Phosphate in Virulence of Candida albicans and Candida glabrata
Abstract
:1. Candida albicans is a Prevalent Human Commensal
2. C. albicans Is Not a Highly Evolved Human Pathogen but It Can Exploit Host Weaknesses
3. Phosphate is Indispensable for C. albicans Growth, a Precondition for its Pathogenesis
4. Phosphate Acquisition Differs Fundamentally between Organisms That Ingest Food, and Those That Absorb It
5. Recognizing a Role of Phosphate in C. albicans Virulence
6. The Transcriptional Regulator that Controls Phosphate Homeostasis is Required for C. albicans Stress Resistance
7. Comparison of Pho4-Regulated Genes in Two Closely Related Yeasts, a Plant Saprobe and An Opportunistic Human Pathogen
8. Cooperation of Transcriptional Regulators, Versus Control by a Single Transcription Factor, as Strategies to Coordinate Genes Required for ATP Biosynthesis
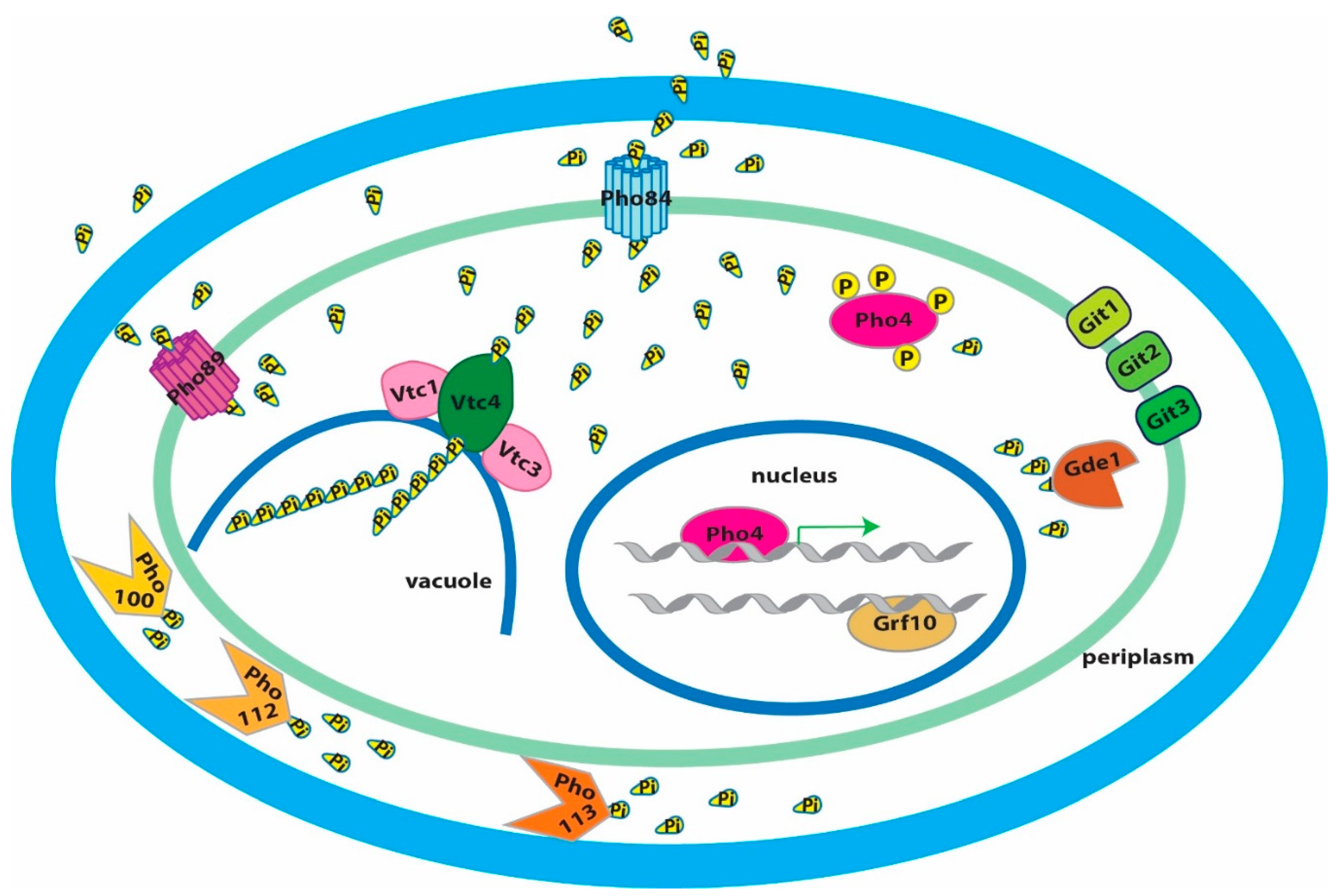
9. The PHO Regulon Controls Transporters of Organic and Inorganic Phosphate Compounds
10. A Pho84 Function Activates C. albicans TORC1, and TORC1 Modulates the PHO Regulon
11. Defects in Virulence and Oxidative Stress Resistance of Cells Lacking Pho84
12. Cell Wall Stress Hypersensitivity of C. albicans Cells Lacking Pho84
13. pho84 Null Mutant Cells Lack Nucleotide Sugars, Substrates of Cell Wall Biosynthetic Enzymes
14. Lack of ATP Impacts C. albicans Carbon Metabolism
15. Metabolites with Roles in Oxidative Stress Management are Decreased in C. albicans Cells Lacking Pho84
16. Perturbation of Phosphate Homeostasis may be Clinically Useful
Funding
Acknowledgments
Conflicts of Interest
References
- Ikeh, M.; Ahmed, Y.; Quinn, J. Phosphate Acquisition and Virulence in Human Fungal Pathogens. Microorganisms 2017, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Lev, S.; Djordjevic, J.T. Why is a functional PHO pathway required by fungal pathogens to disseminate within a phosphate-rich host: A paradox explained by alkaline pH-simulated nutrient deprivation and expanded PHO pathway function. PLoS Pathog. 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Roth, F.J.; Delgado, E.; Ahearn, D.G.; Kalser, M.H. Fungal flora of the normal human small and large intestine. N. Engl. J. Med. 1969, 280, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Arendorf, T.M.; Walker, D.M. The prevalence and intra-oral distribution of Candida albicans in man. Arch. Oral Biol. 1980, 25, 1–10. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef] [Green Version]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Candida albicans genome sequence: A platform for genomics in the absence of genetics. Genome Biol. 2004, 5, 230. [Google Scholar] [CrossRef] [Green Version]
- Bensasson, D.; Dicks, J.; Ludwig, J.M.; Bond, C.J.; Elliston, A.; Roberts, I.N.; James, S.A. Diverse Lineages of Candida albicans Live on Old Oaks. Genetics 2019, 211, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Angebault, C.; Djossou, F.; Abelanet, S.; Permal, E.; Ben Soltana, M.; Diancourt, L.; Bouchier, C.; Woerther, P.-L.; Catzeflis, F.; Andremont, A.; et al. Candida albicans is not always the preferential yeast colonizing humans: A study in Wayampi Amerindians. J. Infect. Dis. 2013, 208, 1705–1716. [Google Scholar] [CrossRef] [Green Version]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Jennison, R.F. Thrush in infancy. Arch. Dis. Child. 1977, 52, 747–749. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.A. Candida (Monilia) albicans: Effect of amino acids, glucose, pH, chlortetracycline (aureomycin), dibasic sodium and calcium phosphates, and anaerobic and aerobic conditions on its growth. AMA Arch. Dermatol. Syphilol. 1954, 70, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Budge, S.; Kaloriti, D.; Tillmann, A.; Jacobsen, M.D.; Yin, Z.; Ene, I.V.; Bohovych, I.; Sandai, D.; Kastora, S.; et al. Stress adaptation in a pathogenic fungus. J. Exp. Biol. 2014, 217, 144–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, G.C.; Pedersen, P.L. Phosphate transport in mitochondria: Past accomplishments, present problems, and future challenges. J. Bioenerg. Biomembr. 1993, 25, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Zara, V.; Dietmeier, K.; Palmisano, A.; Vozza, A.; Rassow, J.; Palmieri, F.; Pfanner, N. Yeast mitochondria lacking the phosphate carrier/p32 are blocked in phosphate transport but can import preproteins after regeneration of a membrane potential. Mol. Cell. Biol. 1996, 16, 6524–6531. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.N.; Flanagan, P.R.; Zeng, J.; Jani, N.M.; Cardenas, M.E.; Moran, G.P.; Köhler, J.R. Phosphate is the third nutrient monitored by TOR in Candida albicans and provides a target for fungal-specific indirect TOR inhibition. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef] [Green Version]
- Tamai, Y.; Toh-e, A.; Oshima, Y. Regulation of inorganic phosphate transport systems in Saccharomyces cerevisiae. J. Bacteriol. 1985, 164, 964–968. [Google Scholar] [CrossRef] [Green Version]
- Lenburg, M.E.; O’Shea, E.K. Signaling phosphate starvation. Trends Biochem. Sci. 1996, 21, 383–387. [Google Scholar] [CrossRef]
- Persson, B.L.; Lagerstedt, J.O.; Pratt, J.R.; Pattison-Granberg, J.; Lundh, K.; Shokrollahzadeh, S.; Lundh, F. Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr. Genet. 2003, 43, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, M.G.; Wanner, B.L.; Crepin, S.; Harel, J. The phosphate regulon and bacterial virulence: A regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 2008, 32, 461–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekabab, S.M.; Harel, J.; Dozois, C.M. Interplay between genetic regulation of phosphate homeostasis and bacterial virulence. Virulence 2014, 5, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Chekabab, S.M.; Jubelin, G.; Dozois, C.M.; Harel, J. PhoB activates Escherichia coli O157:H7 virulence factors in response to inorganic phosphate limitation. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Tomar, P.; Sinha, H. Conservation of PHO pathway in ascomycetes and the role of Pho84. J. Biosci. 2014, 39, 525–536. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, D.M.; Castillo, L.; Nather, K.; Munro, C.A.; Brown, A.J.; Gow, N.A.; Odds, F.C. Property differences among the four major Candida albicans strain clades. Eukaryot. Cell 2009, 8, 373–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrzypek, M.S.; Binkley, J.; Binkley, G.; Miyasato, S.R.; Simison, M.; Sherlock, G. The Candida Genome Database (CGD): Incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res. 2017, 45, D592–D596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fradin, C.; De Groot, P.; MacCallum, D.; Schaller, M.; Klis, F.; Odds, F.C. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 2005, 56, 397–415. [Google Scholar] [CrossRef]
- Thewes, S.; Kretschmar, M.; Park, H.; Schaller, M.; Filler, S.G.; Hube, B. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol. Microbiol. 2007, 63, 1606–1628. [Google Scholar] [CrossRef]
- Zakikhany, K.; Naglik, J.R.; Schmidt-Westhausen, A.; Holland, G.; Schaller, M.; Hube, B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell. Microbiol. 2007, 9, 2938–2954. [Google Scholar] [CrossRef]
- Walker, L.A.; Maccallum, D.M.; Bertram, G.; Gow, N.A.; Odds, F.C.; Brown, A.J. Genome-wide analysis of Candida albicans gene expression patterns during infection of the mammalian kidney. Fungal Genet. Biol. 2009, 46, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Hebecker, B.; Vlaic, S.; Conrad, T.; Bauer, M.; Brunke, S.; Kapitan, M.; Kapitan, M.; Hube, B.; Jacobsen, I.D. Dual-species transcriptional profiling during systemic candidiasis reveals organ-specific host-pathogen interactions. Sci. Rep. 2016, 6, 36055. [Google Scholar] [CrossRef] [Green Version]
- Munoz, J.F.; Delorey, T.; Ford, C.B.; Li, B.Y.; Thompson, D.A.; Rao, R.P.; Cuomo, C.A. Coordinated host-pathogen transcriptional dynamics revealed using sorted subpopulations and single macrophages infected with Candida albicans. Nat. Commun. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urrialde, V.; Prieto, D.; Pla, J.; Alonso-Monge, R. The Candida albicans Pho4 Transcription Factor Mediates Susceptibility to Stress and Influences Fitness in a Mouse Commensalism Model. Front. Microbiol. 2016, 7, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeh, M.A.; Kastora, S.L.; Day, A.M.; Herrero-de-Dios, C.M.; Tarrant, E.; Waldron, K.J.; Banks, A.P.; Bain, J.M.; Lydall, D.; Veal, E.A.; et al. Pho4 mediates phosphate acquisition in Candida albicans and is vital for stress resistance and metal homeostasis. Mol. Biol. Cell 2016. [Google Scholar] [CrossRef]
- Homann, O.R.; Dea, J.; Noble, S.M.; Johnson, A.D. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandeputte, P.; Pradervand, S.; Ischer, F.; Coste, A.T.; Ferrari, S.; Harshman, K.; Sanglard, D. Identification and functional characterization of Rca1, a transcription factor involved in both antifungal susceptibility and host response in Candida albicans. Eukaryot. Cell 2012, 11, 916–931. [Google Scholar] [CrossRef] [Green Version]
- Urrialde, V.; Prieto, D.; Pla, J.; Alonso-Monge, R. The Pho4 transcription factor mediates the response to arsenate and arsenite in Candida albicans. Front. Microbiol. 2015, 6, 118. [Google Scholar] [CrossRef] [Green Version]
- Urrialde, V.; Alburquerque, B.; Guirao-Abad, J.P.; Pla, J.; Arguelles, J.C.; Alonso-Monge, R. Arsenic inorganic compounds cause oxidative stress mediated by the transcription factor PHO4 in Candida albicans. Microbiol. Res. 2017, 203, 10–18. [Google Scholar] [CrossRef]
- Ogawa, N.; DeRisi, J.; Brown, P.O. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell 2000, 11, 4309–4321. [Google Scholar] [CrossRef] [Green Version]
- Orkwis, B.R.; Davies, D.L.; Kerwin, C.L.; Sanglard, D.; Wykoff, D.D. Novel acid phosphatase in Candida glabrata suggests selective pressure and niche specialization in the phosphate signal transduction pathway. Genetics 2010, 186, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Li, C.X.; Gleason, J.E.; Zhang, S.X.; Bruno, V.M.; Cormack, B.P.; Culotta, V.C. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc. Natl. Acad. Sci. USA 2015, 112, E5336–E5342. [Google Scholar] [CrossRef] [Green Version]
- Fidel, P.L.; Vazquez, J.A.; Sobel, J.D. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerwin, C.L.; Wykoff, D.D. Candida glabrata PHO4 is necessary and sufficient for Pho2-independent transcription of phosphate starvation genes. Genetics 2009, 182, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortimer, R.; Polsinelli, M. On the origins of wine yeast. Res. Microbiol. 1999, 150, 199–204. [Google Scholar] [CrossRef]
- Zhou, X.; O’Shea, E.K. Integrated approaches reveal determinants of genome-wide binding and function of the transcription factor Pho4. Mol. Cell 2011, 42, 826–836. [Google Scholar] [CrossRef] [Green Version]
- He, B.Z.; Zhou, X.; O’Shea, E.K. Evolution of reduced co-activator dependence led to target expansion of a starvation response pathway. eLife 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Daignan-Fornier, B.; Fink, G.R. Coregulation of purine and histidine biosynthesis by the transcriptional activators BAS1 and BAS2. Proc. Natl. Acad. Sci. USA 1992, 89, 6746–6750. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.J.P.; Gow, N.A.R.; Warris, A.; Brown, G.D. Memory in Fungal Pathogens Promotes Immune Evasion, Colonisation, and Infection. Trends Microbiol. 2019, 27, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Rebora, K.; Desmoucelles, C.; Borne, F.; Pinson, B.; Daignan-Fornier, B. Yeast AMP pathway genes respond to adenine through regulated synthesis of a metabolic intermediate. Mol. Cell. Biol. 2001, 21, 7901–7912. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, S.; Coulpier, F.; Jourdren, L.; Merle, M.; Beck, S.; Konrad, M.; Daignan-Fornier, B.; Pinson, B. Co-regulation of yeast purine and phosphate pathways in response to adenylic nucleotide variations. Mol. Microbiol. 2008, 68, 1583–1594. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Wangsanut, T.; Fonzi, W.A.; Rolfes, R.J. The GRF10 homeobox gene regulates filamentous growth in the human fungal pathogen Candida albicans. FEMS Yeast Res. 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Wangsanut, T.; Ghosh, A.K.; Metzger, P.G.; Fonzi, W.A.; Rolfes, R.J. Grf10 and Bas1 Regulate Transcription of Adenylate and One-Carbon Biosynthesis Genes and Affect Virulence in the Human Fungal Pathogen Candida albicans. mSphere 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.N.; Michael, M. Princples of Biochemistry, 7th ed.; Macmillan Learning: New York, NY, USA, 2017. [Google Scholar]
- Bishop, A.C.; Sun, T.; Johnson, M.E.; Bruno, V.M.; Patton-Vogt, J. Robust utilization of phospholipase-generated metabolites, glycerophosphodiesters, by Candida albicans: Role of the CaGit1 permease. Eukaryot. Cell 2011, 10, 1618–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, A.C.; Ganguly, S.; Solis, N.V.; Cooley, B.M.; Jensen-Seaman, M.I.; Filler, S.G.; Mitchell, A.P.; Patton-Vogt, J. Glycerophosphocholine utilization by Candida albicans: Role of the Git3 transporter in virulence. J. Biol. Chem. 2013, 288, 33939–33952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samyn, D.R.; Ruiz-Pavon, L.; Andersson, M.R.; Popova, Y.; Thevelein, J.M.; Persson, B.L. Mutational analysis of putative phosphate- and proton-binding sites in the Saccharomyces cerevisiae Pho84 phosphate: H(+) transceptor and its effect on signalling to the PKA and PHO pathways. Biochem. J. 2012, 445, 413–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, R.; Ruiz, A.; Bernal, D.; Chambers, J.R.; Arino, J. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: Evidence for calcium-mediated signalling. Mol. Microbiol. 2002, 46, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Inglis, D.O.; Arnaud, M.B.; Binkley, J.; Shah, P.; Skrzypek, M.S.; Wymore, F.; Binkley, G.; Miyasato, S.R.; Simison, M.; Sherlock, G. The Candida genome database incorporates multiple Candida species: Multispecies search and analysis tools with curated gene and protein information for Candida albicans and Candida glabrata. Nucleic Acids Res. 2012, 40, D667–D674. [Google Scholar] [CrossRef]
- Giots, F.; Donaton, M.C.; Thevelein, J.M. Inorganic phosphate is sensed by specific phosphate carriers and acts in concert with glucose as a nutrient signal for activation of the protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 2003, 47, 1163–1181. [Google Scholar] [CrossRef] [PubMed]
- Popova, Y.; Thayumanavan, P.; Lonati, E.; Agrochao, M.; Thevelein, J.M. Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc. Natl. Acad. Sci. USA 2010, 107, 2890–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schothorst, J.; Kankipati, H.N.; Conrad, M.; Samyn, D.R.; Van Zeebroeck, G.; Popova, Y.; Rubio-Texeira, M.; Persson, B.L.; Thevelein, J.M. Yeast nutrient transceptors provide novel insight in the functionality of membrane transporters. Curr. Genet. 2013, 59, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Mouillon, J.M.; Persson, B.L. New aspects on phosphate sensing and signalling in Saccharomyces cerevisiae. FEMS Yeast Res. 2006, 6, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Barbet, N.C.; Schneider, U.; Helliwell, S.B.; Stansfield, I.; Tuite, M.F.; Hall, M.N. TOR controls translation initiation and early G1 progression in yeast. Mol. Biol. Cell 1996, 7, 25–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loewith, R.; Hall, M.N. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 2011, 189, 1177–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stracka, D.; Jozefczuk, S.; Rudroff, F.; Sauer, U.; Hall, M.N. Nitrogen source activates TOR (target of rapamycin) complex 1 via glutamine and independently of Gtr/Rag proteins. J. Biol. Chem. 2014, 289, 25010–25020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.; Hall, M.N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017, 36, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, T.; Köhler, J.R. Ribosomal protein S6 phosphorylation is controlled by TOR and modulated by PKA in Candida albicans. Mol. Microbiol. 2015, 98, 384–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljungdahl, P.O.; Daignan-Fornier, B. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics 2012, 190, 885–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeckstaens, M.; Llinares, E.; Van Vooren, P.; Marini, A.M. The TORC1 effector kinase Npr1 fine tunes the inherent activity of the Mep2 ammonium transport protein. Nat. Commun. 2014, 5, 3101. [Google Scholar] [CrossRef] [Green Version]
- Bastidas, R.J.; Heitman, J.; Cardenas, M.E. The protein kinase Tor1 regulates adhesin gene expression in Candida albicans. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef] [Green Version]
- Cutler, N.S.; Pan, X.; Heitman, J.; Cardenas, M.E. The TOR signal transduction cascade controls cellular differentiation in response to nutrients. Mol. Biol. Cell 2001, 12, 4103–4113. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, S.N.; Baker, H.; Vezina, C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. 1975, 28, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Baker, H.; Sidorowicz, A.; Sehgal, S.N.; Vezina, C. Rapamycin (AY-22,989), a new antifungal antibiotic. III. In vitro and in vivo evaluation. J. Antibiot. 1978, 31, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Martel, R.R.; Klicius, J.; Galet, S. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can. J. Physiol. Pharmacol. 1977, 55, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Qazi, Y.; Wellen, J.R. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant. Rev. 2014, 28, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.N.; Uppuluri, P.; Broggi, A.; Besold, A.; Ryman, K.; Kambara, H.; Solis, N.; Lorenz, V.; Qi, W.; Acosta-Zaldívar, M.; et al. Intersection of phosphate transport, oxidative stress and TOR signalling in Candida albicans virulence. PLoS Pathog. 2018, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, M.J.; Jakob, U. Oxidative stress protection by polyphosphate--new roles for an old player. Curr. Opin. Microbiol. 2015, 24, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, M.; Neumann, H.; Lenherr, E.D.; Wehner, M.; Rybin, V.; Hassa, P.O.; Uttenweiler, A.; Reinhardt, M.; Schmidt, A.; Seiler, J.; et al. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science 2009, 324, 513–516. [Google Scholar] [CrossRef]
- Gerasimaite, R.; Sharma, S.; Desfougeres, Y.; Schmidt, A.; Mayer, A. Coupled synthesis and translocation restrains polyphosphate to acidocalcisome-like vacuoles and prevents its toxicity. J. Cell Sci. 2014, 127, 5093–5104. [Google Scholar] [CrossRef] [Green Version]
- Broxton, C.N.; Culotta, V.C. SOD Enzymes and Microbial Pathogens: Surviving the Oxidative Storm of Infection. PLoS Pathog. 2016, 12. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.-N.; Acosta-Zaldívar, M.; Qi, W.; Diray-Arce, J.; Walker, L.; Kottom, T.J.; Kelly, R.; Yuan, M.; Asara, J.M.; Lasky-Su, J.A.; et al. Phosphoric metabolites link phosphate import and polysaccharide biosynthesis for Candida albicans cell wall maintenance. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheshachalam, A.; Srivastava, N.; Mitchell, T.; Lacy, P.; Eitzen, G. Granule protein processing and regulated secretion in neutrophils. Front. Immunol. 2014, 5, 448. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Wang, N.; Yao, G.; Mu, C.; Wang, Y.; Sang, J. Blocking beta-1,6-glucan synthesis by deleting KRE6 and SKN1 attenuates the virulence of Candida albicans. Mol. Microbiol. 2018. [Google Scholar] [CrossRef]
- Bushby, S.R.; Hitchings, G.H. Trimethoprim, a sulphonamide potentiator. Br. J. Pharmacol. Chemother. 1968, 33, 72–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLellan, C.A.; Vincent, B.M.; Solis, N.V.; Lancaster, A.K.; Sullivan, L.B.; Hartland, C.L.; Youngsaye, W.; Filler, S.G.; Whitesell, L.; Lindquist, S. Inhibiting mitochondrial phosphate transport as an unexploited antifungal strategy. Nat. Chem. Biol. 2018, 14, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Zhang, Y.M.; Rock, C.O.; Jackowski, S. Coenzyme A: Back in action. Prog. Lipid Res. 2005, 44, 125–153. [Google Scholar] [CrossRef]
- Kowalska, E.; Kujda, M.; Wolak, N.; Kozik, A. Altered expression and activities of enzymes involved in thiamine diphosphate biosynthesis in Saccharomyces cerevisiae under oxidative and osmotic stress. FEMS Yeast Res. 2012, 12, 534–546. [Google Scholar] [CrossRef] [Green Version]
- Huh, W.K.; Lee, B.H.; Kim, S.T.; Kim, Y.R.; Rhie, G.E.; Baek, Y.W.; Hwang, C.S.; Lee, J.S.; Kang, S.O. D-Erythroascorbic acid is an important antioxidant molecule in Saccharomyces cerevisiae. Mol. Microbiol. 1998, 30, 895–903. [Google Scholar] [CrossRef]
- Jung, I.L.; Kim, I.G. Thiamine protects against paraquat-induced damage: Scavenging activity of reactive oxygen species. Environ. Toxicol. Pharmacol. 2003, 15, 19–26. [Google Scholar] [CrossRef]
- Wolak, N.; Kowalska, E.; Kozik, A.; Rapala-Kozik, M. Thiamine increases the resistance of baker’s yeast Saccharomyces cerevisiae against oxidative, osmotic and thermal stress, through mechanisms partly independent of thiamine diphosphate-bound enzymes. FEMS Yeast Res. 2014, 14, 1249–1262. [Google Scholar] [CrossRef] [Green Version]
- Huh, W.K.; Kim, S.T.; Kim, H.; Jeong, G.; Kang, S.O. Deficiency of D-erythroascorbic acid attenuates hyphal growth and virulence of Candida albicans. Infect. Immun. 2001, 69, 3939–3946. [Google Scholar] [CrossRef] [Green Version]
- Schiavi, S.C.; Kumar, R. The phosphatonin pathway: New insights in phosphate homeostasis. Kidney Int. 2004, 65, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kretschmer, M.; Reiner, E.; Hu, G.; Tam, N.; Oliveira, D.L.; Caza, M.; Yeon, J.H.; Kim, J.; Kastrup, C.J.; Jung, W.H.; et al. Defects in phosphate acquisition and storage influence virulence of Cryptococcus neoformans. Infect. Immun. 2014, 82, 2697–2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, R.; Gerasimaite, R.; Jung, J.Y.; Truffault, V.; Pavlovic, I.; Schmidt, A.; Saiardi, A.; Jessen, H.J.; Poirier, Y.; Hothorn, M.; et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 2016, 352, 986–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhler, J.R.; Acosta-Zaldívar, M.; Qi, W. Phosphate in Virulence of Candida albicans and Candida glabrata. J. Fungi 2020, 6, 40. https://doi.org/10.3390/jof6020040
Köhler JR, Acosta-Zaldívar M, Qi W. Phosphate in Virulence of Candida albicans and Candida glabrata. Journal of Fungi. 2020; 6(2):40. https://doi.org/10.3390/jof6020040
Chicago/Turabian StyleKöhler, Julia R., Maikel Acosta-Zaldívar, and Wanjun Qi. 2020. "Phosphate in Virulence of Candida albicans and Candida glabrata" Journal of Fungi 6, no. 2: 40. https://doi.org/10.3390/jof6020040




