Tangeretin Suppresses Fumonisin Production by Modulating an NmrA- and HSCARG-like Protein in Fusarium verticillioides
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening of Medicinal Compounds for Inhibition Against F. verticillioides
2.2. Antifungal and Antimycotoxigenic Properties of Tangeretin
2.2.1. In Vitro Inhibitory Effects of Tangeretin
2.2.2. Protective Efficacy of Tangeretin on Corn
2.2.3. Effect of Tangeretin on Conidial Germination of F. verticillioides
2.3. Biochemical Analysis
2.4. Differential Transcriptomic Analyses
2.5. Function Analysis of Potential Target Genes
2.6. Statistical Analysis
3. Results
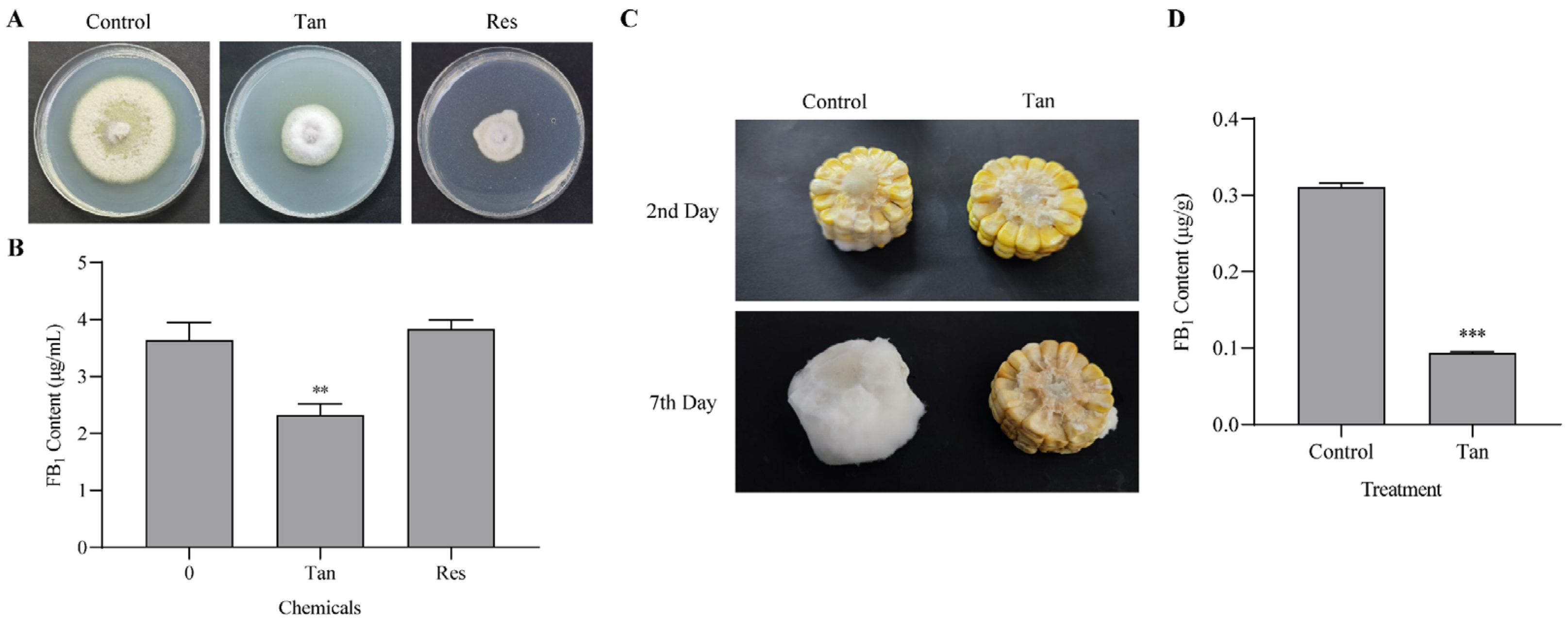
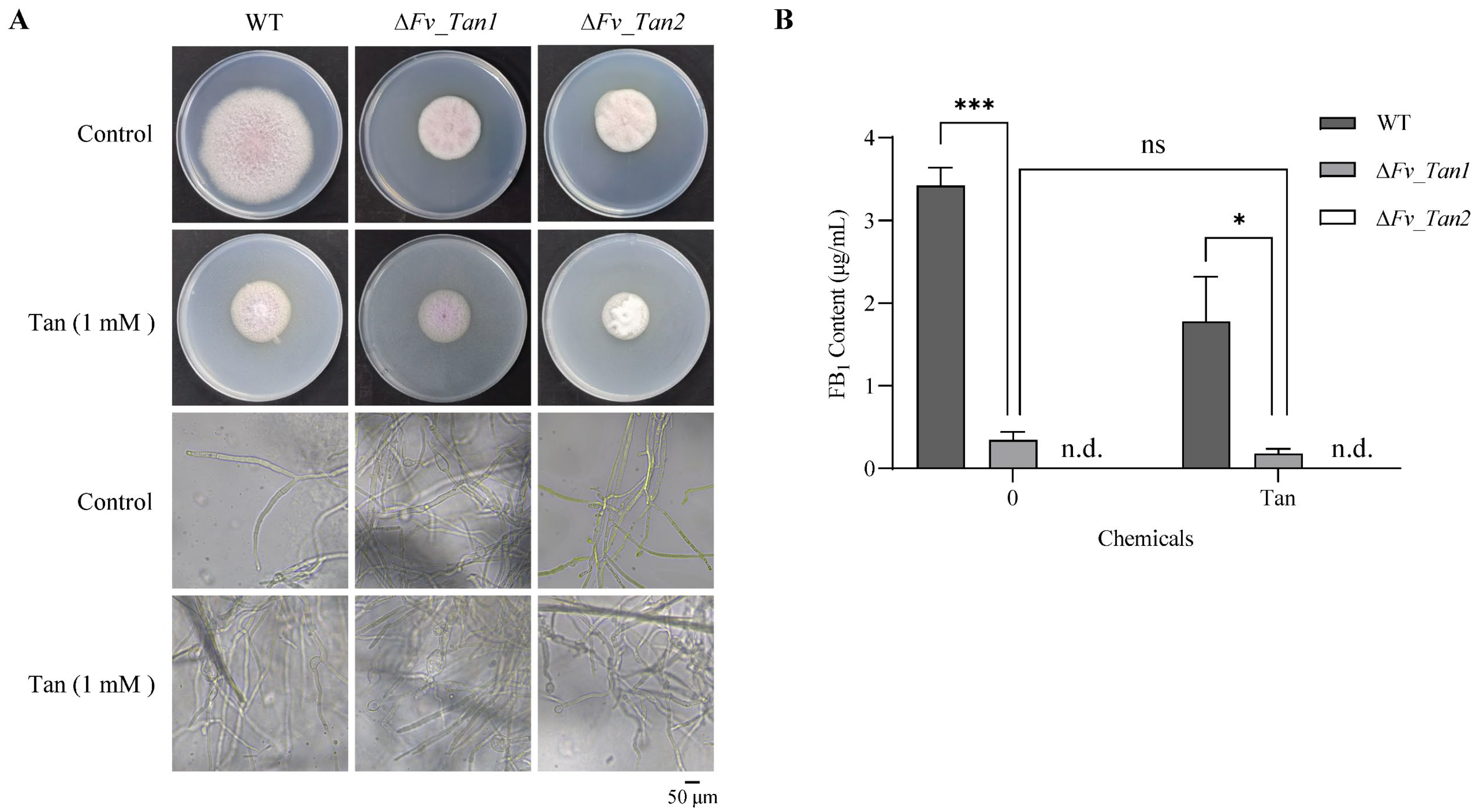
3.1. Suppression of Fungal Growth and Fumonisin Production by Tangeretin
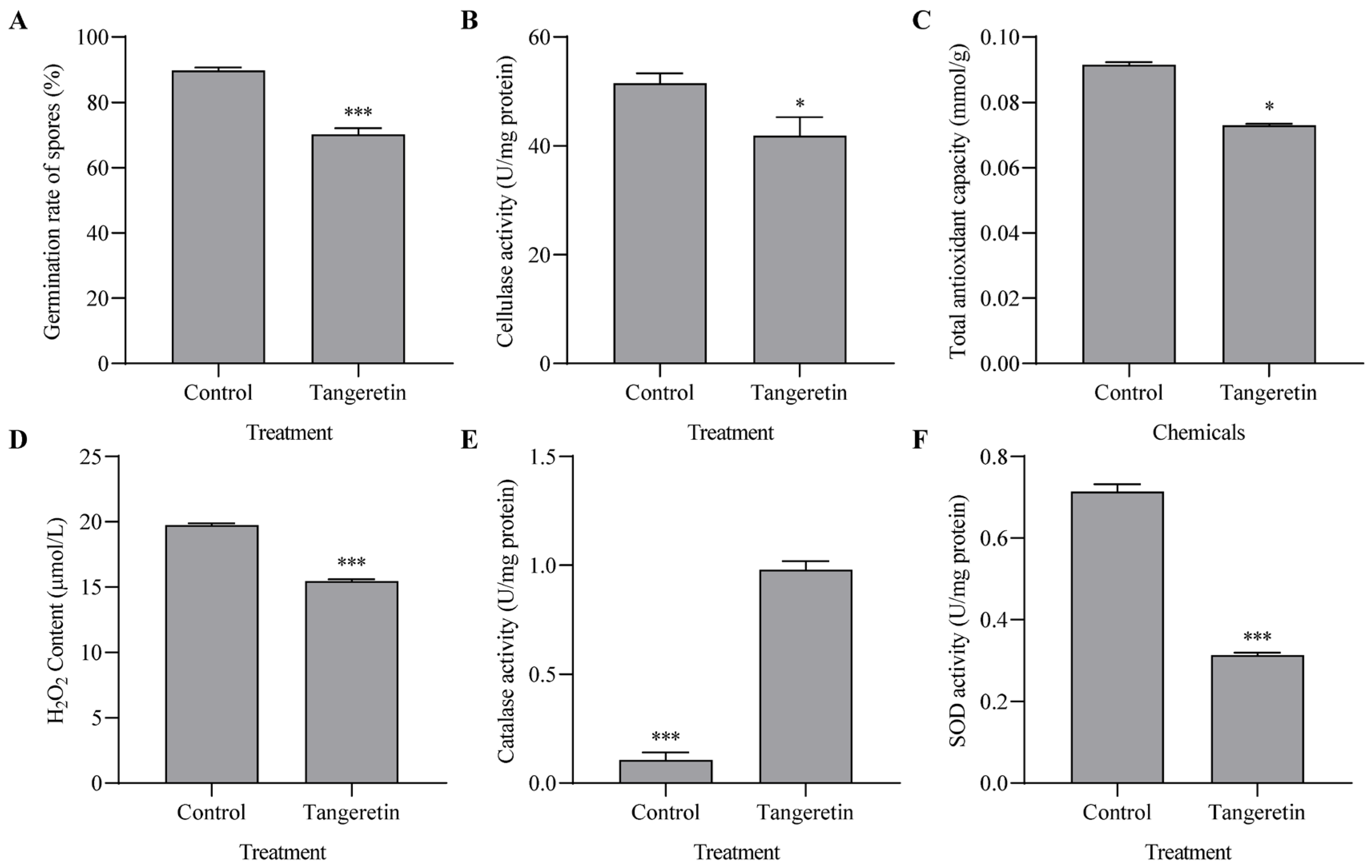
3.2. Interference of Tangeretin in Hyphal Extension, Conidial Development and Redox Homeostasis
3.3. Transcriptomic Analysis of F. verticillioides Reveals Tangeretin Impacts
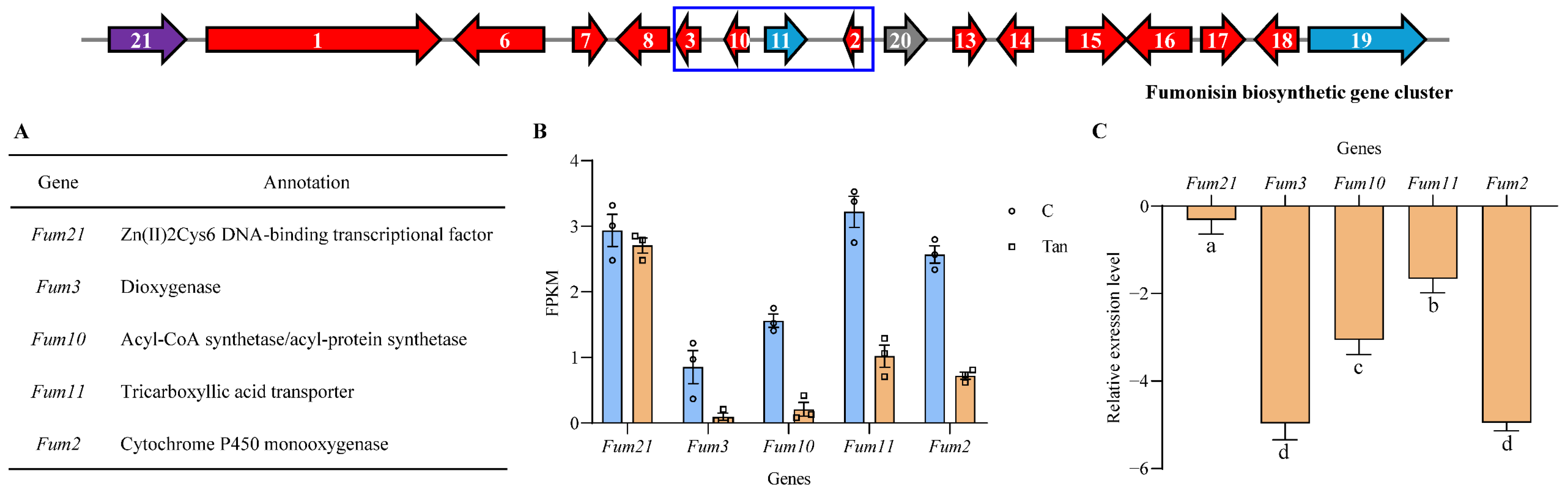
3.4. Effects of Tangeretin on Fumonisin Biosynthetic Gene Cluster
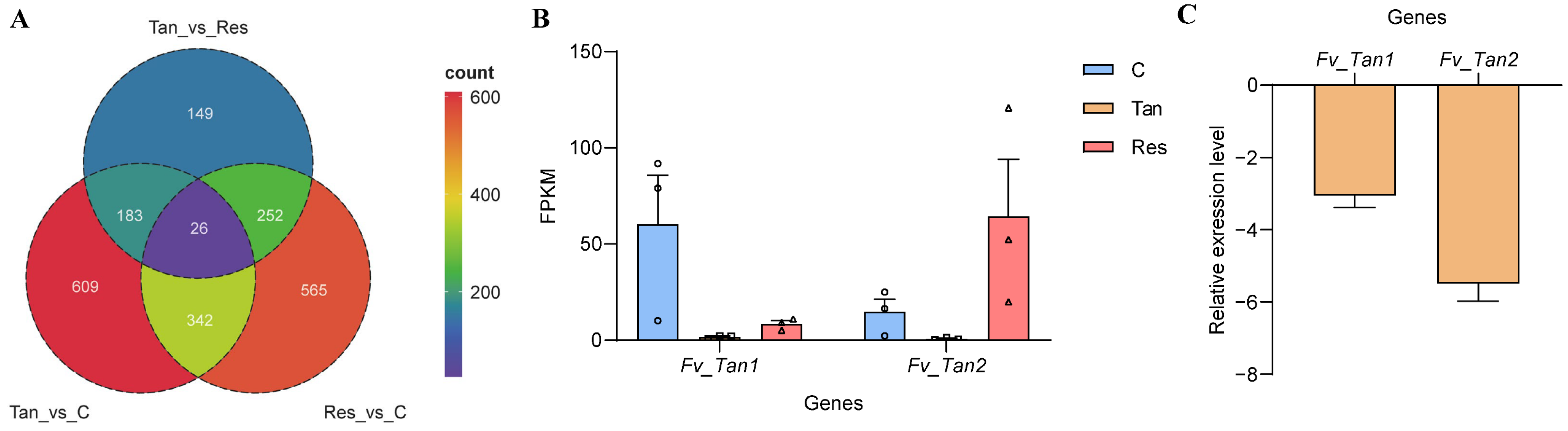
3.5. Identification of Tangeretin’s Potential Target Genes in F. verticillioides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Munkvold, G.P.; Proctor, R.H.; Moretti, A. Mycotoxin production in Fusarium according to contemporary species concepts. Annu. Rev. Phytopathol. 2021, 59, 373–402. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; Logrieco, A.F.; Moretti, A.; Susca, A. A loop-mediated isothermal amplification (lamp) assay for rapid detection of fumonisin producing Aspergillus species. Food Microbiol. 2020, 90, 103469. [Google Scholar] [CrossRef]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef]
- Chen, X.; Abdallah, M.F.; Landschoot, S.; Audenaert, K.; De Saeger, S.; Chen, X.; Rajkovic, A. Aspergillus flavus and Fusarium verticillioides and their main mycotoxins: Global distribution and scenarios of interactions in maize. Toxins 2023, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; WHO: Lyon, France, 2002; ISBN 978-92-832-1282-9.
- Qu, L.; Wang, L.; Ji, H.; Fang, Y.; Lei, P.; Zhang, X.; Jin, L.; Sun, D.; Dong, H. Toxic mechanism and biological detoxification of fumonisins. Toxins 2022, 14, 182. [Google Scholar] [CrossRef]
- Xie, L.; Wu, Y.; Wang, Y.; Jiang, Y.; Yang, B.; Duan, X.; Li, T. Fumonisin B1 induced aggressiveness and infection mechanism of Fusarium proliferatum on banana fruit. Environ. Pollut. 2021, 288, 117793. [Google Scholar] [CrossRef]
- Deepa, N.; Achar, P.N.; Sreenivasa, M.Y. Current perspectives of biocontrol agents for management of Fusarium verticillioides and its fumonisin in cereals-A review. J. Fungi 2021, 7, 776. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A review. Cogent Food Agr. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernández, J.; López-Ibáñez, S.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Plant phytochemicals in food preservation: Antifungal bioactivity: A Review. J. Food Prot. 2020, 83, 163–171. [Google Scholar] [CrossRef]
- Li, T.; Su, X.; Qu, H.; Duan, X.; Jiang, Y. Biosynthesis, regulation, and biological significance of fumonisins in fungi: Current status and prospects. Crit. Rev. Microbiol. 2021, 48, 450–462. [Google Scholar] [CrossRef]
- Brown, D.W.; Butchko, R.A.E.; Busman, M.; Proctor, R.H. The Fusarium verticillioides Fum gene cluster encodes a Zn(II)2Cys6 protein that affects Fum gene expression and fumonisin production. Eukaryotic Cell 2007, 6, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Brown, D.W.; Kleigrewe, K.; Bok, J.W.; Keller, N.P.; Humpf, H.-U.; Tudzynski, B. FfVel1 and FfLae1, components of a velvet-like complex in Fusarium fujikuroi, affect differentiation, secondary metabolism and virulence. Mol. Microbiol. 2010, 77, 972–994. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Pakozdi, K.; Murvai, K.; Pusztahelyi, T.; Kecskemeti, A.; Gaspar, A.; Logrieco, A.F.; Emri, T.; Ádám, A.L.; Leiter, E.; et al. FvatfA regulates growth, stress tolerance as well as mycotoxin and pigment productions in Fusarium verticillioides. Appl. Microbiol. Biot. 2020, 104, 7879–7899. [Google Scholar] [CrossRef]
- Yu, W.; Lin, M.; Yan, H.; Wang, J.; Zhang, S.; Lu, G.; Wang, Z.; Shim, W.-B. The peroxisomal matrix shuttling receptor Pex5 plays a role in FB1 Production and virulence in Fusarium verticillioides. J. Integr. Agr. 2022, 21, 2957–2972. [Google Scholar] [CrossRef]
- Yu, W.; Lin, M.; Peng, M.; Yan, H.; Wang, J.; Zhou, J.; Lu, G.; Wang, Z.; Shim, W.B. Fusarium verticillioides FvPex8 is a key component of the peroxisomal docking/translocation module that serves important roles in fumonisin biosynthesis but not in virulence. Mol. Plant-Microbe Interact. 2021, 34, 803–814. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Tao, H.; Dang, X.; Wang, Y.; Chen, M.; Zhai, Z.; Yu, W.; Xu, L.; Shim, W.-B.; et al. FvBck1, a component of cell wall integrity MAP kinase pathway, is required for virulence and oxidative stress response in sugarcane Pokkah Boeng pathogen. Front. Microbiol. 2015, 6, 1096. [Google Scholar] [CrossRef]
- Kohut, G.; Ádám, A.L.; Fazekas, B.; Hornok, L. N-starvation stress induced FUM gene expression and fumonisin production is mediated via the HOG-type MAPK pathway in Fusarium proliferatum. Int. J. Food Microbiol. 2009, 130, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Glenn, A.E.; Gu, X.; Mitchell, T.R.; Satterlee, T.; Duke, M.V.; Scheffler, B.E.; Gold, S.E. Pyrrocidine, a molecular off switch for fumonisin biosynthesis. PLoS Pathog. 2020, 16, 1–28. [Google Scholar] [CrossRef]
- Yang, K.; Geng, Q.; Luo, Y.; Xie, R.; Sun, T.; Wang, Z.; Qin, L.; Zhao, W.; Liu, M.; Li, Y.; et al. Dysfunction of FadA-cAMP signalling decreases Aspergillus flavus resistance to antimicrobial natural preservative perillaldehyde and AFB1 biosynthesis. Environ. Microbiol. 2022, 24, 11590–11607. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Xu, J.-R. The cAMP signaling pathway in Fusarium verticillioides is important for conidiation, plant infection, and stress responses but not fumonisin production. Mol. Plant-Microbe Interact. 2010, 23, 522–533. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Ghasemipour Afshar, E. Tangeretin: A mechanistic review of its pharmacological and therapeutic effects. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190191. [Google Scholar] [CrossRef]
- Tang, G.; Xia, H.; Liang, J.; Ma, Z.; Liu, W. Spermidine is critical for growth, development, environmental adaptation, and virulence in Fusarium graminearum. Front. Microbiol. 2021, 12, 765398. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Rocha, L.O.; Fontes, L.C.; Carnielli, L.; Reis, T.A.; Corrêa, B. Mycotoxin analysis of industrial beers from Brazil: The influence of fumonisin B1 and deoxynivalenol in beer quality. Food Chem. 2017, 218, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The genome sequence archive family: Toward explosive data growth and diverse data types. Genom. Proteom. Bioinf. 2021, 19, 578–583. [Google Scholar] [CrossRef]
- CNCB-NGDC Members and Partners; Xue, Y.; Bao, Y.; Zhang, Z.; Zhao, W.; Xiao, J.; He, S.; Zhang, G.; Li, Y.; Zhao, G.; et al. Database resources of the national genomics data center, china national center for bioinformation in 2022. Nucleic Acids Res. 2022, 50, D27–D38. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-Seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Pertea, M. GFF utilities: GffRead and GffCompare. F1000Reseach 2020, 9, 304. [Google Scholar] [CrossRef]
- Frazee, A.C.; Pertea, G.; Jaffe, A.E.; Langmead, B.; Salzberg, S.L.; Leek, J.T. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat. Biotechnol. 2015, 33, 243–246. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Möller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, L.; Chen, H.; Li, M.; Zhu, X.; Gao, Q.; Wang, D.; Zhang, Y. A polyketide synthase encoded by the gene An15g07920 is involved in the biosynthesis of ochratoxin A in Aspergillus niger. J. Agric. Food Chem. 2016, 64, 9680–9688. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Du, L.; Zhu, X.; Gerber, R.; Huffman, J.; Lou, L.; Jorgenson, J.; Yu, F.; Zaleta-Rivera, K.; Wang, Q. Biosynthesis of sphinganine-analog mycotoxins. J. Ind. Microbiol. Biotechnol. 2008, 35, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bojja, R.S.; Du, L. Fum3p, a 2-ketoglutarate-dependent dioxygenase required for c-5 hydroxylation of fumonisins in Fusarium verticillioides. Appl. Environ. Microbiol. 2004, 70, 1931–1934. [Google Scholar] [CrossRef]
- Proctor, R.H.; Plattner, R.D.; Desjardins, A.E.; Busman, M.; Butchko, R.A.E. Fumonisin production in the maize pathogen Fusarium verticillioides: Genetic basis of naturally occurring chemical variation. J. Agric. Food Chem. 2006, 54, 2424–2430. [Google Scholar] [CrossRef]
- Butchko, R.A.E.; Plattner, R.D.; Proctor, R.H. Deletion analysis of FUM genes involved in tricarballylic ester formation during fumonisin biosynthesis. J. Agric. Food Chem. 2006, 54, 9398–9404. [Google Scholar] [CrossRef]
- Gao, Z.; Luo, K.; Zhu, Q.; Peng, J.; Liu, C.; Wang, X.; Li, S.; Zhang, H. The natural occurrence, toxicity mechanisms and management strategies of Fumonisin B1: A review. Environ. Pollut. 2023, 320, 121065. [Google Scholar] [CrossRef]
- Müller, S.; Dekant, W.; Mally, A. Fumonisin B1 and the kidney: Modes of action for renal tumor formation by fumonisin B1 in rodents. Food Chem. Toxicol. 2012, 50, 3833–3846. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Wu, X.; Li, M.-M.; Li, G.-Q.; Yang, Y.-T.; Luo, H.-J.; Huang, W.-H.; Chung, H.Y.; Ye, W.-C.; Wang, G.-C.; et al. Antiviral activity of polymethoxylated flavones from “Guangchenpi”, the edible and medicinal pericarps of Citrus reticulata ‘Chachi’. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- del Río, J.A.; Gómez, P.; Baidez, A.G.; Arcas, M.C.; Botía, J.M.; Ortuño, A. Changes in the levels of polymethoxyflavones and flavanones as part of the defense mechanism of Citrus sinensis (Cv. Valencia Late) fruits against Phytophthora citrophthora. J. Agric. Food Chem. 2004, 52, 1913–1917. [Google Scholar] [CrossRef]
- Liang, M.; Ye, H.; Shen, Q.; Jiang, X.; Cui, G.; Gu, W.; Zhang, L.; Naqvi, N.I.; Deng, Y.Z. Tangeretin inhibits fungal ferroptosis to suppress rice blast. JIPB 2021, 63, 2136–2149. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Ullah, S.; Abdi, G.; Shah, G.M.; Zaman, W.; Ayaz, A. Agriculture and environmental management through nanotechnology: Eco-friendly nanomaterial synthesis for soil-plant systems, food safety, and sustainability. Sci. Total Environ. 2024, 926, 171862. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewska, M.; Opaliński, Ł.; Veenhuis, M.; Van Der Klei, I.J. The significance of peroxisomes in secondary metabolite biosynthesis in filamentous fungi. Biotechnol. Lett. 2011, 33, 1921–1931. [Google Scholar] [CrossRef]
- Falter, C.; Reumann, S. The essential role of fungal peroxisomes in plant infection. Mol. Plant Pathol. 2022, 23, 781–794. [Google Scholar] [CrossRef]
- Purdue, P.E.; Lazarow, P.B. Peroxisome biogenesis. Annu. Rev. Cell Dev. Biol. 2001, 17, 701–752. [Google Scholar] [CrossRef]
- Wang, L.; Lai, Y.; Chen, J.; Cao, X.; Zheng, W.; Dong, L.; Zheng, Y.; Li, F.; Wei, G.; Wang, S. The ASH1-PEX16 regulatory pathway controls peroxisome biogenesis for appressorium-mediated insect infection by a fungal pathogen. Proc. Natl. Acad. Sci. USA 2023, 120, e2217145120. [Google Scholar] [CrossRef]
- Han, X.; Qiu, M.; Wang, B.; Yin, W.-B.; Nie, X.; Qin, Q.; Ren, S.; Yang, K.; Zhang, F.; Zhuang, Z.; et al. Functional analysis of the nitrogen metabolite repression regulator gene nmrA in Aspergillus flavus. Front. Microbiol. 2016, 7, 1794. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Geng, Z.; Zhang, Y.; Xue, W.; Ma, L.; Yang, J.; Jin, Y.; Wang, S.; Zhuo, S.; Zhang, Y.; et al. Genome-Wide Characterization of NmrA-like proteins and the regulatory function of soybean GmNmrA6 in response to salt and oxidative stresses. Environ. Exp. Bot. 2023, 213, 105447. [Google Scholar] [CrossRef]
- Jorge, J.M.P.; Martins, C.; Domingos, P.; Martins, T.M.; Hartmann, D.O.; Goldman, G.H.; Pereira, C.S. NmrB (AN9181) expression is activated under oxidative stress conditions acting as a metabolic repressor of Aspergillus nidulans. Front. Microbiol. 2024, 15, 1373469. [Google Scholar] [CrossRef]
- Zang, W.; Zheng, X. Structure and functions of cellular redox sensor HSCARG/NMRAL1, a linkage among redox status, innate immunity, DNA damage response, and cancer. Free Radic. Biol. Med. 2020, 160, 768–774. [Google Scholar] [CrossRef]
- Yaakoub, H.; Mina, S.; Calenda, A.; Bouchara, J.-P.; Papon, N. Oxidative stress response pathways in fungi. Cell. Mol. Life Sci. 2022, 79, 333. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhai, W.; Jiang, D.; Jiang, N.; Yan, J.; Jiang, H.; Wang, M. Tangeretin Suppresses Fumonisin Production by Modulating an NmrA- and HSCARG-like Protein in Fusarium verticillioides. J. Fungi 2025, 11, 313. https://doi.org/10.3390/jof11040313
Wang L, Zhai W, Jiang D, Jiang N, Yan J, Jiang H, Wang M. Tangeretin Suppresses Fumonisin Production by Modulating an NmrA- and HSCARG-like Protein in Fusarium verticillioides. Journal of Fungi. 2025; 11(4):313. https://doi.org/10.3390/jof11040313
Chicago/Turabian StyleWang, Liuqing, Wenlei Zhai, Dongmei Jiang, Nan Jiang, Jiaqi Yan, Haoyun Jiang, and Meng Wang. 2025. "Tangeretin Suppresses Fumonisin Production by Modulating an NmrA- and HSCARG-like Protein in Fusarium verticillioides" Journal of Fungi 11, no. 4: 313. https://doi.org/10.3390/jof11040313
APA StyleWang, L., Zhai, W., Jiang, D., Jiang, N., Yan, J., Jiang, H., & Wang, M. (2025). Tangeretin Suppresses Fumonisin Production by Modulating an NmrA- and HSCARG-like Protein in Fusarium verticillioides. Journal of Fungi, 11(4), 313. https://doi.org/10.3390/jof11040313




