Activity of Amphotericin B and Anidulafungin Combined with Rifampicin, Clarithromycin, Ethylenediaminetetraacetic Acid, N-Acetylcysteine, and Farnesol against Candida tropicalis Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Concentrations Assayed
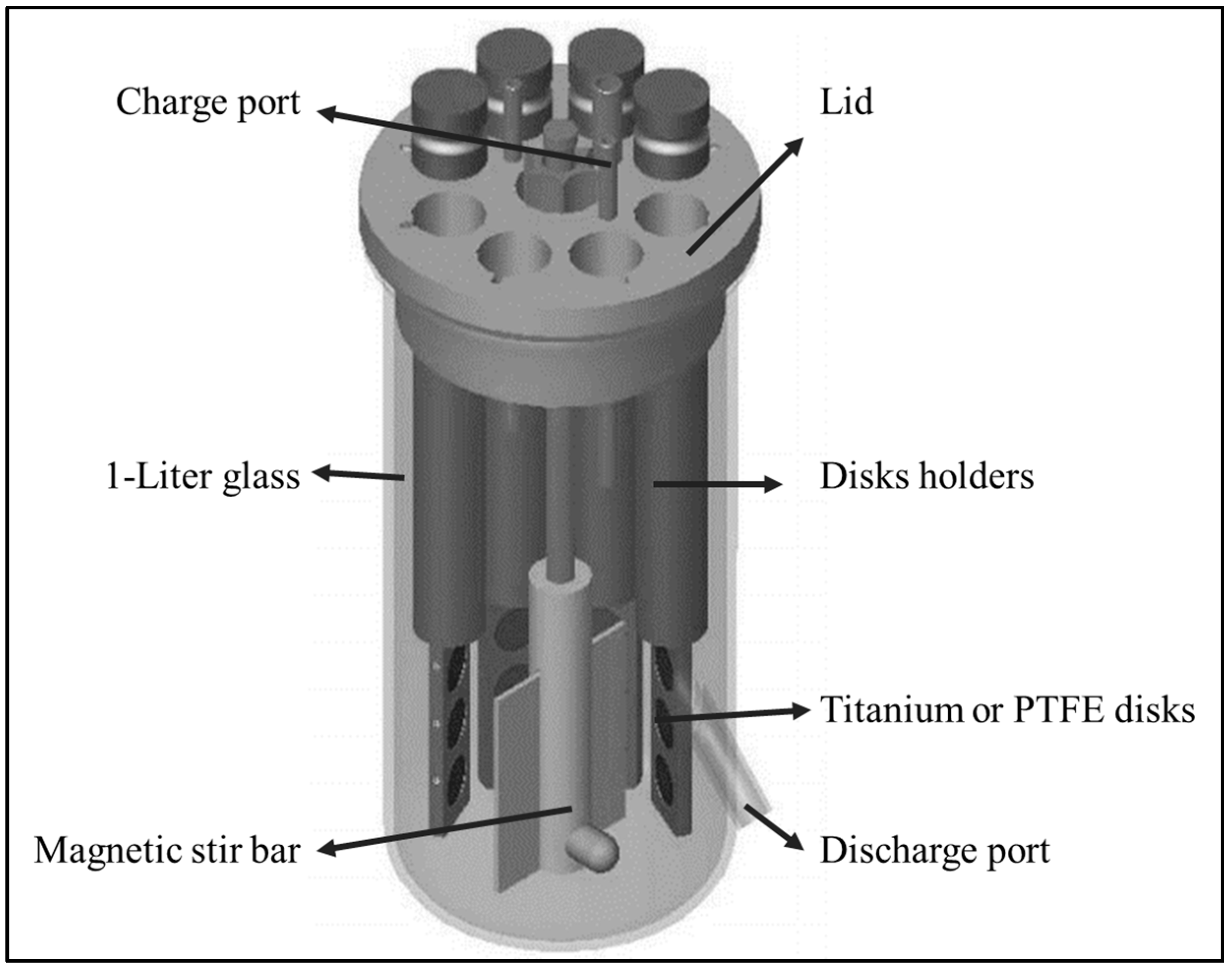
2.2. CBR and Biomaterials
2.3. Isolate Selection and Characterization
2.4. Biofilm Growth Kinetics
2.5. Killing Kinetics of Antifungals plus Anti-Biofilm Compounds
2.6. Data Analysis
3. Results
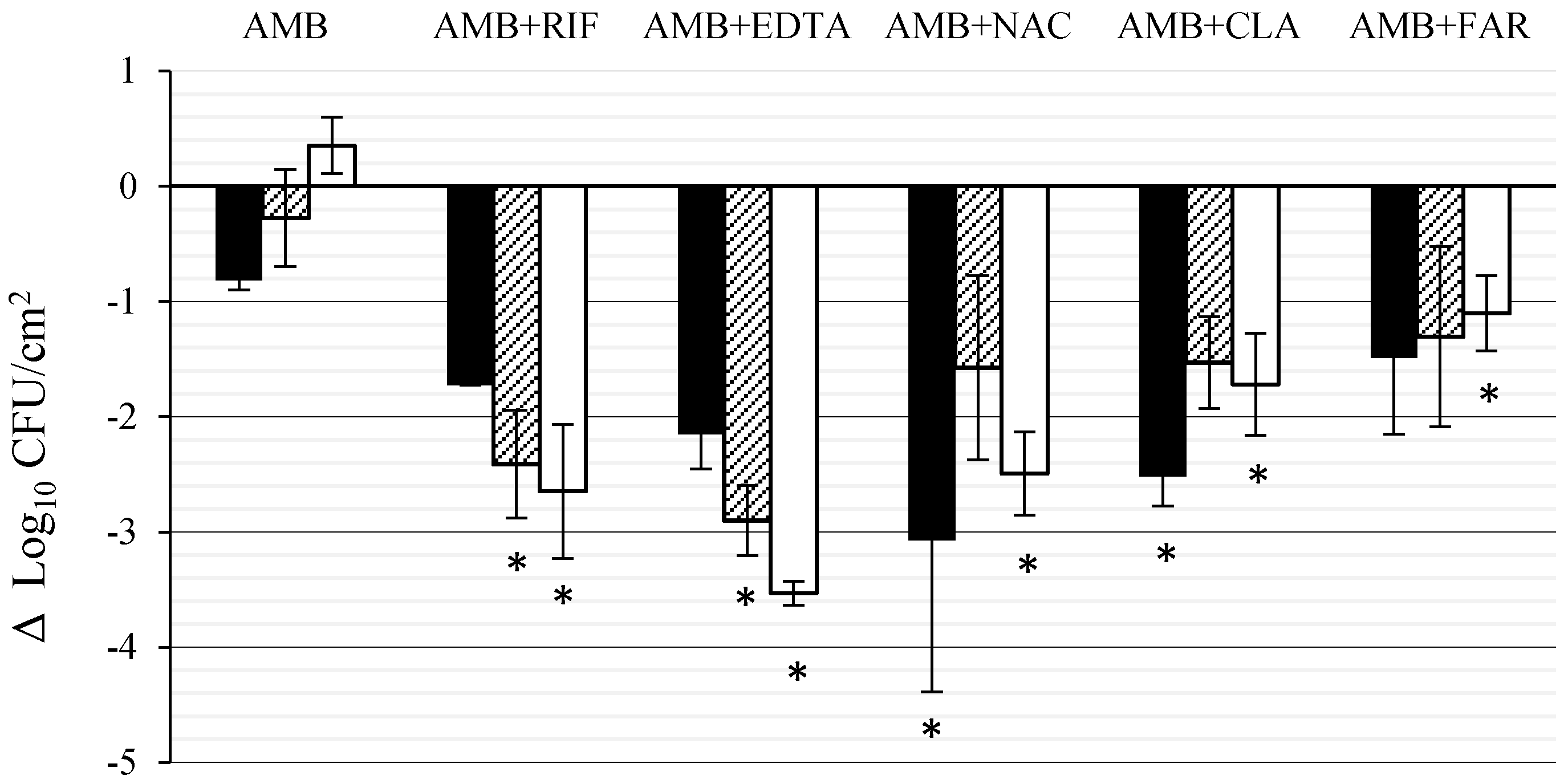
3.1. Effect of AMB Combined with Anti-Biofilm Compounds on C. tropicalis Biofilm Formed on PTFE
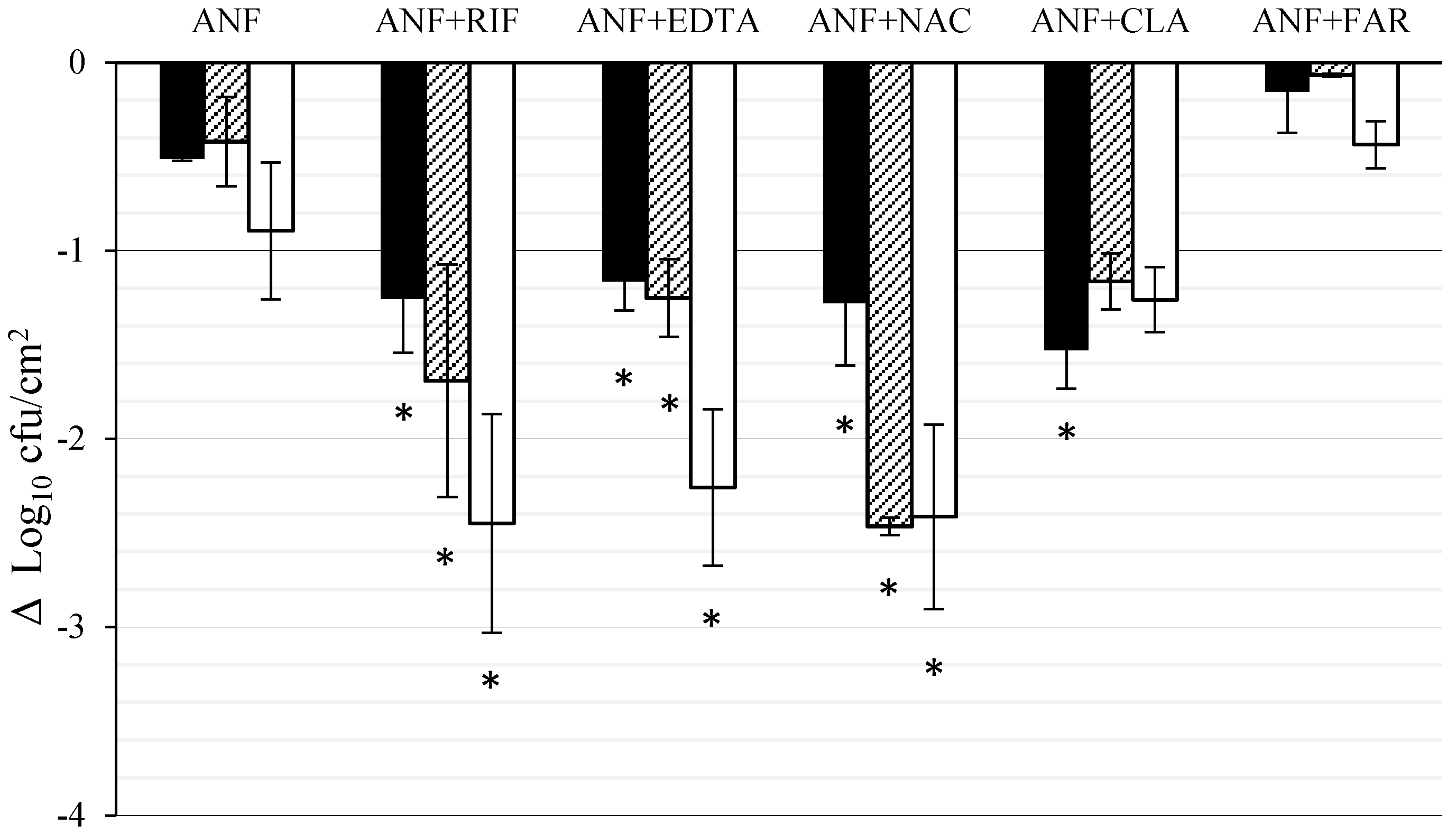
3.2. Effect of ANF Combined with Anti-Biofilm Compounds on C. tropicalis Biofilm Formed on Titanium
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Habash, M.; Reid, G. Microbial biofilms: Their development and significance for medical device-related infections. J. Clin. Pharmacol. 1999, 39, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Fortún, J.; Grill, F.; Martín-Dávila, P.; Blázquez, J.; Tato, M.; Sánchez-Corral, J.; García-San Miguel, L.; Moreno, S. Treatment of long-term intravascular catheter-related bacteraemia with antibiotic-lock therapy. J. Antimicrob. Chemother. 2006, 58, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Patel, R. Clinical practice. Infection associated with prosthetic. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef] [PubMed]
- Pemán, J.; Cantón, E.; Valentín, A. Activity of anidulafungin against Candida biofilms. Rev. Iberoam. Micol. 2008, 25, 124–128. [Google Scholar] [CrossRef]
- Gil, M.L.; Casanova, M.; Martínez, J.P. Changes in the cell wall glycoprotein composition of Candida albicans associated to the inhibition of germ tube formation by EDTA. Arch. Microbiol. 1994, 161, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, A.C.; Hermansson, M.; Elwing, H. N-acetyl-l-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl. Environ. Microbiol. 2003, 69, 4814–4822. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Francés, M.L.; Hernáez, S.; Serrera, A.; Alonso, M.; Rubio, M.F. Effect of amphotericin B alone or in combination with rifampicin or clarithromycin against Candida species biofilms. Int. J. Artif. Organs 2011, 34, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; Lopez-Ribot, J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.S.; Lee, J.Y.; Song, J.H.; Peck, K.R. In vitro evaluation of antibiotic lock technique for the treatment of Candida albicans, C. glabrata, and, C. tropicalis biofilms. J. Korean Med. Sci. 2010, 25, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Lebeaux, D.; Ghigo, J.M.; Beloin, C. Full and broad-spectrum in vivo eradication of catheter-associated biofilms using gentamicin-EDTA antibiotic lock therapy. Antimicrob. Agents Chemother. 2012, 56, 6310–6318. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Peck, K.R.; Oh, W.S.; Song, J.H. In vitro evaluation of the antibiotic lock technique (ALT) for the treatment of catheter-related infections caused by staphylococci. J. Antimicrob. Chemother. 2006, 57, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Dalbøge, C.S.; Nielsen, X.C.; Dalhoff, K.; Alffenaar, J.W.; Duno, M.; Buchard, A.; Uges, D.R.; Jensen, A.G.; Jürgens, G.; Pressler, T.; et al. Pharmacokinetic variability of clarithromycin and differences in CYP3A4 activity in patients with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ruhnke, M.; Meersseman, W.; Paiva, J.A.; Kantecki, M.; Damle, B. Pharmacokinetics of anidulafungin in critically ill patients with candidemia/invasive candidiasis. Antimicrob. Agents Chemother. 2013, 57, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.; Garraffo, R.; Bensalem, M.; Janssen, C.; Bland, S.; Gaillat, J.; Bru, J.P. Pharmacokinetic and dynamic study of levofloxacin and rifampicin in bone and joint infections. Med. Mal. Infect. 2012, 42, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Soldini, D.; Zwahlen, H.; Gabutti, L.; Marzo, A.; Marone, C. Pharmacokinetics of N-acetylcysteine following repeated intravenous infusion in haemodialysed patients. Eur. J. Clin. Pharmacol. 2005, 60, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Wickes, B.L.; López-Ribot, J.L. Inhibition on Candida albicans biofilm formation using divalent cation chelators (EDTA). Mycopathologia 2007, 164, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Goeres, D.M.; Loetterle, L.R.; Hamilton, M.A.; Murga, R.; Kirby, D.W.; Donlan, R.M. Statistical assessment of a laboratory method for growing biofilms. Microbiology 2005, 151, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard, 3rd ed.; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Valentin, A.; Canton, E.; Peman, J.; Martinez, J.P. Voriconazole inhibits biofilm formation in different species of the genus Candida. J. Antimicrob. Chemother. 2012, 67, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Gelis, S.; de Groot, P.W.J.; Castillo, L.; Moragues, M.D.; Sentandreu, R.; Gómez, M.M.; Valentín, E. Pga13 in Candida albicans is localized in the cell wall and influences cell surface properties, morphogenesis and virulence. Fungal Genet. Biol. 2012, 49, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Diekema, D.J.; Messer, S.A.; Pfaller, M.A.; Klepser, M.E. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 2002, 49, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Agents and Chemotherapy. Instructions to Authors; Antimicrobial Agents and Chemotherapy: Washington, DC, USA, 2016; p. 23. [Google Scholar]
- Valentín, A.; Cantón, E.; Pemán, J.; Quindós, G. In vitro activity of amphotericin B and anidulafungin against Candida spp. biofilms. Rev. Iberoam. Micol. 2007, 24, 272–277. [Google Scholar] [CrossRef]
- Walraven, C.J.; Lee, S.A. Antifungal lock therapy. Antimicrob. Agents Chemother. 2013, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Raad, I.I.; Hachem, R.Y.; Hanna, H.A.; Fang, X.; Jiang, Y.; Dvorak, T.; Sherertz, R.J.; Kontoyiannis, D.P. Role of ethylene diamine tetra-acetic acid (EDTA) in catheter lock solutions: EDTA enhances the antifungal activity of amphotericin B lipid complex against Candida embedded in biofilm. Int. J. Antimicrob. Agents 2008, 32, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Kite, P.; Eastwood, K.; Murga, R.; Carr, J.; Arduino, M.J.; Donlan, R.M. Tetrasodium EDTA as a novel central venous catheter lock solution against biofilm. Infect. Control Hosp. Epidemiol. 2005, 26, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Raad, I.; Hanna, H.; Dvorak, T.; Chaiban, G.; Hachem, R. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob. Agents Chemother. 2007, 51, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Darouiche, R.O. Role of antibiofilm-antimicrobial agents in controlling device-related infections. Int. J. Artif. Organs 2011, 34, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Trautner, B.W.; Ramanathan, V.; Darouiche, R.O. Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob. Agents Chemother. 2007, 51, 1556–1558. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Rong, L.; Raad, I.; Versalovic, J. Novel synergistic antibiofilm combinations for salvage of infected catheters. J. Med. Microbiol. 2009, 58, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Widmer, A.F. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 2006, 19, 349–956. [Google Scholar] [CrossRef] [PubMed]
- El-Azizi, M. Enhancement of the in vitro activity of amphotericin B against the biofilms of non-albicans Candida spp. by Rifampicin and Doxycycline. J. Med. Microbiol. 2007, 56, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Gerbaux, C.; Van Bambeke, F.; Montenez, J.P.; Piret, J.; Morlighem, G.; Tulkens, P.M. Hyperactivity of cathepsin B and other lysosomal enzymes in fibroblasts exposed to azithromycin, a dicationic macrolide antibiotic with exceptional tissue accumulation. FEBS Lett. 1996, 394, 307–310. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lee, P.I. Antibiotic-lock therapy and erythromycin for treatment of catheter-related Candida parapsilosis and Staphylococcus aureus infections. J. Antimicrob. Chemother. 2007, 60, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Sohr, R.; Schulz, B.; Fleischhacker, M.; Ruhnke, M. Secretion of, E,E-farnesol and biofilm formation in eight different Candida species. Antimicrob. Agents Chemother. 2008, 52, 1859–1861. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; McCarthy, M.; Alexander, E.L.; Antachopoulos, C.; Meletiadis, J.; Jabra-Rizk, M.A.; Petraitis, V.; Roilides, E.; Walsh, T.J. In vitro interactions between farnesol and fluconazole, amphotericin B or micafungin against Candida albicans biofilms. J. Antimicrob. Chemother. 2015, 70, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Cao, Y.B.; Xu, Z.; Ying, K.; Li, Y.; Xie, Y.; Zhu, Z.Y.; Chen, W.S.; Jiang, Y.Y. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob. Agents Chemother. 2005, 49, 584–589. [Google Scholar] [CrossRef] [PubMed]
| Planktonic MIC | mg/L |
| Amphotericin B | 0.25 |
| Anidulafungin | 0.03 |
| Biofilm MIC 50/90 | |
| Amphotericin B | 0.25/8 |
| Anidulafungin | >16/>16 |
| Ethylenediaminetetraacetic acid | >1000/>1000 |
| Rifampicin | >20/>20 |
| N-acetylcysteine | >80/>80 |
| Clarithromycin | >128/>128 |
| Farnesol (µM) | >200/>200 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Rivero, M.E.; Del Pozo, J.L.; Valentín, A.; De Diego, A.M.; Pemán, J.; Cantón, E. Activity of Amphotericin B and Anidulafungin Combined with Rifampicin, Clarithromycin, Ethylenediaminetetraacetic Acid, N-Acetylcysteine, and Farnesol against Candida tropicalis Biofilms. J. Fungi 2017, 3, 16. https://doi.org/10.3390/jof3010016
Fernández-Rivero ME, Del Pozo JL, Valentín A, De Diego AM, Pemán J, Cantón E. Activity of Amphotericin B and Anidulafungin Combined with Rifampicin, Clarithromycin, Ethylenediaminetetraacetic Acid, N-Acetylcysteine, and Farnesol against Candida tropicalis Biofilms. Journal of Fungi. 2017; 3(1):16. https://doi.org/10.3390/jof3010016
Chicago/Turabian StyleFernández-Rivero, Marcelo Ernesto, José L. Del Pozo, Amparo Valentín, Araceli Molina De Diego, Javier Pemán, and Emilia Cantón. 2017. "Activity of Amphotericin B and Anidulafungin Combined with Rifampicin, Clarithromycin, Ethylenediaminetetraacetic Acid, N-Acetylcysteine, and Farnesol against Candida tropicalis Biofilms" Journal of Fungi 3, no. 1: 16. https://doi.org/10.3390/jof3010016
APA StyleFernández-Rivero, M. E., Del Pozo, J. L., Valentín, A., De Diego, A. M., Pemán, J., & Cantón, E. (2017). Activity of Amphotericin B and Anidulafungin Combined with Rifampicin, Clarithromycin, Ethylenediaminetetraacetic Acid, N-Acetylcysteine, and Farnesol against Candida tropicalis Biofilms. Journal of Fungi, 3(1), 16. https://doi.org/10.3390/jof3010016





