, 270–274. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Beauvais, A.; Latgé, J.-P. Aspergillus Biofilm In Vitro and In Vivo. Microbiol. Spectr. 2015, 3, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifah, D.H.M.; Damra, E.; Khalaf, S.M.H.; Hozzein, W.N. Biogeography of Black Mold Aspergillus niger: Global Situation and Future Perspective under Several Climate Change Scenarios Using MaxEnt Modeling. Diversity 2022, 14, 845. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Dietl, A.-M.; Kontoyiannis, D.P.; Brock, M. Aspergillus terreus Species Complex. Clin. Microbiol. Rev. 2021, 34, e0031120. [Google Scholar] [CrossRef] [PubMed]
- Perea, S.; Patterson, T.F. Antifungal resistance in pathogenic fungi. Clin. Infect. Dis. 2002, 35, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, C.H.; Morelli, K.A.; Schultz, D.; Nadell, C.D.; Cramer, R.A. Fungal biofilm architecture produces hypoxic microenvironments that drive antifungal resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 22473–22483. [Google Scholar] [CrossRef] [PubMed]
- Seidler, M.J.; Salvenmoser, S.; Müller, F.-M.C. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 2008, 52, 4130–4136. [Google Scholar] [CrossRef]
- Loussert, C.; Schmitt, C.; Prevost, M.-C.; Balloy, V.; Fadel, E.; Philippe, B.; Kauffmann-Lacroix, C.; Latgé, J.P.; Beauvais, A. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol. 2010, 12, 405–410. [Google Scholar] [CrossRef]
- Müller, F.-M.C.; Seidler, M.; Beauvais, A. Aspergillus fumigatus biofilms in the clinical setting. Med. Mycol. 2011, 49 (Suppl. S1), S96–S100. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Gutierrez-Correa, M.; Jones, B.; Williams, C. Aspergillus biofilms: Clinical and industrial significance. FEMS Microbiol. Lett. 2011, 324, 89–97. [Google Scholar] [CrossRef]
- Williams, C.; Rajendran, R.; Ramage, G. Aspergillus Biofilms in Human Disease. In Fungal Biofilms and Related Infections; Imbert, C., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 931, pp. 1–11. [Google Scholar]
- Theelen, B.; Cafarchia, C.; Gaitanis, G.; Bassukas, I.D.; Boekhout, T.; Dawson, T.L. Malassezia ecology, pathophysiology, and treatment. Med. Mycol. 2018, 56 (Suppl. S1), S10–S25. [Google Scholar] [CrossRef]
- Cassola, F.; Ramírez, N.; Delarmelina, C.; Duarte, M.C.T. In vitro determination of the susceptibility of Malassezia furfur biofilm to different commercially used antimicrobials. APMIS 2024, 132, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.K. Seborrheic Dermatitis and Malassezia species: How Are They Related? J. Clin. Aesthet. Dermatol. 2009, 2, 14–17. [Google Scholar]
- Rudramurthy, S.M.; Honnavar, P.; Dogra, S.; Yegneswaran, P.P.; Handa, S.; Chakrabarti, A. Association of Malassezia species with dandruff. Indian. J. Med. Res. 2014, 139, 431–437. [Google Scholar]
- Velegraki, A.; Cafarchia, C.; Gaitanis, G.; Iatta, R.; Boekhout, T. Malassezia infections in humans and animals: Pathophysiology, detection, and treatment. PLoS Pathog. 2015, 11, e1004523. [Google Scholar] [CrossRef] [PubMed]
- Zareei, M.; Roudbar Mohammadi, S.; Shahbazi, S.; Roudbary, M.; Borjian Borujeni, Z. Evaluation of the Ability of Malassezia Species in Biofilm Formation. Arch. Clin. Infect. Dis. 2017, 13, e62223. [Google Scholar] [CrossRef]
- Angiolella, L.; Leone, C.; Rojas, F.; Mussin, J.; de Los Angeles Sosa, M.; Giusiano, G. Biofilm, adherence, and hydrophobicity as virulence factors in Malassezia furfur. Med. Mycol. 2018, 56, 110–116. [Google Scholar] [CrossRef]
- Čonková, E.; Proškovcová, M.; Váczi, P.; Malinovská, Z. In Vitro Biofilm Formation by Malassezia pachydermatis Isolates and Its Susceptibility to Azole Antifungals. J. Fungi 2022, 8, 1209. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Walsh, T.J. Uncommon opportunistic fungi: New nosocomial threats. Clin. Microbiol. Infect. 2001, 7 (Suppl. S2), 8–24. [Google Scholar] [CrossRef]
- Walsh, T.J.; Groll, A.; Hiemenz, J.; Fleming, R.; Roilides, E.; Anaissie, E. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 2004, 10 (Suppl. S1), 48–66. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Zepeda, G.; Alvareda, D. Nosocomial urinary infection due to Trichosporon asahii. First two cases in Chile. Rev. Iberoam. Micol. 2003, 20, 21–23. [Google Scholar] [PubMed]
- Lo Passo, C.; Pernice, I.; Celeste, A.; Perdichizzi, G.; Todaro-Luck, F. Transmission of Trichosporon asahii oesophagitis by a contaminated endoscope. Mycoses 2001, 44, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Krzossok, S.; Birck, R.; Henke, S.; Hof, H.; van der Woude, F.J.; Braun, C. Trichosporon asahii infection of a dialysis PTFE arteriovenous graft. Clin. Nephrol. 2004, 62, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Nakayama, M.; Shimizu, Y.; Koganesawa, S.; Kanai, H.; Sugiyama, Y.; Kurakado, S.; Sugita, T. Role of Hog1-mediated stress tolerance in biofilm formation by the pathogenic fungus Trichosporon asahii. Sci. Rep. 2024, 14, 28761. [Google Scholar] [CrossRef]
- de Aguiar Cordeiro, R.; Serpa, R.; Flávia Uchoa Alexandre, C.; de Farias Marques, F.J.; Vladia Silva de Melo, C.; da Silva Franco, J.; José de Jesus Evangelista, A.; Pires de Camargo, Z.; Samia Nogueira Brilhante, R.; Fabio Gadelha Rocha, M.; et al. Trichosporon inkin biofilms produce extracellular proteases and exhibit resistance to antifungals. J. Med. Microbiol. 2015, 64, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.D.A.; Aguiar, A.L.R.; da Silva, B.N.; Pereira, L.M.G.; Portela, F.V.M.; de Camargo, Z.P.; de Lima-Neto, R.G.; de Castelo-Branco, D.S.C.M.; Rocha, M.F.G.; Sidrim, J.J.C. Trichosporon asahii and Trichosporon inkin Biofilms Produce Antifungal-Tolerant Persister Cells. Front. Cell. Infect. Microbiol. 2021, 11, 645812. [Google Scholar] [CrossRef] [PubMed]
- Persister Cell—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/neuroscience/persister-cell (accessed on 15 January 2025).
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006, 44, 2186–2190. [Google Scholar] [CrossRef]
- Coleman, J.J. The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef]
- Krcmery, V.; Jesenska, Z.; Spanik, S.; Gyarfas, J.; Nogova, J.; Botek, R.; Mardiak, J.; Sufliarsky, J.; Sisolakova, J.; Vanickova, M.; et al. Fungaemia due to Fusarium spp. in cancer patients. J. Hosp. Infect. 1997, 36, 223–228. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Bonifaz, A.; Ranque, S.; Sybren de Hoog, G.; Verweij, P.E.; Meis, J.F. Current antifungal treatment of fusariosis. Int. J. Antimicrob. Agents 2018, 51, 326–332. [Google Scholar] [CrossRef]
- Sun, Y.; Chandra, J.; Mukherjee, P.; Szczotka-Flynn, L.; Ghannoum, M.A.; Pearlman, E. A murine model of contact lens-associated fusarium keratitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Laurent, J.; Edel-Hermann, V.; Barbezant, M.; Sixt, N.; Dalle, F.; Aho, S.; Bonnin, A.; Hartemann, P.; Sautour, M. Adaptation of Fusarium oxysporum and Fusarium dimerum to the specific aquatic environment provided by the water systems of hospitals. Water Res. 2015, 76, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Veiga, F.F.; de Castro-Hoshino, L.V.; Sato, F.; Baesso, M.L.; Silva, S.; Negri, M.; Svidzinski, T.I.E. Characterization of a biofilm formed by Fusarium oxysporum on the human nails. Int. J. Dermatol. 2022, 61, 191–198. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Outbreak of Suspected Fungal Meningitis Associated with Surgical Procedures Performed Under Spinal Anaesthesia—The United States of America and Mexico. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON470 (accessed on 16 December 2024).
- Hoenigl, M.; Jenks, J.D.; Egger, M.; Nucci, M.; Thompson, G.R. Treatment of Fusarium Infection of the Central Nervous System: A Review of Past Cases to Guide Therapy for the Ongoing 2023 Outbreak in the United States and Mexico. Mycopathologia 2023, 188, 973–981. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Kos, J.; Radić, B.; Anić, M.; Radović, R.; Kudumija, N.; Vulić, A.; Đekić, S.; Pleadin, J. Impact of Climate Changes on the Natural Prevalence of Fusarium Mycotoxins in Maize Harvested in Serbia and Croatia. Foods 2023, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; He, D.; Wang, L. Advances in Fusarium drug resistance research. J. Glob. Antimicrob. Resist. 2021, 24, 215–219. [Google Scholar] [CrossRef]
- Tejeda-Garibay, S.; Hoyer, K.K. Coccidioidomycosis and Host Microbiome Interactions: What We Know and What We Can Infer from Other Respiratory Infections. J. Fungi 2023, 9, 586. [Google Scholar] [CrossRef]
- Zaheri, S.C.; Field, E.; Orvin, C.A.; Perilloux, D.M.; Klapper, R.J.; Shelvan, A.; Ahmadzadeh, S.; Shekoohi, S.; Kaye, A.D.; Varrassi, G. Valley Fever: Pathogenesis and Evolving Treatment Options. Cureus 2023, 15, e50260. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Stevens, D.A.; Hung, C.-Y.; Beyhan, S.; Taylor, J.W.; Shubitz, L.F.; Duttke, S.H.; Heidari, A.; Johnson, R.H.; Deresinski, S.C.; et al. Coccidioides Species: A Review of Basic Research: 2022. J. Fungi 2022, 8, 859. [Google Scholar] [CrossRef]
- Diep, A.L.; Hoyer, K.K. Host Response to Coccidioides Infection: Fungal Immunity. Front. Cell Infect. Microbiol. 2020, 10, 581101. [Google Scholar] [CrossRef]
- Binnicker, M.J.; Buckwalter, S.P.; Eisberner, J.J.; Stewart, R.A.; McCullough, A.E.; Wohlfiel, S.L.; Wengenack, N.L. Detection of Coccidioides species in clinical specimens by real-time PCR. J. Clin. Microbiol. 2007, 45, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.D.C.O.; Pitangui, N.D.S.; Rodríguez-Arellanes, G.; Taylor, M.L.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Highlights in pathogenic fungal biofilms. Rev. Iberoam. de Micol. 2014, 31, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.A.; Sorrell, T.C. Antifungal agents. Med. J. Aust. 2007, 187, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef]
- Rodrigues, M.L. The Multifunctional Fungal Ergosterol. mBio 2018, 9, e01755-18. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Sawaya, B.P.; Briggs, J.P.; Schnermann, J. Amphotericin B nephrotoxicity: The adverse consequences of altered membrane properties. J. Am. Soc. Nephrol. 1995, 6, 154–164. [Google Scholar] [CrossRef]
- Sabra, R.; Branch, R.A. Amphotericin B Nephrotoxicity. Drug Saf. 1990, 5, 94–108. [Google Scholar] [CrossRef]
- Sud, I.J.; Feingold, D.S. Mechanisms of action of the antimycotic imidazoles. J. Investig. Dermatol. 1981, 76, 438–441. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2016, 7, 2173. [Google Scholar] [CrossRef] [PubMed]
- Rakhshan, A.; Rahmati Kamel, B.; Saffaei, A.; Tavakoli-Ardakani, M. Hepatotoxicity Induced by Azole Antifungal Agents: A Review Study. Iran. J. Pharm. Res. 2023, 22, e130336. [Google Scholar] [CrossRef] [PubMed]
- Sawistowska-Schröder, E.T.; Kerridge, D.; Perry, H. Echinocandin inhibition of 1,3-beta-D-glucan synthase from Candida albicans. FEBS Lett. 1984, 173, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Echinocandins: A new class of antifungal. J. Antimicrob. Chemother. 2002, 49, 889–891. [Google Scholar] [CrossRef]
- Maligie, M.A.; Selitrennikoff, C.P. Cryptococcus neoformans resistance to echinocandins: (1,3)beta-glucan synthase activity is sensitive to echinocandins. Antimicrob. Agents Chemother. 2005, 49, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.D.; Stover, K.R. Antifungal-Associated Drug-Induced Cardiac Disease. Clin. Infect. Dis. 2015, 61 (Suppl. S6), S662–S668. [Google Scholar] [CrossRef]
- Zhu, P.; Li, Y.; Guo, T.; Liu, S.; Tancer, R.J.; Hu, C.; Zhao, C.; Xue, C.; Liao, G. New antifungal strategies: Drug combination and co-delivery. Adv. Drug Deliv. Rev. 2023, 198, 114874. [Google Scholar] [CrossRef] [PubMed]
- Nowosielski, M.; Hoffmann, M.; Wyrwicz, L.S.; Stepniak, P.; Plewczynski, D.M.; Lazniewski, M.; Ginalski, K.; Rychlewski, L. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J. Chem. Inf. Model. 2011, 51, 455–462. [Google Scholar] [CrossRef]
- Petranyi, G.; Ryder, N.S.; Stütz, A. Allylamine Derivatives: New Class of Synthetic Antifungal Agents Inhibiting Fungal Squalene Epoxidase. Science 1984, 224, 1239–1241. [Google Scholar] [CrossRef]
- Chen, S.; Li, S.; Liu, Z.; Wu, Y.; Tu, Y.; Li, J. Comparison of the effects of three different anti-fungus drugs on Candida albicans of murine vaginal mucosa. J. Huazhong Univ. Sci. Technol. Med. Sci. 2007, 27, 209–212. [Google Scholar] [CrossRef]
- Coelho, C.; Casadevall, A. Cryptococcal therapies and drug targets: The old, the new and the promising. Cell Microbiol. 2016, 18, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Tits, J.; Cammue, B.P.A.; Thevissen, K. Combination Therapy to Treat Fungal Biofilm-Based Infections. Int. J. Mol. Sci. 2020, 21, 8873. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef]
- Chapman, S.W.; Sullivan, D.C.; Cleary, J.D. In search of the holy grail of antifungal therapy. Trans. Am. Clin. Clim. Assoc. 2008, 119, 197–215, discussion 215–216. [Google Scholar]
- Abassi, M.; Boulware, D.R.; Rhein, J. Cryptococcal Meningitis: Diagnosis and Management Update. Curr. Trop. Med. Rep. 2015, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Fan, F.; Liu, Y.; Liu, Y.; Lv, R.; Sun, W.; Ding, W.; Cai, Y.; Li, W.; Liu, X.; Qu, W. Candida albicans biofilms: Antifungal resistance, immune evasion, and emerging therapeutic strategies. Int. J. Antimicrob. Agents 2022, 60, 106673. [Google Scholar] [CrossRef] [PubMed]
- Tseung, K.S.Y.N.H.; Zhao, J. Update on the Fungal Biofilm Drug Resistance and Its Alternative Treatment. J. Biosci. Med. 2016, 4, 37–47. [Google Scholar]
- Bermas, A.; Geddes-McAlister, J. Combatting the evolution of antifungal resistance in Cryptococcus neoformans. Mol. Microbiol. 2020, 114, 721–734. [Google Scholar] [CrossRef]
- Williams, D.W.; Kuriyama, T.; Silva, S.; Malic, S.; Lewis, M.A.O. Candida biofilms and oral candidosis: Treatment and prevention. Periodontol. 2000 2011, 55, 250–265. [Google Scholar] [CrossRef]
- Nett, J.E.; Pohl, C.H. Editorial: Fungal Biofilms in Infection and Disease. Front. Cell. Infect. Microbiol. 2021, 11, 753650. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Srinivasan, A.; Ramasubramanian, A.K.; López-Ribot, J.L. From Biology to Drug Development: New Approaches to Combat the Threat of Fungal Biofilms. Microbiol. Spectr. 2015, 3, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Collignon, P.J. Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Walraven, C.J.; Lee, S.A. Antifungal lock therapy. Antimicrob. Agents Chemother. 2013, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sherertz, R.J.; Boger, M.S.; Collins, C.A.; Mason, L.; Raad, I.I. Comparative in vitro efficacies of various catheter lock solutions. Antimicrob. Agents Chemother. 2006, 50, 1865–1868. [Google Scholar] [CrossRef]
- Sousa, C.; Henriques, M.; Oliveira, R. Mini-review: Antimicrobial central venous catheters—Recent advances and strategies. Biofouling 2011, 27, 609–620. [Google Scholar] [CrossRef]
- Kovács, R.; Majoros, L. Antifungal lock therapy: An eternal promise or an effective alternative therapeutic approach? Lett. Appl. Microbiol. 2022, 74, 851–862. [Google Scholar] [CrossRef]
- Schinabeck, M.K.; Long, L.A.; Hossain, M.A.; Chandra, J.; Mukherjee, P.K.; Mohamed, S.; Ghannoum, M.A. Rabbit model of Candida albicans biofilm infection: Liposomal amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 2004, 48, 1727–1732. [Google Scholar] [CrossRef]
- Vargas-Cruz, N.; Reitzel, R.A.; Rosenblatt, J.; Chaftari, A.-M.; Wilson Dib, R.; Hachem, R.; Kontoyiannis, D.P.; Raad, I.I. Nitroglycerin-Citrate-Ethanol Catheter Lock Solution Is Highly Effective for In Vitro Eradication of Candida auris Biofilm. Antimicrob. Agents Chemother. 2019, 63, e00299-19. [Google Scholar] [CrossRef]
- Gupta, P.; Meher, M.K.; Tripathi, S.; Poluri, K.M. Nanoformulations for dismantling fungal biofilms: The latest arsenals of antifungal therapy. Mol. Asp. Med. 2024, 98, 101290. [Google Scholar] [CrossRef]
- Fukushima, K.; Rasyida, A.; Yang, M.-C. Characterization, degradation and biocompatibility of PBAT based nanocomposites. Appl. Clay Sci. 2013, 80–81, 291–298. [Google Scholar] [CrossRef]
- Jo, Y.-K.; Kim, B.H.; Jung, G. Antifungal Activity of Silver Ions and Nanoparticles on Phytopathogenic Fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, S.K.; Sarojini, S.; Umesh, M. Anti-Biofilm Activities of Nanocomposites: Current Scopes and Limitations. In Bio-manufactured Nanomaterials; Pal, K., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 83–94. [Google Scholar]
- Panigrahy, S.K.; Nandha, A.; Chaturvedi, M.; Mishra, P.K. Novel nanocomposites with advanced materials and their role in waste water treatment. Next Sustain. 2024, 4, 100042. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, A.U. Nanoparticles vs. biofilms: A battle against another paradigm of antibiotic resistance. Med. Chem. Commun. 2016, 7, 1479–1498. [Google Scholar] [CrossRef]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Falkner, R.; Jaspers, N. Regulating Nanotechnologies: Risk, Uncertainty and the Global Governance Gap. Glob. Environ. Politics 2012, 12, 30–55. [Google Scholar] [CrossRef]
- Ramage, G.; Martínez, J.P.; López-Ribot, J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006, 6, 979–986. [Google Scholar] [CrossRef]
- Narayana, S.V.V.S.; Srihari, S.V.V.P. A Review on Surface Modifications and Coatings on Implants to Prevent Biofilm. Regen. Eng. Transl. Med. 2020, 6, 330–346. [Google Scholar]
- Krishnan, S. Biofilm Formation on Medical Devices and Infection: Preventive Approaches. In Biofilm and Materials Science; Kanematsu, H., Barry, D.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 93–108. [Google Scholar]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podestà, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formation. PLoS ONE 2011, 6, e25029. [Google Scholar] [CrossRef]
- Coad, B.R.; Kidd, S.E.; Ellis, D.H.; Griesser, H.J. Biomaterials surfaces capable of resisting fungal attachment and biofilm formation. Biotechnol. Adv. 2014, 32, 296–307. [Google Scholar] [CrossRef]
- Junter, G.-A.; Thébault, P.; Lebrun, L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016, 30, 13–25. [Google Scholar] [CrossRef]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy. Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, O.; Kaplan, J.B.; Ghigo, J.-M. Antibiofilm polysaccharides. Environ. Microbiol. 2013, 15, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Delattin, N.; De Brucker, K.; Craik, D.J.; Cheneval, O.; Fröhlich, M.; Veber, M.; Girandon, L.; Davis, T.R.; Weeks, A.E.; Kumamoto, C.A.; et al. Plant-derived decapeptide OSIP108 interferes with Candida albicans biofilm formation without affecting cell viability. Antimicrob. Agents Chemother. 2014, 58, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gongora, D.; Woods, M.; Prosser, R.S.; Geddes-McAlister, J. Natural compounds from freshwater mussels disrupt fungal virulence determinants and influence fluconazole susceptibility in the presence of macrophages in Cryptococcus neoformans. Microbiol. Spectr. 2024, 12, e0284123. [Google Scholar] [CrossRef]
- Martinez, L.R.; Mihu, M.R.; Tar, M.; Cordero, R.J.B.; Han, G.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. Demonstration of antibiofilm and antifungal efficacy of chitosan against candidal biofilms, using an in vivo central venous catheter model. J. Infect. Dis. 2010, 201, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Du, L.; King, J.B.; Hall, B.E.; Cichewicz, R.H. Small-molecule suppressors of Candida albicans biofilm formation synergistically enhance the antifungal activity of amphotericin B against clinical Candida isolates. ACS Chem. Biol. 2013, 8, 840–848. [Google Scholar] [CrossRef]
- Hedrick, T.L.; Adams, J.D.; Sawyer, R.G. Implant-associated infections: An overview. J. Long. Term. Eff. Med. Implant. 2006, 16, 83–99. [Google Scholar] [CrossRef]
- Oliva, A.; Miele, M.C.; Al Ismail, D.; Di Timoteo, F.; De Angelis, M.; Rosa, L.; Cutone, A.; Venditti, M.; Mascellino, M.T.; Valenti, P.; et al. Challenges in the Microbiological Diagnosis of Implant-Associated Infections: A Summary of the Current Knowledge. Front. Microbiol. 2021, 12, 750460. [Google Scholar] [CrossRef]
- Mikziński, P.; Kraus, K.; Widelski, J.; Paluch, E. Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI). Microorganisms 2024, 12, 1198. [Google Scholar] [CrossRef]
- Cleaver, L.; Garnett, J.A. How to study biofilms: Technological advancements in clinical biofilm research. Front. Cell. Infect. Microbiol. 2023, 13, 1335389. [Google Scholar] [CrossRef] [PubMed]
- Tsikopoulos, A.; Tsikopoulos, K.; Sidiropoulos, K.; Meroni, G.; Triaridis, S.; Drago, L.; Papaioannidou, P. Development and Prevention of Biofilm on Cochlear Implants: A Systematic Review. Medicina 2024, 60, 1959. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Islam, M.A.; Sharun, K.; Emran, T.B.; Al-Tawfiq, J.A.; Dhama, K. Recent advances in the treatment of biofilms induced surgical site infections. Int. J. Surg. 2023, 109, 65–67. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action, 1st ed.; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).