In Vitro Anti-Biofilm Activities of Citral and Thymol Against Candida Tropicalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organisms, Media and Growth Conditions
2.2. Anti-Fungal and Anti-Biofilm Susceptibility Tests
2.3. Field Emission Scanning Electron Microscope (FE-SEM) and Confocal Laser Scanning Microscope (CLSM)
2.4. Measurement of Reactive Oxygen Species (ROS) Levels
2.5. RNA Isolation, cDNA Synthesis and Real-Time Expression Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Citral and Thymol Effectively Inhibited C. tropicalis and Eradicated Biofilm
3.2. FE-SEM and CLSM Analysis Displayed Damage to The Biofilm in The Presence of Citral and Thymol
3.3. Citral and Thymol Indicated No Direct Binding to The Cell Membrane but Thymol Acts via Cell Wall
3.4. Thymol and Citral Generated Reactive Oxygen Species
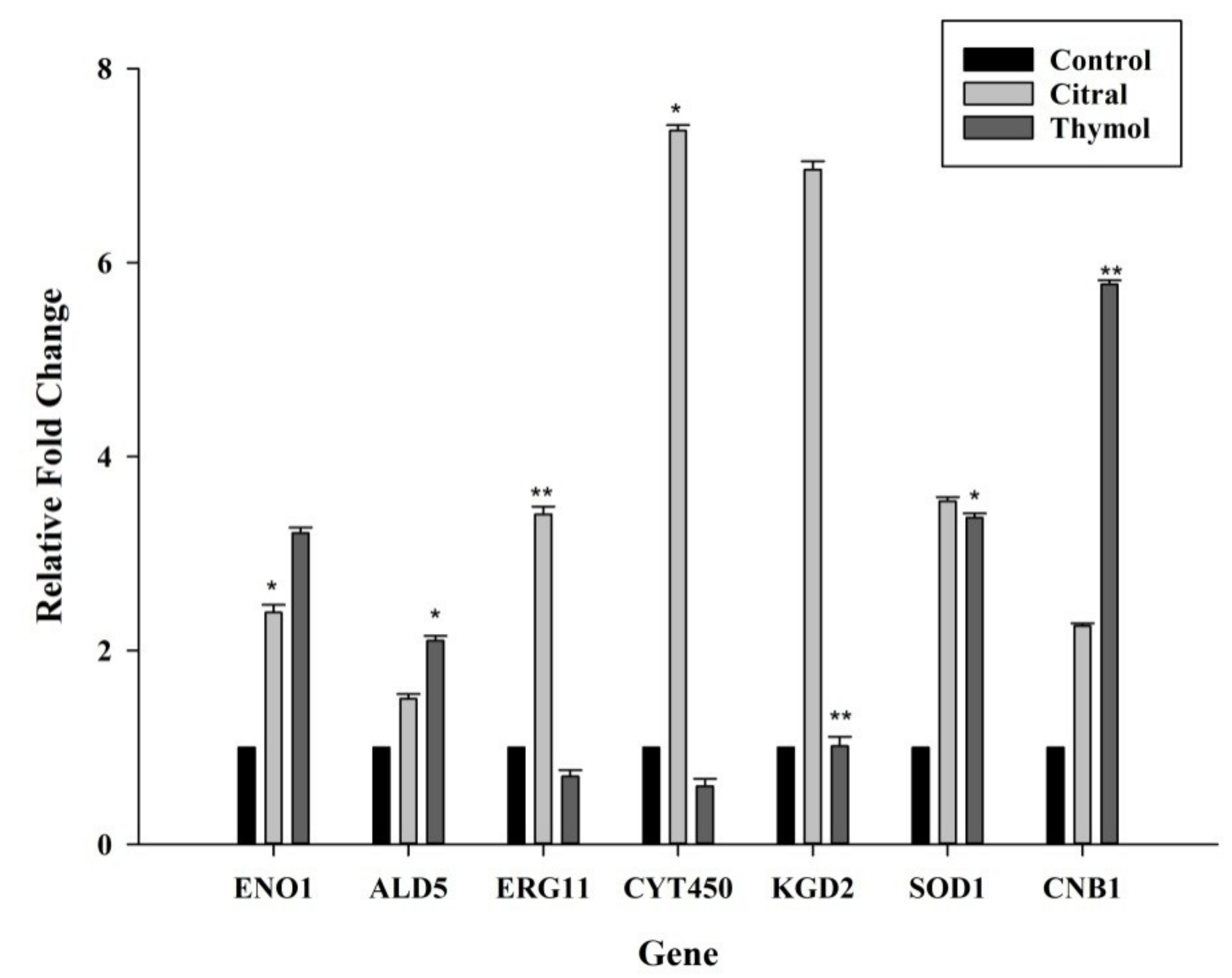
3.5. Citral Upregulated ERG11/CYT450 Genes Whereas Thymol Upregulated CNB1 and SOD1 Genes
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krasner, R.I. The Microbial Challenge: Human-Microbe Interactions; ASM Press: Washington DC, USA, 2002; ISBN 1555812414. [Google Scholar]
- Kothavade, R.J.; Kura, M.M.; Valand, A.G.; Panthaki, M.H. Candida tropicalis: Its prevalence, pathogenicity and increasing resistance to fluconazole. J. Med. Microbiol. 2010, 59, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Chander, J.; Singla, N.; Sidhu, S.K.; Gombar, S. Epidemiology of Candida blood stream infections: Experience of a tertiary care centre in North India. J. Infect. Dev. Ctries. 2013, 7, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Silva, S.; Henriques, M.; Oliveira, R. Insights into Candida tropicalis nosocomial infections and virulence factors. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Y.; Tan, A.L.; Tee, N.W.S.; Ng, L.S.Y.; Chee, C.W.J. The increased role of non-albicans species in candidaemia: Results from a 3-year surveillance study. Mycoses 2010, 53, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Tascini, C.; Sozio, E.; Corte, L.; Sbrana, F.; Scarparo, C.; Ripoli, A.; Bertolino, G.; Merelli, M.; Tagliaferri, E.; Corcione, A.; et al. The role of biofilm forming on mortality in patients with candidemia: A study derived from real world data. Infect. Dis. 2018, 50, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, E.G.; Andes, D.R. Candida Biofilm Tolerance: Comparison of Planktonic and Biofilm Resistance Mechanisms. In Candida albicans: Cellular and Molecular Biology; Springer: Cham, Switzerland, 2017; pp. 77–92. [Google Scholar]
- Andes, D.R. In Vivo Candida Device Biofilm Models. In Candida albicans: Cellular and Molecular Biology; Springer: Cham, Switzerland, 2017; pp. 93–113. [Google Scholar]
- Nett, J.E.; Andes, D. Fungal biofilms: In vivo models for discovery of anti-biofilm drugs. Microbiol. Spectr. 2015, 3, E30. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. De Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirci, F.; Bueno, J. Essential Oils against Microbial Resistance Mechanisms, Challenges and Applications in Drug Discovery. In Essential Oils and Nanotechnology for Treatment of Microbial Diseases; CRC Press: Boca Raton, FL, USA, 2017; pp. 155–170. [Google Scholar]
- Da Silva, N.B.; de Lucena Rangel, M.; Almeida, B.B.; de Castro, R.D.; Valença, A.M.G.; Cavalcanti, A.L. Antifungal Activity of the Essential Oil of Cymbopogon citratus (DC) Stapf. An in vitro study. J. Oral Res. 2017, 6, 319–323. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular Evolution of Antifungal Drug Resistance. Annu. Rev. Microbiol. 2017, 71, 753–775. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 4th ed.; CLSI Document M27; CLSI: Wayne, PA, USA, 2017; ISBN 1-56238-826-6. [Google Scholar]
- Ramage, G.; VandeWalle, K.; Wickes, B.L.; López-Ribot, J.L. Standardized Method for In Vitro Antifungal Susceptibility Testing of Candida albicans Biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Cain, M.T.; Crawford, K.; Andes, D.R. Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by XTT assay. J. Clin. Microbiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; Gattuso, M.; Pérez, P.; Zacchino, S. Evidence for the mechanism of action of the antifungal phytolaccoside B isolated from Phytolacca tetramera Hauman. J. Nat. Prod. 2008, 71, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. In vitro growth and analysis of Candida biofilms. Nat. Protoc. 2008, 3, 1909. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersboll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, D.G. The antimicrobial peptide arenicin-1 promotes generation of reactive oxygen species and induction of apoptosis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 1246–1251. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Chatrath, A.; Kumari, P.; Gangwar, R.; Prasad, R. Investigation of Differentially Expressed Proteins of Candida tropicalis Biofilm in Response to Citral. J. Proteomics Bioinform. 2018, 11, 57–61. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Sandai, D.; Tabana, Y.M.; El Ouweini, A.; Ayodeji, I.O. Resistance of Candida albicans biofilms to drugs and the host immune system. Jundishapur J. Microbiol. 2016, 9, e37385. [Google Scholar] [CrossRef] [PubMed]
- Basak, G.; Das, D.; Das, N. Dual role of acidic diacetate sophorolipid as biostabilizer for ZnO nanoparticle synthesis and biofunctionalizing agent against Salmonella enterica and Candida albicans. J. Microbiol. Biotechnol. 2014, 24, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Alfatah, M.; Ganesan, K.; Bhattacharyya, M.S. Inhibitory effect of sophorolipid on Candida albicans biofilm formation and hyphal growth. Sci. Rep. 2016, 6, 23575. [Google Scholar] [CrossRef] [PubMed]
- Millard, P.J.; Roth, B.L.; Thi, H.P.; Yue, S.T.; Haugland, R.P. Development of the FUN-1 family of fluorescent probes for vacuole labeling and viability testing of yeasts. Appl. Environ. Microbiol. 1997, 63, 2897–2905. [Google Scholar] [PubMed]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Caola, I.; Nollo, G.; Rinaldi, M.; Fracchia, L. Inhibition of Candida albicans biofilm by lipopeptide AC7 coated medical-grade silicone in combination with farnesol. AIMS Bioeng. 2018, 5, 192–208. [Google Scholar] [CrossRef]
- Sousa, J.; Costa, A.; Leite, M.; Guerra, F.; Silva, V.; Menezes, C.; Pereira, F.; Lima, E. Antifungal activity of citral by disruption of ergosterol biosynthesis in fluconazole resistant Candida tropicalis. Int. J. Trop. Dis. Heal. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, A.; Khan, L.A.; Padoa, C.J.; van Vuuren, S.; Manzoor, N. Effect of two monoterpene phenols on antioxidant defense system in Candida albicans. Microb. Pathog. 2015, 80, 50–56. [Google Scholar] [CrossRef]
- Chang, W.-Q.; Wu, X.-Z.; Cheng, A.-X.; Zhang, L.; Ji, M.; Lou, H.-X. Retigeric acid B exerts antifungal effect through enhanced reactive oxygen species and decreased cAMP. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-C.; Hsiao, T.-Y.; Chen, C.-T.; Yang, Y.-L. Candida albicans ENO1 null mutants exhibit altered drug susceptibility, hyphal formation, and virulence. J. Microbiol. 2013, 51, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lu, H.; Jiang, Y.; Cao, Y. CaIPF19998 reduces drug susceptibility by enhancing the ability of biofilm formation and regulating redox homeostasis in Candida albicans. Curr. Microbiol. 2013, 67, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2015, 5, a019752. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Gam, L.H.; Yong, V.C.; Rosli, R.; Ng, K.P.; Chong, P.P. Immunoproteomic analysis of antibody response to cell wall-associated proteins of C andida tropicalis. J. Appl. Microbiol. 2014, 117, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.E.B.; Giacomelli, S.R.; Altenhofen, D.; Alves, S.H.; Morsch, V.M.; Schetinger, M.R.C. Fluconazole and amphotericin-B resistance are associated with increased catalase and superoxide dismutase activity in Candida albicans and Candida dubliniensis. Rev. Soc. Bras. Med. Trop. 2013, 46, 752–758. [Google Scholar] [CrossRef] [PubMed]




| Gene | Gene ID | Amplicon Length (bp) | Tm (°C) | Sequence 5’ to 3’ |
|---|---|---|---|---|
| enolase | ENO1 | 176 | 50.2 | F: TATTGCCATGGATGTTGCTT |
| enolase | ENO1 | R: CTTCAGCGAATGGATCTTCA | ||
| alcohol dehydrogenase | ALD5 | 196 | 51.8 | F: TTGTTACCGGTGGTGCTAGA |
| alcohol dehydrogenase | ALD5 | R: GAGTGAATACCAGCAGCCAA | ||
| sterol 14-demethylase | ERG11 | 120 | 50.5 | F: ACTCATGGGGTTGCCAATGT |
| sterol 14-demethylase | ERG11 | R: AGTTGAGCAAATGAACGGTC | ||
| Cytochrome P450 52A1 | CYT450 | 169 | 54.0 | F: GTTCTGCTGTGTTTCCAGCC |
| Cytochrome P450 52A1 | CYT450 | R:AGACCCAGAGAATGTCAAGGC | ||
| 2-oxoglutarate dehydrogenase complex | KGD2 | 135 | 55.0 | F: GGTGCATTCTCCAAGGCTGT |
| 2-oxoglutarate dehydrogenase complex | KGD2 | R: CAAACCCTTTGGTGTGGCAA | ||
| superoxide dismutase | SOD1 | 140 | 54.0 | F: TTCAAGGTTCTGGTTGGGCT |
| superoxide dismutase | SOD1 | R: AGCATGTTCCCAAGCATCAA | ||
| calcineurin subunit B | CNB1 | 112 | 55.0 | F: AGATGGGTCAGGGGAAATTGAC |
| calcineurin subunit B | CNB1 | R: ACGACCATCACCATCTGTGTC | ||
| glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 153 | 54.0 | F: GTCAACGATCCATTCATTGC |
| glyceraldehyde-3-phosphate dehydrogenase | GAPDH | R: AGCTGGGTCTCTTTCTTGGA |
| Drug | Sorbitol | Ergosterol | ||||
|---|---|---|---|---|---|---|
| Control | Absence | Presence | Absence | Presence | ||
| Citral | #MIC50 | + | 32 | 32 | 32 | 32 |
| #BIC50 | + | 64 | 64 | 64 | 64 | |
| #BEC50 | + | 128 | 128 | 128 | 128 | |
| Thymol | #MIC50 | + | 16 | 32 | 16 | 16 |
| #BIC50 | + | 32 | 64 | 32 | 32 | |
| #BEC50 | + | 128 | 128 | 128 | 128 | |
| Amphotericin B | #MIC50 | + | NA | NA | 1 | 16 |
| #BIC50 | + | NA | NA | 4 | 32 | |
| #BEC50 | + | NA | NA | 8 | 64 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatrath, A.; Gangwar, R.; Kumari, P.; Prasad, R. In Vitro Anti-Biofilm Activities of Citral and Thymol Against Candida Tropicalis. J. Fungi 2019, 5, 13. https://doi.org/10.3390/jof5010013
Chatrath A, Gangwar R, Kumari P, Prasad R. In Vitro Anti-Biofilm Activities of Citral and Thymol Against Candida Tropicalis. Journal of Fungi. 2019; 5(1):13. https://doi.org/10.3390/jof5010013
Chicago/Turabian StyleChatrath, Apurva, Rashmi Gangwar, Poonam Kumari, and Ramasare Prasad. 2019. "In Vitro Anti-Biofilm Activities of Citral and Thymol Against Candida Tropicalis" Journal of Fungi 5, no. 1: 13. https://doi.org/10.3390/jof5010013
APA StyleChatrath, A., Gangwar, R., Kumari, P., & Prasad, R. (2019). In Vitro Anti-Biofilm Activities of Citral and Thymol Against Candida Tropicalis. Journal of Fungi, 5(1), 13. https://doi.org/10.3390/jof5010013




