Identification of Cryptic Species of Four Candida Complexes in a Culture Collection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Isolates and Phenotypic Identification
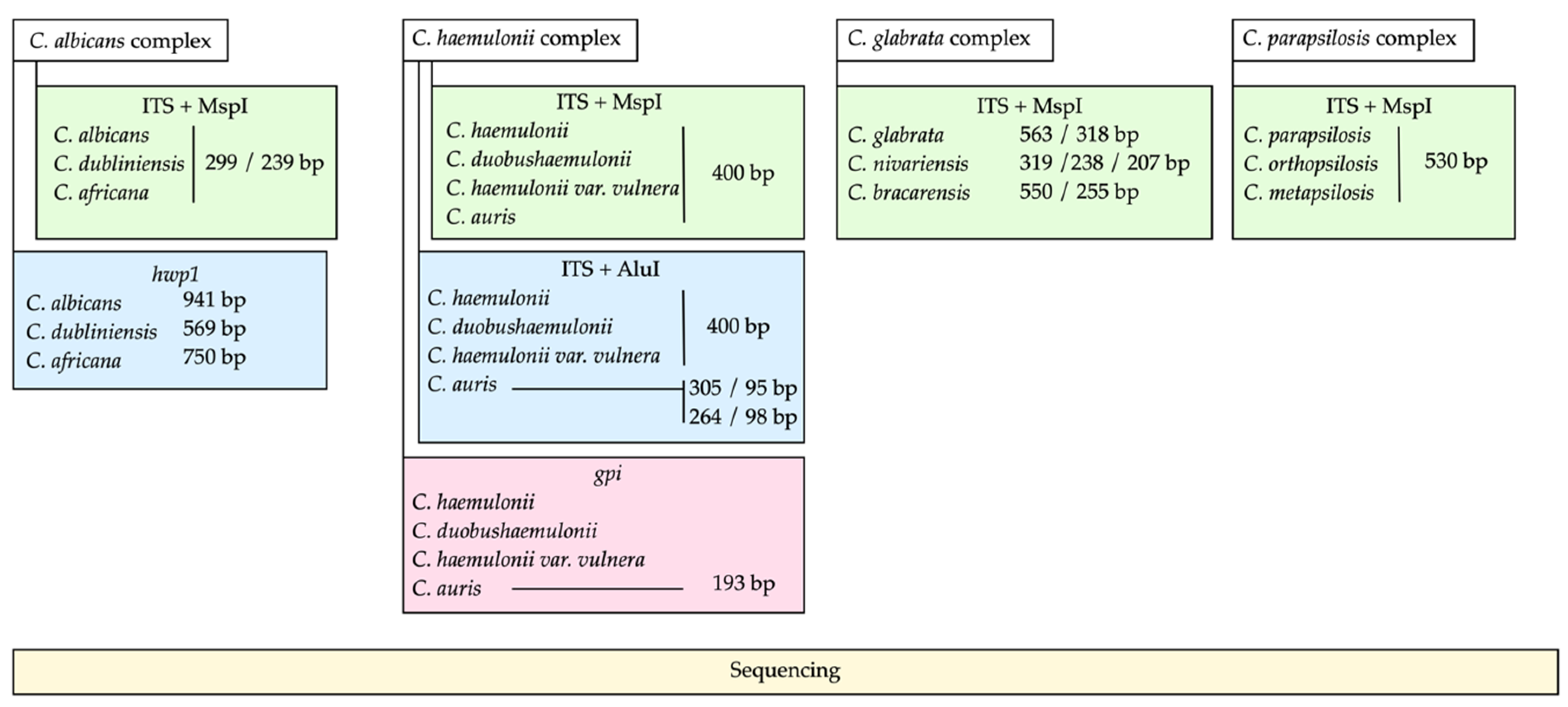
2.2. PCR-RFLP of the ITS Region and MspI
2.3. PCR of the hwp1 Gene for Identification of Species from C. albicans Complex
2.4. PCR-RFLP for the Identification of C. auris within the C. haemulonii Complex
2.5. PCR of the gpi Gene for Identification of C. auris
2.6. Sequencing of PCR Products
3. Results
3.1. Identification of Cryptic Species within the C. albicans Complex
3.2. Identification of Cryptic Species of the C. haemulonii Complex
3.3. Identification of Cryptic Species of the C. glabrata and C. parapsilosis Complexes
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quindos, G.; Marcos-Arias, C.; San-Millan, R.; Mateo, E.; Eraso, E. The continuous changes in the aetiology and epidemiology of invasive candidiasis: From familiar Candida albicans to multiresistant Candida auris. Int. Microbiol. 2018, 21, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Slavin, M.; Marriott, D.; Halliday, C.; Kidd, S.; Arthur, I.; Bak, N.; Heath, C.H.; Kennedy, K.; Morrissey, C.O.; et al. Changing epidemiology of candidaemia in Australia. J. Antimicrob. Chemother. 2017, 72, 1103–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quindos, G. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev. Iberoam. Micol. 2014, 31, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Santolaya, M.E.; Thompson, L.; Benadof, D.; Tapia, C.; Legarraga, P.; Cortes, C.; Rabello, M.; Valenzuela, R.; Rojas, P.; Rabagliati, R.; et al. A prospective, multi-center study of Candida bloodstream infections in Chile. PLoS ONE 2019, 14, e0212924. [Google Scholar] [CrossRef] [Green Version]
- Mucci, M.J.; Cuestas, M.L.; Landanburu, M.F.; Mujica, M.T. Prevalence of Candida albicans, Candida dubliniensis and Candida africana in pregnant women suffering from vulvovaginal candidiasis in Argentina. Rev. Iberoam. Micol. 2017, 34, 72–76. [Google Scholar] [CrossRef]
- Liu, M.; Huang, S.; Guo, L.; Li, H.; Wang, F.; Zhang, Q.I.; Song, G. Clinical features and risk factors for blood stream infections of Candida in neonates. Exp. Ther. Med. 2015, 10, 1139–1144. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Kishi, M.; Suda, M.; Sakata, K.; Shimoda, H.; Miura, H.; Ogawa, A.; Kobayashi, S. Prevalence of Candida albicans and non-albicans on the tongue dorsa of elderly people living in a post-disaster area: A cross-sectional survey. BMC Oral Health 2017, 17, 51. [Google Scholar] [CrossRef] [Green Version]
- Diba, K.; Makhdoomi, K.; Nasri, E.; Vaezi, A.; Javidnia, J.; Gharabagh, D.J.; Jazani, N.H.; Reza Chavshin, A.; Badiee, P.; Badali, H.; et al. Emerging Candida species isolated from renal transplant recipients: Species distribution and susceptibility profiles. Microb. Pathog. 2018, 125, 240–245. [Google Scholar] [CrossRef]
- Pendleton, K.M.; Dickson, R.P.; Newton, D.W.; Hoffman, T.C.; Yanik, G.A.; Huffnagle, G.B. Respiratory Tract Colonization by Candida species Portends Worse Outcomes in Immunocompromised Patients. Clin. Pulm. Med. 2018, 25, 197–201. [Google Scholar] [CrossRef]
- Gomez, J.; Garcia-Vazquez, E.; Espinosa, C.; Ruiz, J.; Canteras, M.; Hernandez-Torres, A.; Banos, V.; Herrero, J.A.; Valdes, M. Nosocomial candidemia at a general hospital: The change of epidemiological and clinical characteristics. A comparative study of 2 cohorts (1993–1998 versus 2002–2005). Rev. Iberoam. Micol. 2009, 26, 184–188. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20 (Suppl. 6), 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, J.; Dingle, T.C.; Bull, A.; Shokoples, S.; Laverdiere, M.; Baxter, M.R.; Adam, H.J.; Karlowsky, J.A.; Zhanel, G.G.; Canadian Antimicrobial Resistance Alliance (CARA); et al. Species distribution and antifungal susceptibility of invasive Candida isolates from Canadian hospitals: Results of the CANWARD 2011-16 study. J. Antimicrob. Chemother. 2019, 74, iv48–iv54. [Google Scholar] [CrossRef] [PubMed]
- Criseo, G.; Scordino, F.; Romeo, O. Current methods for identifying clinically important cryptic Candida species. J. Microbiol. Methods 2015, 111, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J.; Westerneng, T.J.; Haynes, K.A.; Bennett, D.E.; Coleman, D.C. Candida dubliniensis sp. nov.: Phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 1995, 141 Pt 7, 1507–1521. [Google Scholar] [CrossRef] [Green Version]
- Tietz, H.J.; Hopp, M.; Schmalreck, A.; Sterry, W.; Czaika, V. Candida africana sp. nov., a new human pathogen or a variant of Candida albicans? Mycoses 2001, 44, 437–445. [Google Scholar] [CrossRef]
- Correia, A.; Sampaio, P.; James, S.; Pais, C. Candida bracarensis sp. nov., a novel anamorphic yeast species phenotypically similar to Candida glabrata. Int. J. Syst. Evol. Microbiol. 2006, 56, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Alcoba-Florez, J.; Mendez-Alvarez, S.; Cano, J.; Guarro, J.; Perez-Roth, E.; del Pilar Arevalo, M. Phenotypic and molecular characterization of Candida nivariensis sp. nov., a possible new opportunistic fungus. J. Clin. Microbiol. 2005, 43, 4107–4111. [Google Scholar] [CrossRef] [Green Version]
- Tavanti, A.; Davidson, A.D.; Gow, N.A.; Maiden, M.C.; Odds, F.C. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 2005, 43, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Cendejas-Bueno, E.; Kolecka, A.; Alastruey-Izquierdo, A.; Theelen, B.; Groenewald, M.; Kostrzewa, M.; Cuenca-Estrella, M.; Gomez-Lopez, A.; Boekhout, T. Reclassification of the Candida haemulonii complex as Candida haemulonii (C. haemulonii group I), C. duobushaemulonii sp. nov. (C. haemulonii group II), and C. haemulonii var. vulnera var. nov.: Three multiresistant human pathogenic yeasts. J. Clin. Microbiol. 2012, 50, 3641–3651. [Google Scholar] [CrossRef] [Green Version]
- Sugita, T.; Takashima, M.; Poonwan, N.; Mekha, N. Candida pseudohaemulonii Sp. Nov., an amphotericin B-and azole-resistant yeast species, isolated from the blood of a patient from Thailand. Microbiol. Immunol. 2006, 50, 469–473. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Snyder, G.M.; Wright, S.B. The Epidemiology and Prevention of Candida auris. Curr. Infect. Dis. Rep. 2019, 21, 19. [Google Scholar] [CrossRef]
- Rhodes, J.; Fisher, M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019, 52, 84–89. [Google Scholar] [CrossRef]
- Haas, M.; Grenouillet, F.; Loubersac, S.; Ariza, B.; Pepin-Puget, L.; Alvarez-Moreno, C.A.; Valderrama-Beltran, S.L.; Lavergne, R.A.; Le Pape, P.; Morio, F. Identification of cryptic Candida species by MALDI-TOF mass spectrometry, not all MALDI-TOF systems are the same: Focus on the C. parapsilosis species complex. Diagn. Microbiol. Infect. Dis. 2016, 86, 385–386. [Google Scholar] [CrossRef]
- Gamarra, S.; Dudiuk, C.; Mancilla, E.; Vera Garate, M.V.; Guerrero, S.; Garcia-Effron, G. Molecular tools for cryptic Candida species identification with applications in a clinical laboratory. Biochem. Mol. Biol. Educ. 2013, 41, 180–186. [Google Scholar] [CrossRef]
- Reyes-Montes, M.D.R.; Acosta-Altamirano, G.; Duarte-Escalante, E.; Salazar, E.G.; Martinez-Herrera, E.; Arenas, R.; Gonzalez, G.; Frias-De-Leon, M.G. Usefulness of a multiplex PCR for the rapid identification of Candida glabrata species complex in Mexican clinical isolates. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e37. [Google Scholar] [CrossRef] [Green Version]
- Neji, S.; Hadrich, I.; Ilahi, A.; Trabelsi, H.; Chelly, H.; Mahfoudh, N.; Cheikhrouhou, F.; Sellami, H.; Makni, F.; Ayadi, A. Molecular Genotyping of Candida parapsilosis Species Complex. Mycopathologia 2018, 183, 765–775. [Google Scholar] [CrossRef]
- Dudiuk, C.; Morales-Lopez, S.E.; Podesta, V.; Macedo, D.; Leonardelli, F.; Vitale, R.G.; Tosello, M.E.; Cabeza, M.S.; Biasoli, M.; Gamarra, S.; et al. Multiplex PCR designed to differentiate species within the Candida glabrata complex. Rev. Iberoam. Micol. 2017, 34, 43–45. [Google Scholar] [CrossRef]
- Fidler, G.; Leiter, E.; Kocsube, S.; Biro, S.; Paholcsek, M. Validation of a simplex PCR assay enabling reliable identification of clinically relevant Candida species. BMC Infect. Dis. 2018, 18, 393. [Google Scholar] [CrossRef]
- Arastehfar, A.; Fang, W.; Pan, W.; Liao, W.; Yan, L.; Boekhout, T. Identification of nine cryptic species of Candida albicans, C. glabrata, and C. parapsilosis complexes using one-step multiplex PCR. BMC Infect. Dis. 2018, 18, 480. [Google Scholar] [CrossRef]
- Montes, K.; Ortiz, B.; Galindo, C.; Figueroa, I.; Braham, S.; Fontecha, G. Identification of Candida Species from Clinical Samples in a Honduran Tertiary Hospital. Pathogens 2019, 8, 237. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sachu, A.; Mohan, K.; Vinod, V.; Dinesh, K.; Karim, S. Simple low cost differentiation of Candida auris from Candida haemulonii complex using CHROMagar Candida medium supplemented with Pal’s medium. Rev. Iberoam. Micol. 2017, 34, 109–111. [Google Scholar] [CrossRef]
- Ortiz, B.; Perez-Aleman, E.; Galo, C.; Fontecha, G. Molecular identification of Candida species from urinary infections in Honduras. Rev. Iberoam. Micol. 2018, 35, 73–77. [Google Scholar] [CrossRef]
- Mirhendi, H.; Makimura, K.; Khoramizadeh, M.; Yamaguchi, H. A one-enzyme PCR-RFLP assay for identification of six medically important Candida species. Nippon Ishinkin Gakkai Zasshi 2006, 47, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Romeo, O.; Criseo, G. First molecular method for discriminating between Candida africana, Candida albicans, and Candida dubliniensis by using hwp1 gene. Diagn. Microbiol. Infect. Dis. 2008, 62, 230–233. [Google Scholar] [CrossRef]
- Ruiz-Gaitan, A.C.; Fernandez-Pereira, J.; Valentin, E.; Tormo-Mas, M.A.; Eraso, E.; Peman, J.; de Groot, P.W.J. Molecular identification of Candida auris by PCR amplification of species-specific GPI protein-encoding genes. Int. J. Med. Microbiol. 2018, 308, 812–818. [Google Scholar] [CrossRef]
- Padovan, A.C.; Chaves, G.M.; Colombo, A.L.; Briones, M.R. A novel allele of HWP1, isolated from a clinical strain of Candida albicans with defective hyphal growth and biofilm formation, has deletions of Gln/Pro and Ser/Thr repeats involved in cellular adhesion. Med. Mycol. 2009, 47, 824–835. [Google Scholar] [CrossRef] [Green Version]
- Sadrossadati, S.Z.; Ghahri, M.; Imani Fooladi, A.A.; Sayyahfar, S.; Beyraghi, S.; Baseri, Z. Phenotypic and genotypic characterization of Candida species isolated from candideamia in Iran. Curr. Med. Mycol. 2018, 4, 14–20. [Google Scholar] [CrossRef]
- Ambaraghassi, G.; Dufresne, P.J.; Dufresne, S.F.; Vallieres, E.; Munoz, J.F.; Cuomo, C.A.; Berkow, E.L.; Lockhart, S.R.; Luong, M.L. Identification of Candida auris using the updated 8.01 VITEK(R)2 yeast identification system: A multi-laboratory evaluation study. J. Clin. Microbiol. 2019. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Sales, J.A.; da Silva, M.L.Q.; de Oliveira, J.S.; Pereira, L.A.; Pereira-Neto, W.A.; Cordeiro, R.A.; Sidrim, J.J.C.; Castelo-Branco, D.; Rocha, M.F.G. Antifungal susceptibility and virulence of Candida parapsilosis species complex: An overview of their pathogenic potential. J. Med. Microbiol. 2018, 67, 903–914. [Google Scholar] [CrossRef]
- Bineshian, F.; Yadegari, M.H.; Sharifi, Z.; Akbari Eidgahi, M.; Nasr, R. Identification of Candida Species Using MP65 Gene and Evaluation of the Candida albicans MP65 Gene Expression in BALB/C Mice. Jundishapur J. Microbiol. 2015, 8, e18984. [Google Scholar] [CrossRef] [Green Version]
- Hazirolan, G.; Altun, H.U.; Gumral, R.; Gursoy, N.C.; Otlu, B.; Sancak, B. Prevalence of Candida africana and Candida dubliniensis, in vulvovaginal candidiasis: First Turkish Candida africana isolates from vulvovaginal candidiasis. J. Mycol. Med. 2017, 27, 376–381. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, A.; Chen, X.; Wang, G.; Feng, X. Molecular Characterization of Candida africana in Genital Specimens in Shanghai, China. BioMed Res. Int. 2015, 2015, 185387. [Google Scholar] [CrossRef] [Green Version]
- Romeo, O.; Criseo, G. Molecular epidemiology of Candida albicans and its closely related yeasts Candida dubliniensis and Candida africana. J. Clin. Microbiol. 2009, 47, 212–214. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Fan, S.; Liu, X.; Li, J. Prevalence of Candida albicans-closely related yeasts, Candida africana and Candida dubliniensis, in vulvovaginal candidiasis. Med. Mycol. 2014, 52, 636–640. [Google Scholar] [CrossRef] [Green Version]
- Ngouana, T.K.; Krasteva, D.; Drakulovski, P.; Toghueo, R.K.; Kouanfack, C.; Ambe, A.; Reynes, J.; Delaporte, E.; Boyom, F.F.; Mallie, M.; et al. Investigation of minor species Candida africana, Candida stellatoidea and Candida dubliniensis in the Candida albicans complex among Yaounde (Cameroon) HIV-infected patients. Mycoses 2015, 58, 33–39. [Google Scholar] [CrossRef]
- Ngouana, T.K.; Drakulovski, P.; Krasteva, D.; Toghueo, R.K.; Kouanfack, C.; Reynes, J.; Delaporte, E.; Boyom, F.F.; Mallie, M.; Bertout, S. Genetic diversity of the Hwp1 gene and HIS3, EF3, CDC3 microsatellites and antifungal susceptibility profiles of Candida albicans isolates from Yaounde HIV-infected patients. Med. Mycol. 2017, 55, 546–554. [Google Scholar]
- Naeimi, B.; Mirhendi, H.; Khamisipour, G.; Sadeghzadeh, F.; Ahmadi, B. Candida africana in recurrent vulvovaginal candidiasis (RVVC) patients: Frequency and phenotypic and genotypic characteristics. J. Med. Microbiol. 2018, 67, 1601–1607. [Google Scholar] [CrossRef]
- Dieng, Y.; Sow, D.; Ndiaye, M.; Guichet, E.; Faye, B.; Tine, R.; Lo, A.; Sylla, K.; Ndiaye, M.; Abiola, A.; et al. Identification of three Candida africana strains in Senegal. J. Mycol. Med. 2012, 22, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Molecular phylogenetics and epidemiology of Candida albicans. Future Microbiol. 2010, 5, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.E.; Lockhart, S.R. Recent Taxonomic Developments with Candida and Other Opportunistic Yeasts. Curr. Fungal Infect. Rep. 2012, 6, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Gil-Alonso, S.; Jauregizar, N.; Canton, E.; Eraso, E.; Quindos, G. Comparison of the in vitro activity of echinocandins against Candida albicans, Candida dubliniensis, and Candida africana by time-kill curves. Diagn. Microbiol. Infect. Dis. 2015, 82, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of candidemia in Latin America: A laboratory-based survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, R.; Caceres, D.H.; Perez, M.; Garcia, N.; Castillo, W.; Santiago, E.; Borace, J.; Lockhart, S.R.; Berkow, E.L.; Hayer, L.; et al. Emerging Multidrug-Resistant Candida duobushaemulonii Infections in Panama Hospitals: Importance of Laboratory Surveillance and Accurate Identification. J. Clin. Microbiol. 2018, 56, e00371-18. [Google Scholar] [CrossRef] [Green Version]
- Arauz, A.B.; Caceres, D.H.; Santiago, E.; Armstrong, P.; Arosemena, S.; Ramos, C.; Espinosa-Bode, A.; Borace, J.; Hayer, L.; Cedeno, I.; et al. Isolation of Candida auris from 9 patients in Central America: Importance of accurate diagnosis and susceptibility testing. Mycoses 2018, 61, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, P.A.; Rivera, S.M.; Escandon, P.; Caceres, D.H.; Chow, N.; Stuckey, M.J.; Diaz, J.; Gomez, A.; Velez, N.; Espinosa-Bode, A.; et al. Hospital-Associated Multicenter Outbreak of Emerging Fungus Candida auris, Colombia, 2016. Emerg. Infect. Dis. 2019, 25, 1339. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, J.N., Jr.; Assy, J.G.; Levin, A.S.; Del Negro, G.M.; Giudice, M.C.; Tringoni, M.P.; Thomaz, D.Y.; Motta, A.L.; Abdala, E.; Pierroti, L.C.; et al. Candida haemulonii Complex Species, Brazil, January 2010-March 2015. Emerg. Infect. Dis. 2016, 22, 561–563. [Google Scholar] [CrossRef] [Green Version]
- Boatto, H.F.; Cavalcanti, S.D.; Del Negro, G.M.; Girao, M.J.; Francisco, E.C.; Ishida, K.; Gompertz, O.F. Candida duobushaemulonii: An emerging rare pathogenic yeast isolated from recurrent vulvovaginal candidiasis in Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.S.; Figueiredo-Carvalho, M.H.; Barbedo, L.S.; Ziccardi, M.; Chaves, A.L.; Zancope-Oliveira, R.M.; Pinto, M.R.; Sgarbi, D.B.; Dornelas-Ribeiro, M.; Branquinha, M.H.; et al. Candida haemulonii complex: Species identification and antifungal susceptibility profiles of clinical isolates from Brazil. J. Antimicrob. Chemother. 2015, 70, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda-Zapico, I.; Eraso, E.; Hernandez-Almaraz, J.L.; Lopez-Soria, L.M.; Carrillo-Munoz, A.J.; Hernandez-Molina, J.M.; Quindos, G. Prevalence and antifungal susceptibility patterns of new cryptic species inside the species complexes Candida parapsilosis and Candida glabrata among blood isolates from a Spanish tertiary hospital. J. Antimicrob. Chemother. 2011, 66, 2315–2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposto, M.C.; Prigitano, A.; Romeo, O.; Criseo, G.; Trovato, L.; Tullio, V.; Fadda, M.E.; Tortorano, A.M.; Group, F.W. Looking for Candida nivariensis and C. bracarensis among a large Italian collection of C. glabrata isolates: Results of the FIMUA working group. Mycoses 2013, 56, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, M.; Alanazi, A.F.; Ahmad, S.; Al-Sweih, N.; Khan, Z. Lack of detection of Candida nivariensis and Candida bracarensis among 440 clinical Candida glabrata sensu lato isolates in Kuwait. PLoS ONE 2019, 14, e0223920. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Messer, S.A.; Gherna, M.; Bishop, J.A.; Merz, W.G.; Pfaller, M.A.; Diekema, D.J. Identification of Candida nivariensis and Candida bracarensis in a large global collection of Candida glabrata isolates: Comparison to the literature. J. Clin. Microbiol. 2009, 47, 1216–1217. [Google Scholar] [CrossRef] [Green Version]
- Morales-Lopez, S.E.; Taverna, C.G.; Bosco-Borgeat, M.E.; Maldonado, I.; Vivot, W.; Szusz, W.; Garcia-Effron, G.; Cordoba, S.B. Candida glabrata species complex prevalence and antifungal susceptibility testing in a culture collection: First description of Candida nivariensis in Argentina. Mycopathologia 2016, 181, 871–878. [Google Scholar] [CrossRef]
- Romeo, O.; Delfino, D.; Costanzo, B.; Cascio, A.; Criseo, G. Molecular characterization of Italian Candida parapsilosis isolates reveals the cryptic presence of the newly described species Candida orthopsilosis in blood cultures from newborns. Diagn. Microbiol. Infect. Dis. 2012, 72, 234–238. [Google Scholar] [CrossRef]
- Lovero, G.; Borghi, E.; Balbino, S.; Cirasola, D.; De Giglio, O.; Perdoni, F.; Caggiano, G.; Morace, G.; Montagna, M.T. Molecular Identification and Echinocandin Susceptibility of Candida parapsilosis Complex Bloodstream Isolates in Italy, 2007–2014. PLoS ONE 2016, 11, e0150218. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Ahmad, S.; Hagen, F.; Meis, J.F.; Al-Sweih, N.; Khan, Z. Simple, Low-Cost Detection of Candida parapsilosis Complex Isolates and Molecular Fingerprinting of Candida orthopsilosis Strains in Kuwait by ITS Region Sequencing and Amplified Fragment Length Polymorphism Analysis. PLoS ONE 2015, 10, e0142880. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, S.R.; Messer, S.A.; Pfaller, M.A.; Diekema, D.J. Geographic distribution and antifungal susceptibility of the newly described species Candida orthopsilosis and Candida metapsilosis in comparison to the closely related species Candida parapsilosis. J. Clin. Microbiol. 2008, 46, 2659–2664. [Google Scholar] [CrossRef] [Green Version]


| Clinical Sample | No. of Samples (%) | C. albicans Complex | C. glabrata Complex | C. parapsilosis Complex | C. haemulonii Complex |
|---|---|---|---|---|---|
| Urine | 38 (35.19) | 21 | 16 | 1 | - |
| Sputum | 28 (25.93) | 25 | 1 | 2 | - |
| Vaginal swab | 16 (14.81) | 10 | 4 | - | 2 |
| Blood | 7 (6.48) | 2 | - | 5 | - |
| Catheter | 7 (6.48) | 3 | 1 | 3 | - |
| Stool | 2 (1.85) | - | - | 2 | - |
| Cutaneous secretion | 5 (4.63) | 1 | 2 | 1 | 1 |
| Otic secretion | 1 (0.93) | - | - | 1 | - |
| Oral swab | 2 (1.85) | 2 | - | - | - |
| Cerebrospinal fluid (CSF) | 2 (1.85) | 2 | - | - | - |
| Total (%) | 108 (100%) | 66 (61.11%) | 24 (22.22%) | 15 (13.88%) | 3 (2.78%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontecha, G.; Montes, K.; Ortiz, B.; Galindo, C.; Braham, S. Identification of Cryptic Species of Four Candida Complexes in a Culture Collection. J. Fungi 2019, 5, 117. https://doi.org/10.3390/jof5040117
Fontecha G, Montes K, Ortiz B, Galindo C, Braham S. Identification of Cryptic Species of Four Candida Complexes in a Culture Collection. Journal of Fungi. 2019; 5(4):117. https://doi.org/10.3390/jof5040117
Chicago/Turabian StyleFontecha, Gustavo, Kathy Montes, Bryan Ortiz, Celeste Galindo, and Sharleen Braham. 2019. "Identification of Cryptic Species of Four Candida Complexes in a Culture Collection" Journal of Fungi 5, no. 4: 117. https://doi.org/10.3390/jof5040117
APA StyleFontecha, G., Montes, K., Ortiz, B., Galindo, C., & Braham, S. (2019). Identification of Cryptic Species of Four Candida Complexes in a Culture Collection. Journal of Fungi, 5(4), 117. https://doi.org/10.3390/jof5040117






