RETRACTED: Efficacy of Chelerythrine against Mono- and Dual-Species Biofilms of Candida albicans and Staphylococcus aureus and Its Properties of Inducing Hypha-to-Yeast Transition of C. albicans
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strains and Cultural Conditions
2.3. Minimal Inhibitory Concentrations (MICs)
2.4. Minimum Biofilm Inhibitory Concentrations (MBIC90S)
2.5. Biofilm Inhibition Assay
2.6. Biofilm Composition by CLSM
2.7. Antibiotics Diffusion within Biofilms
2.8. Minimal Biofilm Eradication Concentration
2.9. Effect of Chelerythrine on C. albicans Hyphal Growth and Mature Hyphae
2.10. Statistical Analysis
3. Results
3.1. Minimum Inhibitory Concentrations (MICS) and Minimum Biofilm Inhibitory Concentration (MBIC90S) of Chelerythrine against C. albicans and S. aureus
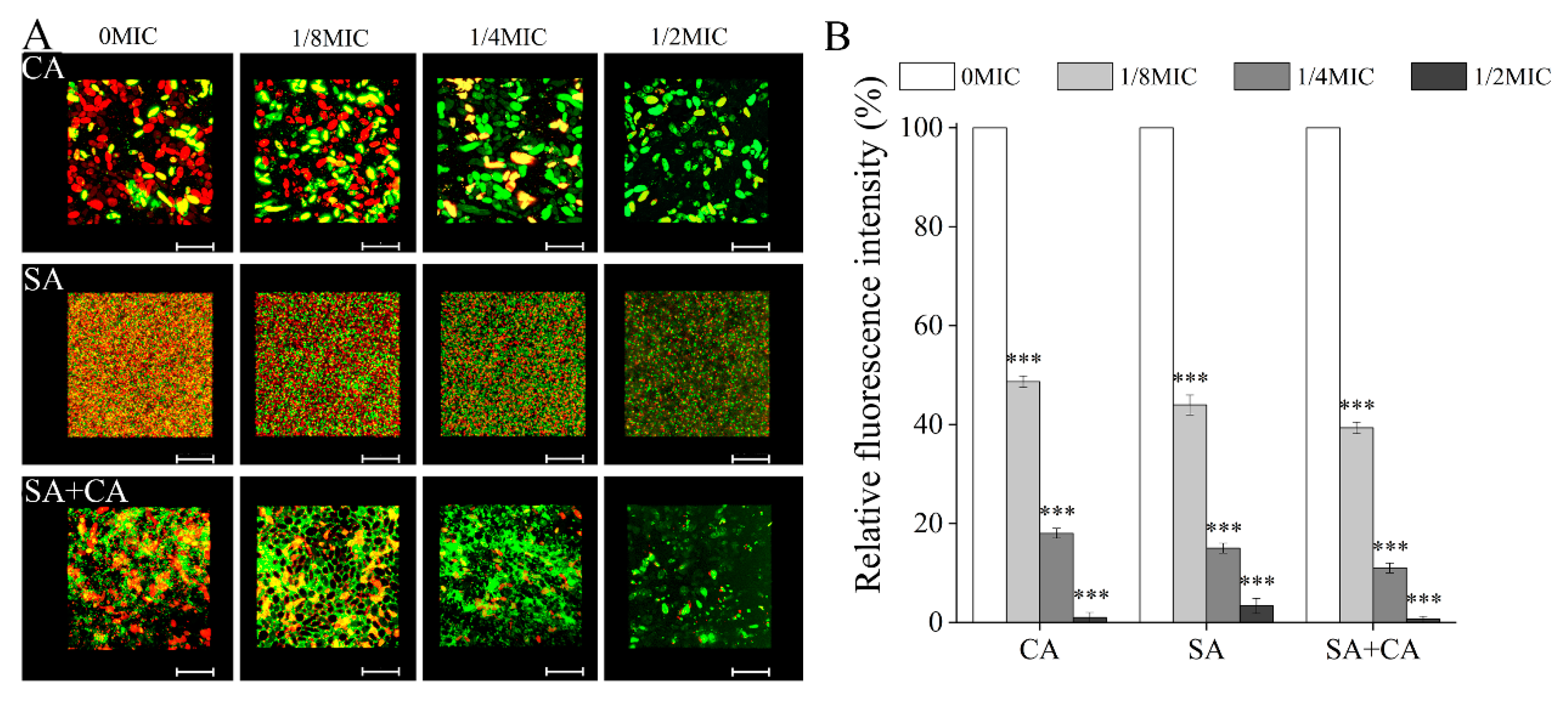
3.2. Chelerythrine Decreased the Biofilm Formation of C. albicans and S. aureus Mono- and Dual-Species
3.3. Sub-MIC Chelerythrine Changed the Biofilm Community Structure of C. albicans and S. aureus Mono- and Dual-Species
3.4. eDNA Levels within S. aureus Mono-Species and C. albicans and S. aureus Dual-Species Biofilms Decreases in Response to Chelerythrine
3.5. Chelerythrine Reduced the Biofilm Formation of C. albicans and S. aureus Mono- and Dual-Species by Mediating Extracellular Proteins Levels
3.6. Chelerythrine Decreases the Biofilm Formation of C. albicans and S. aureus Mono- and Dual-Species by Mediating Extracellular Polysaccharides Levels
3.7. Chelerythrine Treatment Reduces Mono- and Dual-Species Biofilms Tolerance to Gatifloxacin
3.8. Chelerythrine Eradicates Efficiently Preformed Biofilms of C. albicans and S. aureus Mono- and Dual-Species
3.9. Chelerythrine Inhibits the Hyphae Formation of C. albicans
3.10. Chelerythrine Induce the Hypha-to-Yeast Transition in C. albicans
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Diekema, D.J.; Messer, S.A.; Brueggemann, A.B.; Coffman, S.L.; Doern, G.V.; Herwaldt, L.A.; Pfaller, M.A. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 2002, 40, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.K.; Garneau-Tsodikova, S. Synergistic combinations of azoles and antihistamines against Candida species in vitro. Med. Mycol. 2019, 57, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; Costerton, J.W.; Shirtliff, M.E. Polymicrobial interactions: Impact on pathogenesis and human disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, J.; Jiang, N. Oral cavity infection: An adverse effect after the treatment of oral cancer in aged individuals. J. Appl. Oral. Sci. 2014, 22, 261–267. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Carolus, H.; Van Dyck, K.; Van Dijck, P. Candida albicans and Staphylococcus species: A threatening twosome. Front. Microbiol. 2019, 10, 2162. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharikova, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. MBio 2016, 7, e01365-16. [Google Scholar] [CrossRef]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; Gomes, F.; Rodrigues, C.F. Candida spp./bacteria mixed biofilms. J. Fungi 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.V.; Mitchell, A.P.; Andes, D.R. Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harb. Perspect. Med. 2014, 4, a019729. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.F.; Zhou, P.; Li, W.F.; Xu, H.B. Effects of chelerythrine, a specific inhibitor of cyclooxygenase-2, on acute inflammation in mice. Fitoterapia 2011, 82, 620–625. [Google Scholar] [CrossRef]
- Miao, F.; Yang, X.J.; Ma, Y.N.; Zheng, F.; Song, X.P.; Zhou, L. Structural modification of sanguinarine and chelerythrine and their in vitro acaricidal activity against Psoroptes cuniculi. Chem. Pharm. Bull. 2012, 60, 1508–1513. [Google Scholar] [CrossRef]
- He, N.; Wang, P.Q.; Wang, P.Y.; Ma, C.Y.; Kang, W.Y. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. BMC Complement. Altern. Med. 2018, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Yi, Y.L.; Zhang, C.; Wu, S.Q.; Shi, C.B.; Wang, G.X. Bioassay-guided isolation and identification of active compounds from Macleaya microcarpa (Maxim) Fedde against fish pathogenic bacteria. Aquac. Res. 2013, 44, 1221–1228. [Google Scholar] [CrossRef]
- Tantapakul, C.; Phakhodee, W.; Ritthiwigrom, T.; Yossathera, K.; Deachathai, S.; Laphookhieo, S. Antibacterial compounds from Zanthoxylum rhetsa. Arch. Pharmacal Res. 2012, 35, 1139–1142. [Google Scholar] [CrossRef]
- Gong, Y.; Li, S.; Wang, W.; Li, Y.; Ma, W.; Sun, S. In vitro and in vivo activity of chelerythrine against Candida albicans and underlying mechanisms. Future Microbiol. 2019, 14, 1545–1557. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Li, X.; Yin, L.; Ramage, G.; Li, B.; Tao, Y.; Zhi, Q.; Lin, H.; Zhou, Y. Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans. Microbiologyopen 2019, 8, e937. [Google Scholar] [CrossRef]
- Rho, D.; Chauret, N.; Laberge, N.; Archambault, J. Growth characteristics of Sanguinaria canadensis L. cell suspensions and immobilized cultures for production of benzophenanthridine alkaloids. Appl. Microbiol. Biotechnol. 1992, 36, 611–617. [Google Scholar] [CrossRef]
- Wright, C.S. Structural comparison of the two distinct sugar binding sites in wheat germ agglutinin isolectin II. J. Mol. Biol. 1984, 178, 91–104. [Google Scholar] [CrossRef]
- Jegal, U.; Lee, J.H.; Lee, J.; Jeong, H.; Kim, M.J.; Kim, K.H. Ultrasound-assisted gatifloxacin delivery in mouse cornea, in vivo. Sci. Rep. 2019, 9, 15532. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Zhang, J.; Wang, W.; Wang, T.; Liu, M.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and antibiofilm activities of paeoniflorin against carbapenem-resistant Klebsiella pneumoniae. J. Appl. Microbiol. 2019, 128, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Ikeh, M.A.C.; Fidel, P.L., Jr.; Noverr, M.C. Identification of specific components of the eicosanoid biosynthetic and signaling pathway involved in pathological inflammation during intra-abdominal infection with Candida albicans and Staphylococcus aureus. Infect. Immun. 2018, 86, e00144-18. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.Q.; Liu, P.; Liu, W.G.; Gao, Y.; Sun, S.J. In vitro interactions between fluconazole and minocycline against mixed cultures of Candida albicans and Staphylococcus aureus. J. Microbiol. Immunol. 2015, 48, 655–661. [Google Scholar] [CrossRef]
- Shin, D.S.; Eom, Y.B. Efficacy of zerumbone against dual-species biofilms of Candida albicans and Staphylococcus aureus. Microb. Pathog. 2019, 137, 103768. [Google Scholar] [CrossRef]
- She, P.F.; Liu, Y.Q.; Wang, Y.X.; Tan, F.; Luo, Z.; Wu, Y. Antibiofilm efficacy of the gold compound auranofin on dual species biofilms of Staphylococcus aureus and Candida sp. J. Appl. Microbiol. 2019, 128, 88–101. [Google Scholar] [CrossRef]
- Yang, X.; Sha, K.; Xu, G.; Tian, H.; Wang, X.; Chen, S.; Wang, Y.; Li, J.; Chen, J.; Huang, N. Subinhibitory concentrations of allicin decrease uropathogenic Escherichia coli (UPEC) biofilm formation, adhesion ability, and swimming motility. Int. J. Mol. Sci. 2016, 17, 979. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef]
- Lopez-Ribot, J.L. Large-scale biochemical profiling of the Candida albicans biofilm matrix: New compositional, structural, and functional insights. Mbio 2014, 5, e01781-14. [Google Scholar] [CrossRef]
- Sugimoto, S.; Sato, F.; Miyakawa, R.; Chiba, A.; Onodera, S.; Hori, S.; Mizunoe, Y. Broad impact of extracellular DNA on biofilm formation by clinically isolated methicillin-resistant and -sensitive strains of Staphylococcus aureus. Sci. Rep. 2018, 8, 2254. [Google Scholar] [CrossRef] [PubMed]
- Teirlinck, E.; Xiong, R.H.; Brans, T.; Forier, K.; Fraire, J.; Van Acker, H.; Matthijs, N.; De Rycke, R.; De Smedt, S.C.; Coenye, T.; et al. Laser-induced vapour nanobubbles improve drug diffusion and efficiency in bacterial biofilms. Nat. Commun. 2018, 9, 4518. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Alfatah, M.; Ganesan, K.; Bhattacharyya, M.S. Inhibitory effect of sophorolipid on Candida albicans biofilm formation and hyphal growth. Sci. Rep. 2016, 6, 23575. [Google Scholar] [CrossRef] [PubMed]
- Vediyappan, G.; Dumontet, V.; Pelissier, F.; d’Enfert, C. Gymnemic acids inhibit hyphal growth and virulence in Candida albicans. PLoS ONE 2013, 8, e74189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tomar, M.S.; Acharya, A. Chelerythrine delayed tumor growth and increased survival duration of Dalton’s lymphoma bearing BALB/c H(2d) mice by activation of NK cells in vivo. J. Cancer Res. Ther. 2015, 11, 904–910. [Google Scholar] [CrossRef]
- Chmura, S.J.; Dolan, M.E.; Cha, A.; Mauceri, H.J.; Kufe, D.W.; Weichselbaum, R.R. In vitro and in vivo activity of protein kinase C inhibitor chelerythrine chloride induces tumor cell toxicity and growth delay in vivo. Clin. Cancer Res. 2000, 6, 737–742. [Google Scholar]
- Kosina, P.; Walterova, D.; Ulrichova, J.; Lichnovsky, V.; Stiborova, M.; Rydlova, H.; Vicar, J.; Krecman, V.; Brabec, M.J.; Simanek, V. Sanguinarine and chelerythrine: Assessment of safety on pigs in ninety days feeding experiment. Food Chem. Toxicol. 2004, 42, 85–91. [Google Scholar] [CrossRef]









| Strain | MICS (μg/mL) | MBIC90S (μg/mL) |
|---|---|---|
| Candida albicans SC5314 | 4 | 2 |
| Staphylococcus aureus ATCC25923 | 4 | 2 |
| Dual species | 6 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, W.; Zhang, J.; Wang, W.; Liu, M.; Fu, Y.; Li, X.; Wang, T.; Li, Y. RETRACTED: Efficacy of Chelerythrine against Mono- and Dual-Species Biofilms of Candida albicans and Staphylococcus aureus and Its Properties of Inducing Hypha-to-Yeast Transition of C. albicans. J. Fungi 2020, 6, 45. https://doi.org/10.3390/jof6020045
Qian W, Zhang J, Wang W, Liu M, Fu Y, Li X, Wang T, Li Y. RETRACTED: Efficacy of Chelerythrine against Mono- and Dual-Species Biofilms of Candida albicans and Staphylococcus aureus and Its Properties of Inducing Hypha-to-Yeast Transition of C. albicans. Journal of Fungi. 2020; 6(2):45. https://doi.org/10.3390/jof6020045
Chicago/Turabian StyleQian, Weidong, Jianing Zhang, Wenjing Wang, Miao Liu, Yuting Fu, Xiang Li, Ting Wang, and Yongdong Li. 2020. "RETRACTED: Efficacy of Chelerythrine against Mono- and Dual-Species Biofilms of Candida albicans and Staphylococcus aureus and Its Properties of Inducing Hypha-to-Yeast Transition of C. albicans" Journal of Fungi 6, no. 2: 45. https://doi.org/10.3390/jof6020045
APA StyleQian, W., Zhang, J., Wang, W., Liu, M., Fu, Y., Li, X., Wang, T., & Li, Y. (2020). RETRACTED: Efficacy of Chelerythrine against Mono- and Dual-Species Biofilms of Candida albicans and Staphylococcus aureus and Its Properties of Inducing Hypha-to-Yeast Transition of C. albicans. Journal of Fungi, 6(2), 45. https://doi.org/10.3390/jof6020045





