Molecular Mechanism of Aflatoxin B1 Synthesis Related AfVerB Regulating the Development, AFB1 Biosyntheis and Virulence of Aspergillus flavus Mainly Through Its CYP Domain
Abstract
1. Introduction
2. Materials and Methods
2.1. The A. flavus Strains and Media Used in This Study
2.2. The Construction of A. flavus Strains
2.3. Phenotype Analysis
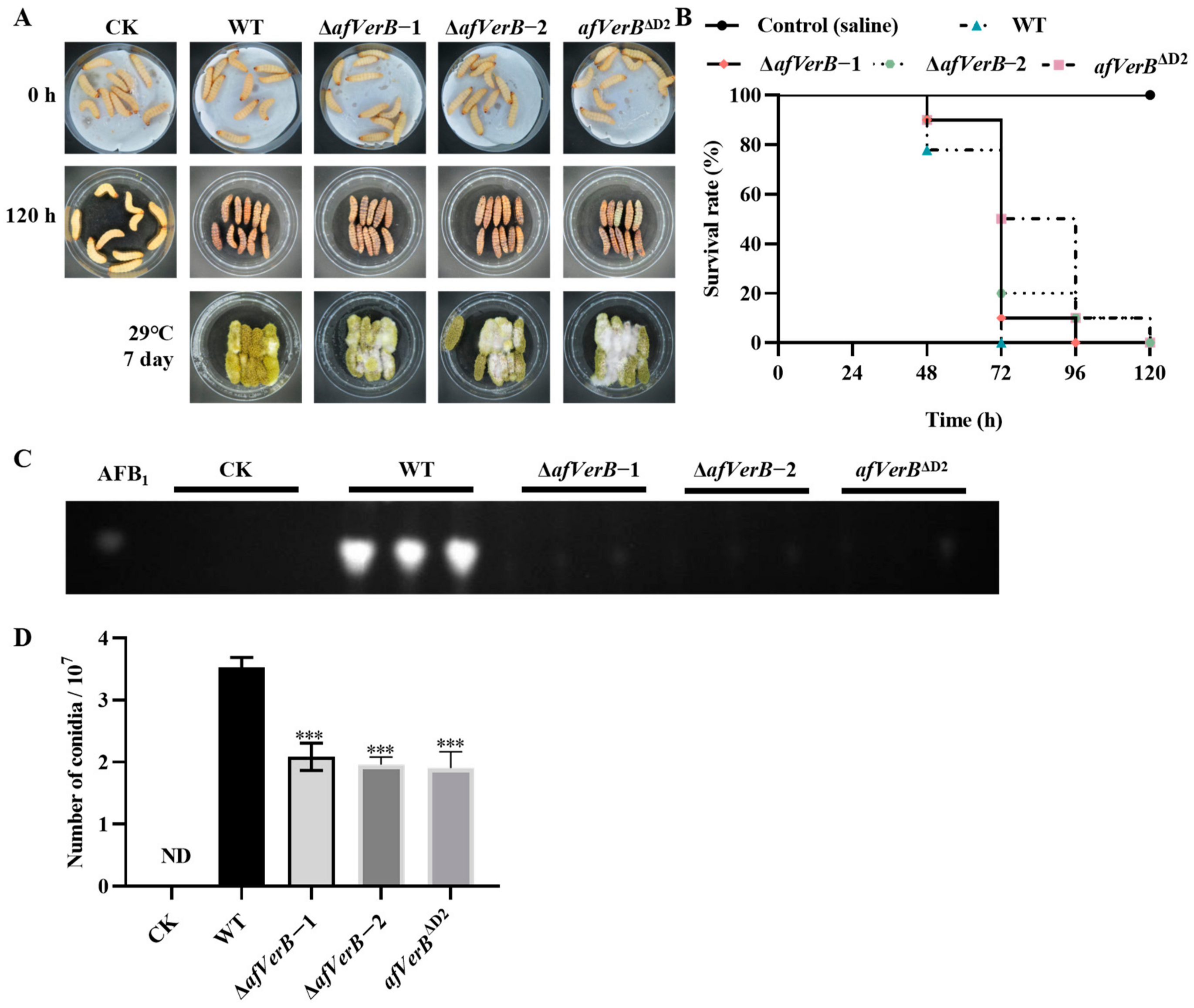
2.4. Analysis of AFB1 Production
2.5. Crop Colonization Analysis
2.6. Stress Analysis
2.7. Insect Infection Model
2.8. Agar Spotting Assays
2.9. RT-qPCR Assays
2.10. Statistical Analysis
3. Results
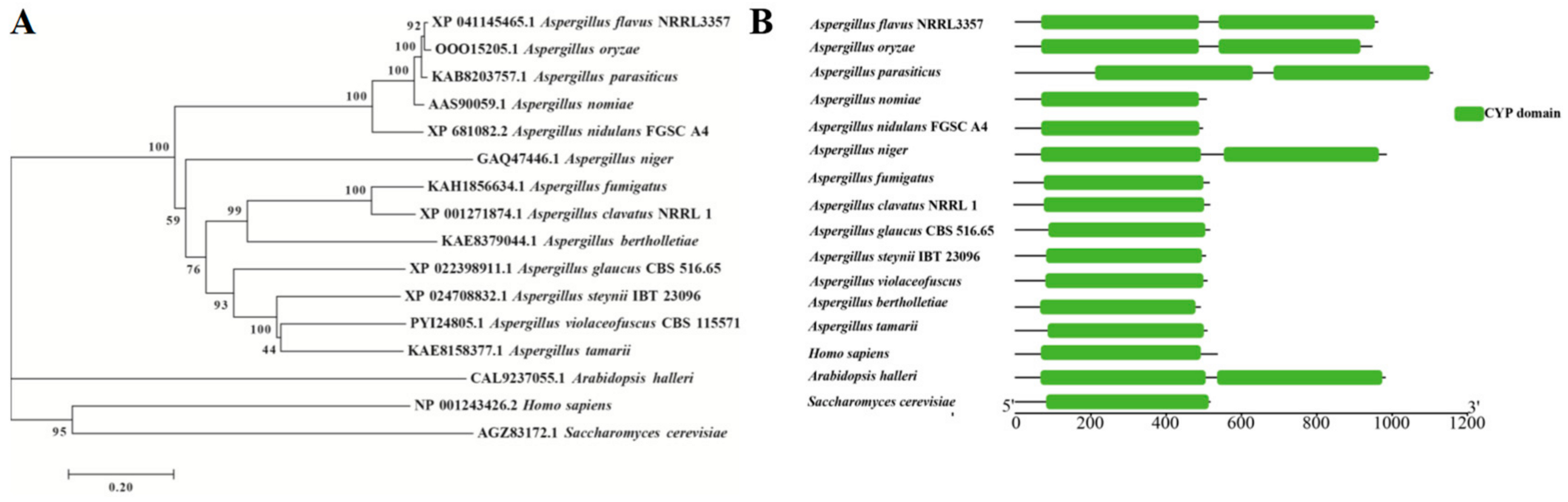
3.1. Bioinformatics Analysis and Strain Construction
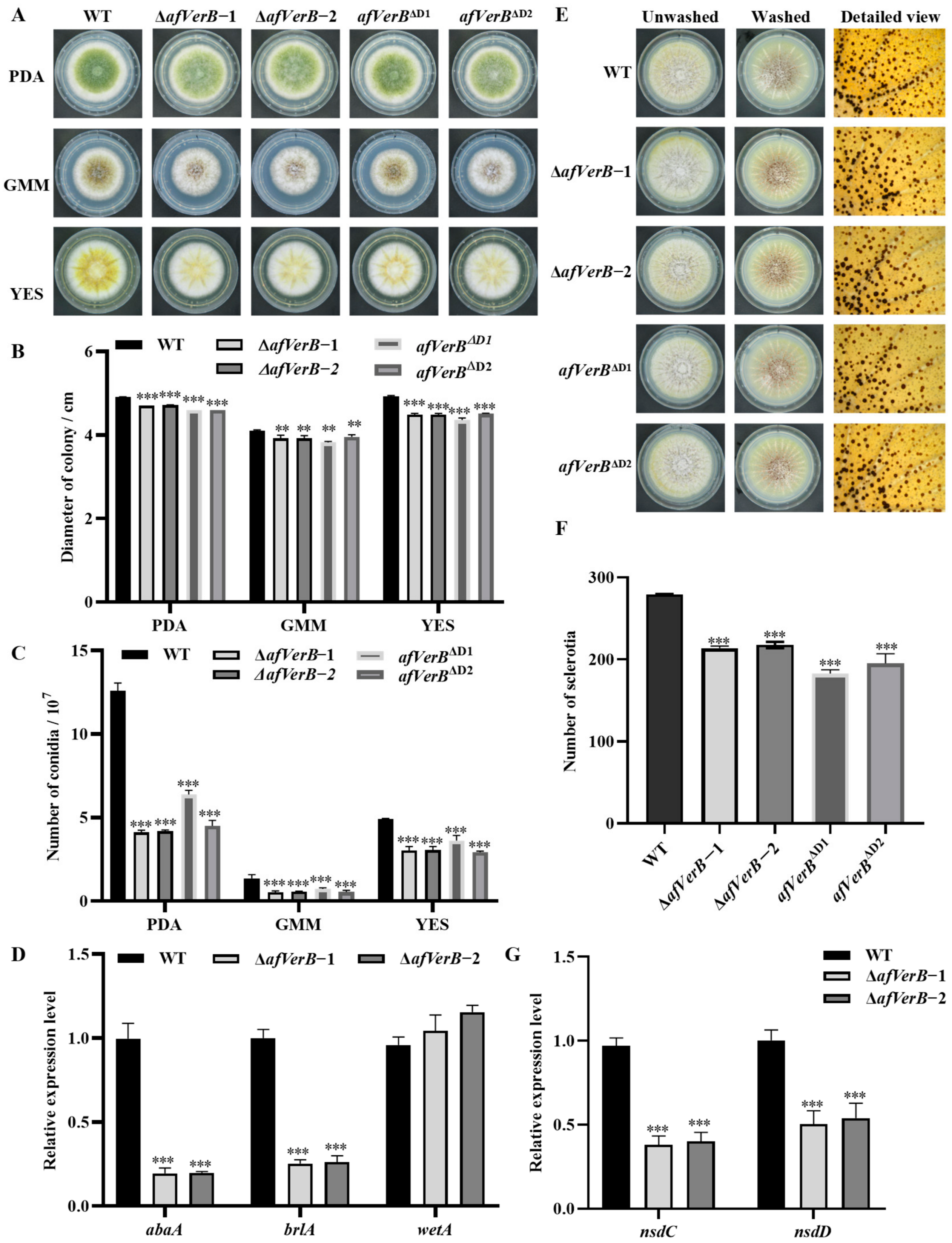
3.2. AfVerB Is Deeply Involved in Fungal Growth and Conidiation Through Its Both CYP Domains
3.3. AfVerB Is Involved in the Formation of Sclerotia via Both CYP Domains
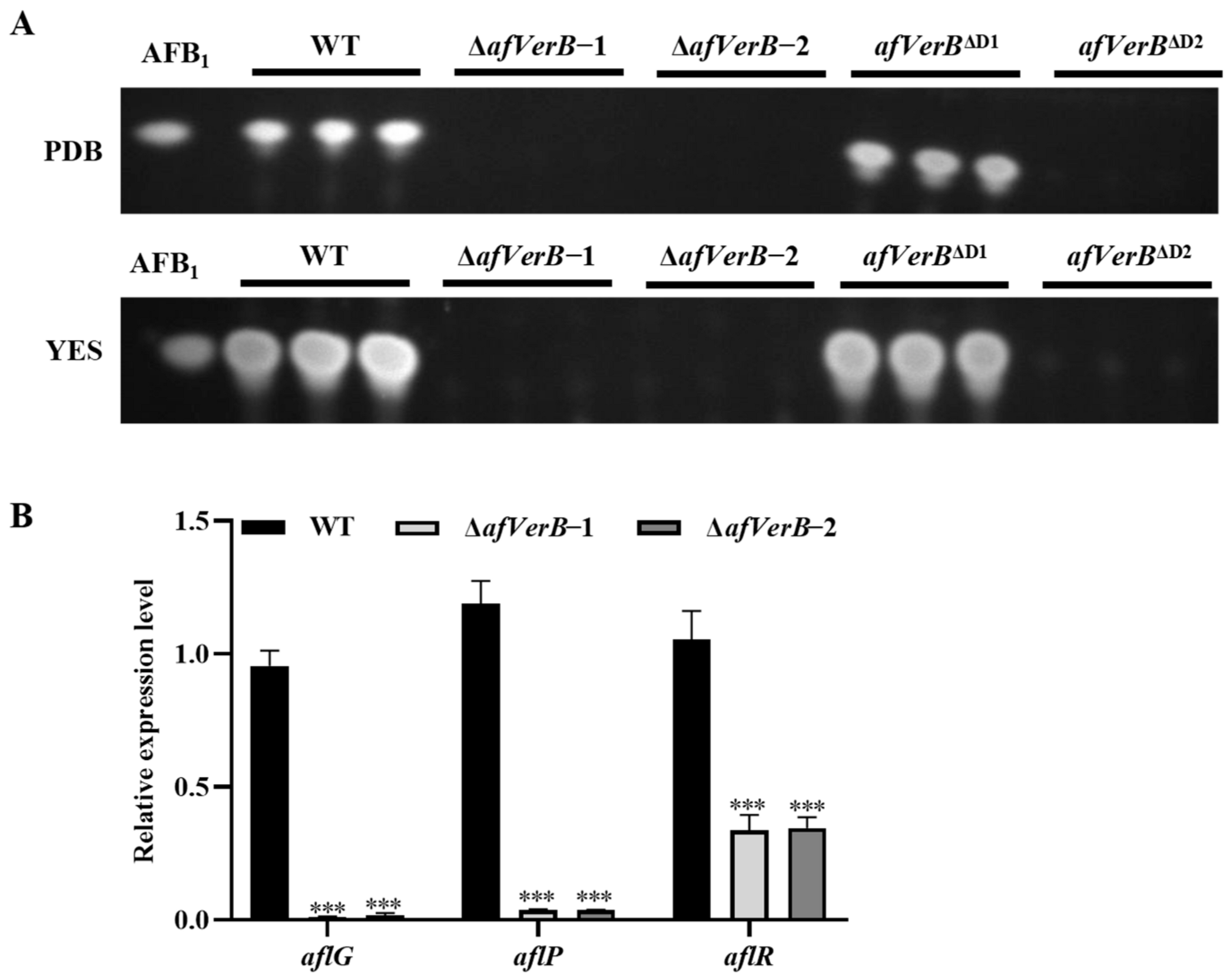
3.4. AfVerB Affects AFB1 Biosynthesis Through Its Second CYP Domain
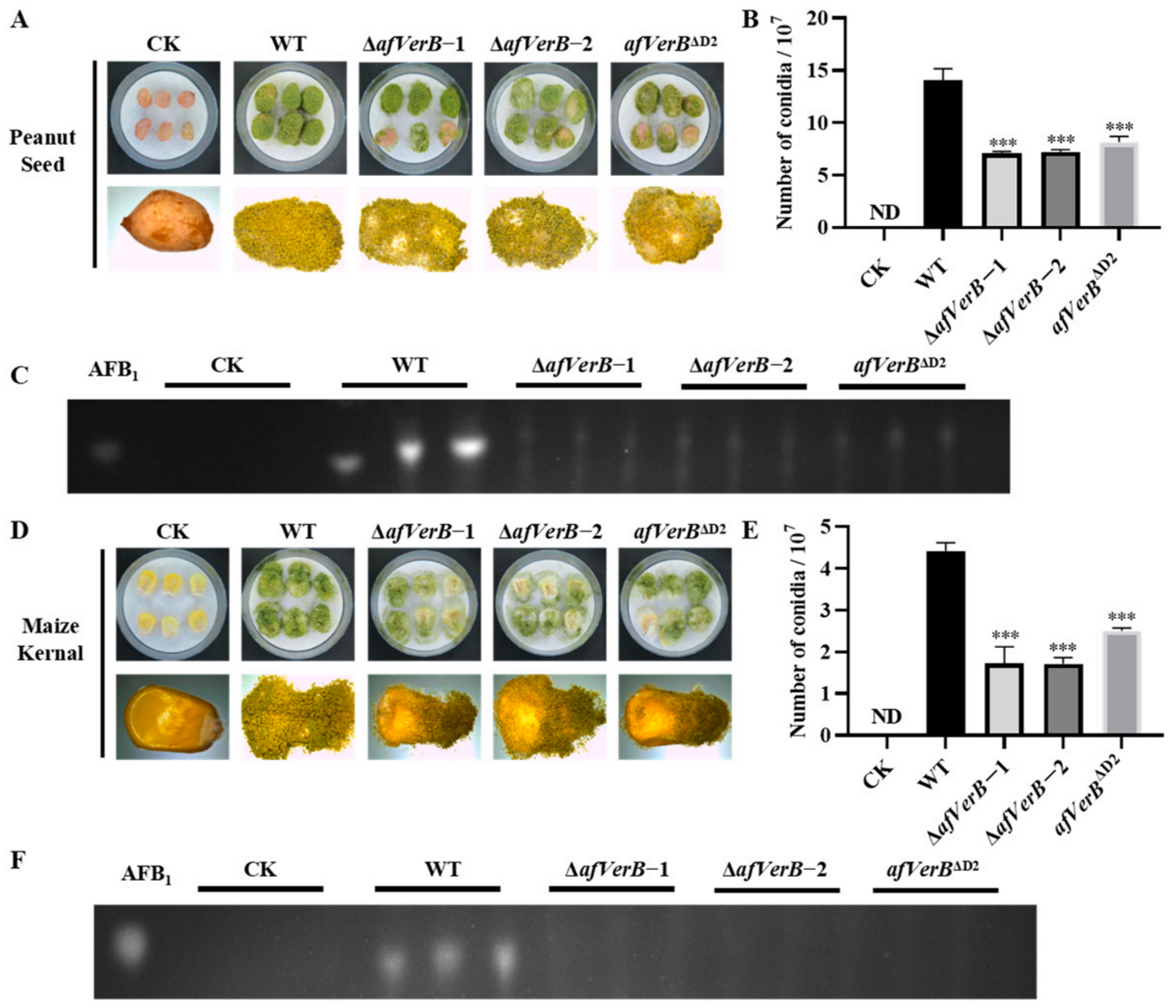
3.5. AfVerB Is Deeply Involved in the Colonization of Crop Grains by A. flavus
3.6. AfVerB Is Involved in Fungal Virulence to G. Mellonella
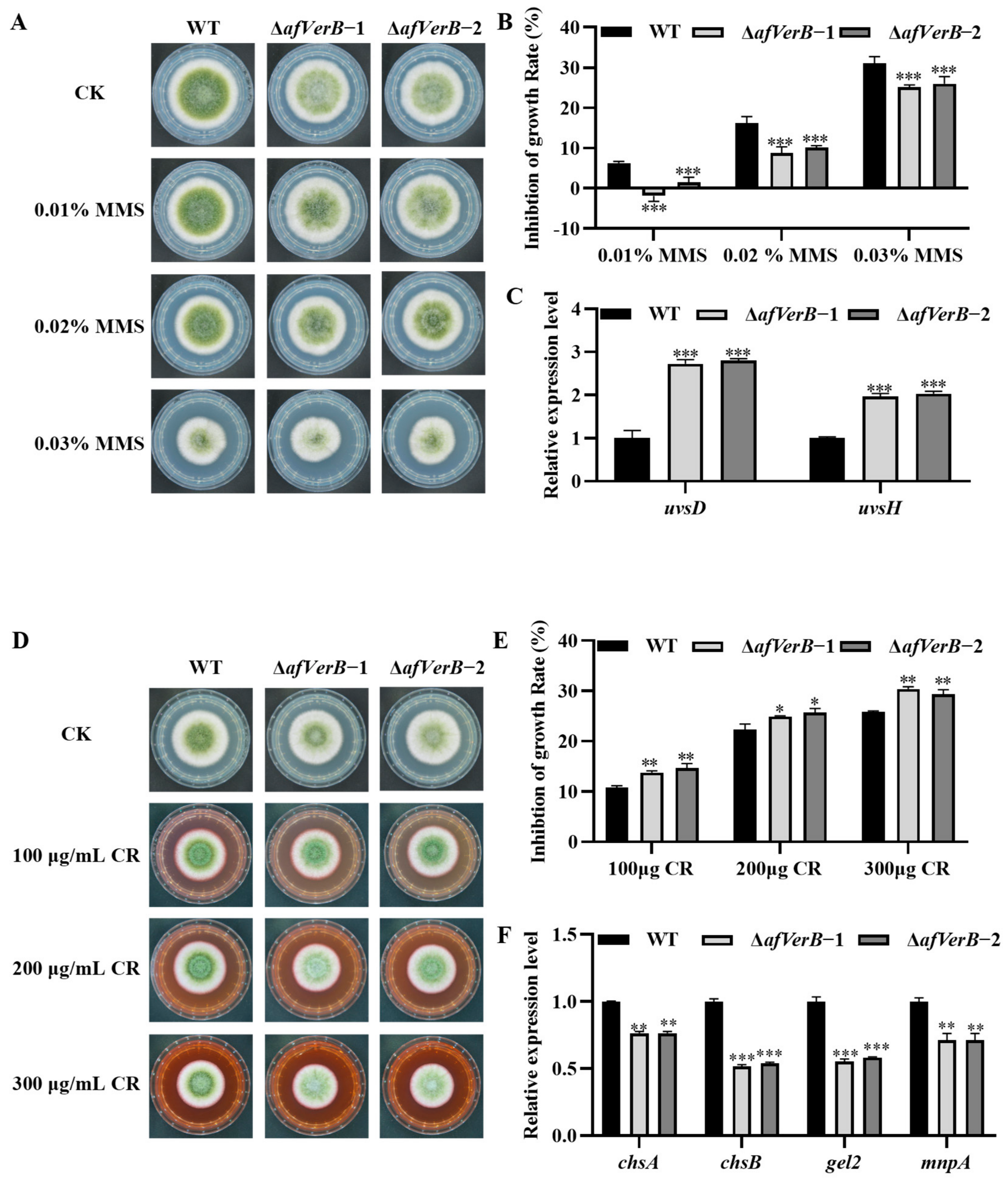
3.7. AfVerB Plays a Critical Role in the Response of A. flavus to Stresses
3.8. AfVerB Is Involved in the Response of A. flavus to Antifungal Drugs
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Paul, R.A.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. Invasive Aspergillosis by Aspergillus flavus: Epidemiology, Diagnosis, Antifungal Resistance, and Management. J. Fungi 2019, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Beltran, A.; Moral, J.; Picot, A.; Puckett, R.D.; Cotty, P.J.; Michailides, T.J. Atoxigenic Aspergillus flavus Isolates Endemic to Almond, Fig, and Pistachio Orchards in California with Potential to Reduce Aflatoxin Contamination in these Crops. Plant Dis. 2019, 103, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Williams, W.P. Toward elucidation of genetic and functional genetic mechanisms in corn host resistance to Aspergillus flavus infection and aflatoxin contamination. Front. Microbiol. 2014, 5, 364. [Google Scholar] [CrossRef]
- Cary, J.W.; Ehrlich, K.C.; Bland, J.M.; Montalbano, B.G. The aflatoxin biosynthesis cluster gene, aflX, encodes an oxidoreductase involved in conversion of versicolorin A to demethylsterigmatocystin. Appl. Environ. Microbiol. 2006, 72, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, D.; Cary, J.W.; Ehrlich, K.; Yu, J.; Cleveland, T.E. Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia 2006, 162, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.K. Lack of interaction between AFLR and AFLJ contributes to nonaflatoxigenicity of Aspergillus sojae. J. Biotechnol. 2004, 107, 245–253. [Google Scholar] [CrossRef]
- Wang, P.; Xu, J.; Chang, P.K.; Liu, Z.; Kong, Q. New Insights of Transcriptional Regulator AflR in Aspergillus flavus Physiology. Microbiol. Spectr. 2022, 10, e0079121. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Chang, P.K.; Li, C.; Hu, Z.; Zheng, M.; Sun, Q.; Shan, S. Identification of AflR Binding Sites in the Genome of Aspergillus flavus by ChIP-Seq. J. Fungi 2020, 6, 52. [Google Scholar] [CrossRef]
- Abbas, A.; Prajapati, R.K.; Aalto-Setala, E.; Baykov, A.A.; Malinen, A.M. Aflatoxin biosynthesis regulators AflR and AflS: DNA binding affinity, stoichiometry, and kinetics. Biochem. J. 2024, 481, 805–821. [Google Scholar] [CrossRef]
- Jia, K.; Yan, L.; Jia, Y.; Xu, S.; Yan, Z.; Wang, S. aflN Is Involved in the Biosynthesis of Aflatoxin and Conidiation in Aspergillus flavus. Toxins 2021, 13, 831. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yue, Y.; Ren, S.; Yang, M.; Zhang, Y.; Cao, X.; Wang, Y.; Zhang, J.; Ge, F.; Wang, S. Lysine acetylation contributes to development, aflatoxin biosynthesis and pathogenicity in Aspergillus flavus. Environ. Microbiol. 2019, 21, 4792–4807. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, D.; Lin, H.; Ma, D.; Fu, W.; Yao, Y.; Pan, X.; Wang, S.; Zhuang, Z. Role of RNA Modifications, Especially m6A, in Aflatoxin Biosynthesis of Aspergillus flavus. J. Agric. Food Chem. 2024, 72, 726–741. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Chang, P.K.; Yu, J.; Cotty, P.J. Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 2004, 70, 6518–6524. [Google Scholar] [CrossRef] [PubMed]
- Yabe, K.; Nakamura, Y.; Nakajima, H.; Ando, Y.; Hamasaki, T. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 1991, 57, 1340–1345. [Google Scholar] [CrossRef]
- Liang, L.; Wang, X.; Lan, H.; Wei, S.; Lei, Y.; Zhang, S.; Zhai, H.; Hu, Y.; Lv, Y. Comprehensive analysis of aflatoxin B(1) biosynthesis in Aspergillus flavus via transcriptome-wide m(6)A methylome response to cycloleucine. J. Hazard. Mater. 2024, 461, 132677. [Google Scholar] [CrossRef]
- Liang, L.; Wang, X.; Wei, S.; Lei, Y.; Zhang, S.; Zhai, H.; Hu, Y.; Lv, Y. m(6)A methyltransferase AflIme4 orchestrates mycelial growth, development and aflatoxin B(1) biosynthesis in Aspergillus flavus. Microbiol. Res. 2024, 283, 127710. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Markoglou, A.N.; Doukas, E.G.; Ziogas, B.N. Phenylpyrrole-resistance and aflatoxin production in Aspergillus parasiticus Speare. Int. J. Food Microbiol. 2008, 127, 268–275. [Google Scholar] [CrossRef]
- Markoglou, A.N.; Doukas, E.G.; Malandrakis, A.A. Effect of anilinopyrimidine resistance on aflatoxin production and fitness parameters in Aspergillus parasiticus Speare. Int. J. Food Microbiol. 2011, 146, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Doukas, E.G.; Markoglou, A.N.; Vontas, J.G.; Ziogas, B.N. Effect of DMI-resistance mechanisms on cross-resistance patterns, fitness parameters and aflatoxin production in Aspergillus parasiticus Speare. Fungal Genet. Biol. 2012, 49, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Pan, X.; Zhang, M.; Liu, Y.; Huang, C.; Li, Y.; Hao, L.; Wang, S. Set2 family regulates mycotoxin metabolism and virulence via H3K36 methylation in pathogenic fungus Aspergillus flavus. Virulence 2022, 13, 1358–1378. [Google Scholar] [CrossRef]
- Wu, D.; Yang, C.; Yao, Y.; Ma, D.; Lin, H.; Hao, L.; Xin, W.; Ye, K.; Sun, M.; Hu, Y.; et al. SntB triggers the antioxidant pathways to regulate development and aflatoxin biosynthesis in Aspergillus flavus. eLife 2024, 13, RP94743. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, D.; Yang, S.; Fu, W.; Ma, D.; Yao, Y.; Lin, H.; Yuan, J.; Yang, Y.; Zhuang, Z. Regulation of Fungal Morphogenesis and Pathogenicity of Aspergillus flavus by Hexokinase AfHxk1 through Its Domain Hexokinase_2. J. Fungi 2023, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhang, M.; Yang, C.; Pan, X.; Wu, D.; Lin, H.; Ma, D.; Yao, Y.; Fu, W.; Chang, J.; et al. The epigenetic regulator Set9 harmonizes fungal development, secondary metabolism, and colonization capacity of Aspergillus flavus. Int. J. Food Microbiol. 2023, 403, 110298. [Google Scholar] [CrossRef]
- Pan, X.; Hao, L.; Yang, C.; Lin, H.; Wu, D.; Chen, X.; Zhang, M.; Ma, D.; Wang, Y.; Fu, W.; et al. SWD1 epigenetically chords fungal morphogenesis, aflatoxin biosynthesis, metabolism, and virulence of Aspergillus flavus. J. Hazard. Mater. 2023, 455, 131542. [Google Scholar] [CrossRef]
- Long, N.; Orasch, T.; Zhang, S.; Gao, L.; Xu, X.; Hortschansky, P.; Ye, J.; Zhang, F.; Xu, K.; Gsaller, F.; et al. The Zn2Cys6-type transcription factor LeuB cross-links regulation of leucine biosynthesis and iron acquisition in Aspergillus fumigatus. PLoS Genet. 2018, 14, e1007762. [Google Scholar] [CrossRef]
- Du, W.; Zhai, P.; Wang, T.; Bromley, M.J.; Zhang, Y.; Lu, L. The C2H2 Transcription Factor SltA Contributes to Azole Resistance by Coregulating the Expression of the Drug Target Erg11A and the Drug Efflux Pump Mdr1 in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2021, 65, e01839-20. [Google Scholar] [CrossRef]
- Choy, H.L.; Gaylord, E.A.; Doering, T.L. Ergosterol distribution controls surface structure formation and fungal pathogenicity. mBio 2023, 14, e0135323. [Google Scholar] [CrossRef]
- Perez-Cantero, A.; Lopez-Fernandez, L.; Guarro, J.; Capilla, J. Azole resistance mechanisms in Aspergillus: Update and recent advances. Int. J. Antimicrob. Agents 2020, 55, 105807. [Google Scholar] [CrossRef]
- Wang, X.; Mohammad, I.S.; Fan, L.; Zhao, Z.; Nurunnabi, M.; Sallam, M.A.; Wu, J.; Chen, Z.; Yin, L.; He, W. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharm. Sin. B 2021, 11, 2585–2604. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Jakobek, J.L.; Ramirez-Prado, J.H.; Horn, B.W. Recombination, balancing selection and adaptive evolution in the aflatoxin gene cluster of Aspergillus parasiticus. Mol. Ecol. 2007, 16, 4401–4417. [Google Scholar] [CrossRef] [PubMed]
- Schamann, A.; Soukup, S.T.; Geisen, R.; Kulling, S.; Schmidt-Heydt, M. Comparative analysis of the genomes and aflatoxin production patterns of three species within the Aspergillus section Flavi reveals an undescribed chemotype and habitat-specific genetic traits. Commun. Biol. 2024, 7, 1134. [Google Scholar] [CrossRef]
- Buitimea-Cantua, G.V.; Magana-Barajas, E.; Buitimea-Cantua, N.E.; Leija Gutierrez, H.M.; Del Refugio Rocha-Pizana, M.; Rosas-Burgos, E.C.; Hernandez-Morales, A.; Molina-Torres, J. Down-regulation of aflatoxin biosynthetic genes in Aspergillus parasiticus by Heliopsis longipes roots and affinin for reduction of aflatoxin production. J. Environ. Sci. Health B 2021, 56, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.X.; Mouhoumed, A.Z.; Tong, S.M.; Ying, S.H.; Feng, M.G. BrlA and AbaA Govern Virulence-Required Dimorphic Switch, Conidiation, and Pathogenicity in a Fungal Insect Pathogen. mSystems 2019, 4, e00140-19. [Google Scholar] [CrossRef]
- Prade, R.A.; Timberlake, W.E. The Aspergillus nidulans brlA regulatory locus consists of overlapping transcription units that are individually required for conidiophore development. EMBO J. 1993, 12, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- Mead, M.E.; Borowsky, A.T.; Joehnk, B.; Steenwyk, J.L.; Shen, X.X.; Sil, A.; Rokas, A. Recurrent Loss of abaA, a Master Regulator of Asexual Development in Filamentous Fungi, Correlates with Changes in Genomic and Morphological Traits. Genome Biol. Evol. 2020, 12, 1119–1130. [Google Scholar] [CrossRef]
- Wu, M.Y.; Mead, M.E.; Kim, S.C.; Rokas, A.; Yu, J.H. WetA bridges cellular and chemical development in Aspergillus flavus. PLoS ONE 2017, 12, e0179571. [Google Scholar] [CrossRef]
- Chang, P.K.; Cary, J.W.; Lebar, M.D. Biosynthesis of conidial and sclerotial pigments in Aspergillus species. Appl. Microbiol. Biotechnol. 2020, 104, 2277–2286. [Google Scholar] [CrossRef]
- Horn, B.W.; Gell, R.M.; Singh, R.; Sorensen, R.B.; Carbone, I. Sexual Reproduction in Aspergillus flavus Sclerotia: Acquisition of Novel Alleles from Soil Populations and Uniparental Mitochondrial Inheritance. PLoS ONE 2016, 11, e0146169. [Google Scholar] [CrossRef]
- Alves de Castro, P.; Valero, C.; Chiaratto, J.; Colabardini, A.C.; Pardeshi, L.; Pereira Silva, L.; Almeida, F.; Campos Rocha, M.; Nascimento Silva, R.; Malavazi, I.; et al. Novel Biological Functions of the NsdC Transcription Factor in Aspergillus fumigatus. mBio 2021, 12, e03102–e03120. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kong, Q.; Yao, Y.; Xu, S.; Xie, X. Fusion expression and anti-Aspergillus flavus activity of a novel inhibitory protein DN-AflR. Int. J. Food Microbiol. 2019, 290, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hadi, A.; Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. R. Soc. Interface 2012, 9, 757–767. [Google Scholar] [CrossRef]
- Carbone, I.; Ramirez-Prado, J.H.; Jakobek, J.L.; Horn, B.W. Gene duplication, modularity and adaptation in the evolution of the aflatoxin gene cluster. BMC Evol. Biol. 2007, 7, 111. [Google Scholar] [CrossRef]
- Wang-Buhler, J.L.; Lee, S.J.; Chung, W.G.; Stevens, J.F.; Tseng, H.P.; Hseu, T.H.; Hu, C.H.; Westerfield, M.; Yang, Y.H.; Miranda, C.L.; et al. CYP2K6 from zebrafish (Danio rerio): Cloning, mapping, developmental/tissue expression, and aflatoxin B1 activation by baculovirus expressed enzyme. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Ates, M.B.; Ortatatli, M. Corrigendum to ‘Phase-1 bioactivation mechanisms of aflatoxin through AhR, CAR and PXR nuclear receptors and the interactions with Nigella sativa seeds and thymoquinone in broilers’ [Ecotoxicol. Environ. Saf. 208 (2021) 11174]. Ecotoxicol. Environ. Saf. 2021, 210, 111898. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Fan, J.; Zhu, G.; Lu, L. Aspergillus fumigatus Elongator complex subunit 3 affects hyphal growth, adhesion and virulence through wobble uridine tRNA modification. PLoS Pathog. 2022, 18, e1010976. [Google Scholar] [CrossRef]
- Abarca, M.L.; Bragulat, M.R.; Castella, G.; Cabanes, F.J. Impact of some environmental factors on growth and ochratoxin A production by Aspergillus niger and Aspergillus welwitschiae. Int. J. Food Microbiol. 2019, 291, 10–16. [Google Scholar] [CrossRef]
- Raitt, D.C.; Johnson, A.L.; Erkine, A.M.; Makino, K.; Morgan, B.; Gross, D.S.; Johnston, L.H. The Skn7 response regulator of Saccharomyces cerevisiae interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress. Mol. Biol. Cell 2000, 11, 2335–2347. [Google Scholar] [CrossRef]
- Vargas-Perez, I.; Sanchez, O.; Kawasaki, L.; Georgellis, D.; Aguirre, J. Response regulators SrrA and SskA are central components of a phosphorelay system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryot. Cell 2007, 6, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Hoshi, Y.; Maeda, T.; Nakajima, T.; Abe, K. Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol. Microbiol. 2005, 56, 1246–1261. [Google Scholar] [CrossRef]
- Babudri, N.; Politi, M.G. Different action of MMS and EMS in UV-sensitive strains of Aspergillus nidulans. Mutat. Res. 1989, 217, 211–217. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, B.J.; Kang, H.S. The Aspergillus uvsH gene encodes a product homologous to yeast RAD18 and Neurospora UVS-2. Mol. Gen. Genet. 1995, 248, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, H.; Wang, J.; Wei, S.; Zhai, H.; Zhang, S.; Hu, Y. Afper1 contributes to cell development and aflatoxin biosynthesis in Aspergillus flavus. Int. J. Food Microbiol. 2022, 377, 109828. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, T.; Wang, Y.; Chen, G.; Fang, X.; Zhou, G.; Wang, J. ChsA, a Class II Chitin Synthase, Contributes to Asexual Conidiation, Mycelial Morphology, Cell Wall Integrity, and the Production of Enzymes and Organic Acids in Aspergillus niger. J. Fungi 2023, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; McIntyre, M.; Hansen, K.; Nielsen, J. Metabolic engineering of the morphology of Aspergillus oryzae by altering chitin synthesis. Appl. Environ. Microbiol. 2002, 68, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lu, Y.; Ouyang, H.; Zhou, H.; Yan, J.; Du, T.; Jin, C. N-Glycosylation of Gel1 or Gel2 is vital for cell wall beta-glucan synthesis in Aspergillus fumigatus. Glycobiology 2013, 23, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Kim, H.; Han, D.M.; Jahng, K.Y.; Chae, K.S. Expression of the mnpA gene that encodes the mannoprotein of Aspergillus nidulans is dependent on fadA and flbA as well as veA. Fungal Genet. Biol. 2003, 38, 228–236. [Google Scholar] [CrossRef]
- Godet, C.; Goudet, V.; Laurent, F.; Le Moal, G.; Gounant, V.; Frat, J.P.; Cateau, E.; Roblot, F.; Cadranel, J. Nebulised liposomal amphotericin B for Aspergillus lung diseases: Case series and literature review. Mycoses 2015, 58, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Zhang, C.; Wang, H.; Wei, X.; Chen, P.; Lu, L. Mitochondrial dysfunctions trigger the calcium signaling-dependent fungal multidrug resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Buechel, E.R.; Pinkett, H.W. Transcription factors and ABC transporters: From pleiotropic drug resistance to cellular signaling in yeast. FEBS Lett. 2020, 594, 3943–3964. [Google Scholar] [CrossRef]
- Andrade, A.C.; Van Nistelrooy, J.G.; Peery, R.B.; Skatrud, P.L.; De Waard, M.A. The role of ABC transporters from Aspergillus nidulans in protection against cytotoxic agents and in antibiotic production. Mol. Gen. Genet. 2000, 263, 966–977. [Google Scholar] [CrossRef]
- Li, Y.; Dai, M.; Lu, L.; Zhang, Y. The C(2)H(2)-Type Transcription Factor ZfpA, Coordinately with CrzA, Affects Azole Susceptibility by Regulating the Multidrug Transporter Gene atrF in Aspergillus fumigatus. Microbiol. Spectr. 2023, 11, e0032523. [Google Scholar] [CrossRef]
- Lotfali, E.; Ghajari, A.; Kordbacheh, P.; Zaini, F.; Mirhendi, H.; Mohammadi, R.; Noorbakhsh, F.; Rezaie, S. Regulation of ERG3, ERG6, and ERG11 Genes in Antifungal-Resistant isolates of Candida parapsilosis. Iran. Biomed. J. 2017, 21, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Joseph, L.; Parker, J.E.; Asadzadeh, M.; Kelly, S.L.; Meis, J.F.; Khan, Z. ERG6 and ERG2 Are Major Targets Conferring Reduced Susceptibility to Amphotericin B in Clinical Candida glabrata Isolates in Kuwait. Antimicrob. Agents Chemother. 2019, 63, e01900-18. [Google Scholar] [CrossRef]
- Schroeder, L.; Ikui, A.E. Tryptophan confers resistance to SDS-associated cell membrane stress in Saccharomyces cerevisiae. PLoS ONE 2019, 14, e0199484. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, M.J.; Yu, J.H.; Shin, K.S. Heterotrimeric G-protein signalers and RGSs in Aspergillus fumigatus. Pathogens 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, X.D.; Tian, S.G.; Zhang, C.J.; Lu, Z.Y.; Chen, Y.; Chen, F.Y.; Li, Z.W.; Su, X.T.; Han, X.L.; et al. Role of the small GTPase Rho1 in cell wall integrity, stress response, and pathogenesis of Aspergillus fumigatus. Fungal Genet. Biol. 2018, 120, 30–41. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, K.; Zhou, S.; Wu, D.; Ma, D.; Yao, Y.; Yang, C.; Sun, M.; Yang, S.; Fu, W.; Xin, W.; et al. Molecular Mechanism of Aflatoxin B1 Synthesis Related AfVerB Regulating the Development, AFB1 Biosyntheis and Virulence of Aspergillus flavus Mainly Through Its CYP Domain. J. Fungi 2025, 11, 293. https://doi.org/10.3390/jof11040293
Ye K, Zhou S, Wu D, Ma D, Yao Y, Yang C, Sun M, Yang S, Fu W, Xin W, et al. Molecular Mechanism of Aflatoxin B1 Synthesis Related AfVerB Regulating the Development, AFB1 Biosyntheis and Virulence of Aspergillus flavus Mainly Through Its CYP Domain. Journal of Fungi. 2025; 11(4):293. https://doi.org/10.3390/jof11040293
Chicago/Turabian StyleYe, Kangfu, Song Zhou, Dandan Wu, Dongmei Ma, Yanfang Yao, Chi Yang, Minghui Sun, Sile Yang, Wangzhuo Fu, Wenwen Xin, and et al. 2025. "Molecular Mechanism of Aflatoxin B1 Synthesis Related AfVerB Regulating the Development, AFB1 Biosyntheis and Virulence of Aspergillus flavus Mainly Through Its CYP Domain" Journal of Fungi 11, no. 4: 293. https://doi.org/10.3390/jof11040293
APA StyleYe, K., Zhou, S., Wu, D., Ma, D., Yao, Y., Yang, C., Sun, M., Yang, S., Fu, W., Xin, W., Yuan, J., Zhuang, Z., & Yang, Y. (2025). Molecular Mechanism of Aflatoxin B1 Synthesis Related AfVerB Regulating the Development, AFB1 Biosyntheis and Virulence of Aspergillus flavus Mainly Through Its CYP Domain. Journal of Fungi, 11(4), 293. https://doi.org/10.3390/jof11040293





