Molecular Tracking and Remote Sensing to Evaluate New Chemical Treatments Against the Maize Late Wilt Disease Causal Agent, Magnaporthiopsis maydis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiments for Assessing Fungicide Efficiency in Controlling Late Wilt
2.1.1. The Spring 2018 Field Experiment for Assessing Azoxystrobin Spraying during and after Land Tillage
2.1.2. The Summer 2018 Field Experiment for Assessing Seed Coating and Fungicide Application by Dripline Irrigation
2.2. Remote Sensing for Evaluating the Efficacy of Treatments Based on High-Resolution Visible-Channel and Thermal Aerial Imaging of the Cornfields
2.3. Molecular Diagnosis of the Late Wilt Pathogen
2.3.1. Plant Material
2.3.2. DNA Extraction and qPCR
DNA Extraction
qPCR-Based Method
2.4. Statistical Analyses
3. Results
3.1. The Spring 2018 Field Experiment for Assessing Azoxystrobin Spraying during and after Land Tillage
3.2. The Summer 2018 Field Experiment for Assessing Seed Coating and Fungicide Application by Dripline Irrigation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samra, A.S.; Sabet, K.A.; Hingorani, M.K. A new wilt disease of maize in Egypt. Plant Dis. Rep. 1962, 46, 481–483. [Google Scholar]
- El-Shafey, H.A.; Claflin, L.E. Late Wilt; APS Press: St. Paul, MN, USA, 1999; pp. 43–44. [Google Scholar]
- Degani, O.; Movshowitz, D.; Dor, S.; Meerson, A.; Goldblat, Y.; Rabinovitz, O. Evaluating azoxystrobin seed coating against maize late wilt disease using a sensitive qPCR-based method. Plant Dis. 2019, 103, 238–248. [Google Scholar] [CrossRef] [Green Version]
- El-Naggarr, A.A.A.; Sabryr, A.M.; Yassin, M.A. Impact of late wilt disease caused by Harpophora maydis on maize yield. J. Biol. Chem. Environ. Sci. 2015, 10, 577–595. [Google Scholar]
- Tej, R.; Rodríguez-Mallol, C.; Rodríguez-Arcos, R.; Karray-Bouraoui, N.; Molinero-Ruiz, L. Inhibitory effect of lycium europaeum extracts on phytopathogenic soil-borne fungi and the reduction of late wilt in maize. Eur. J. Plant Pathol. 2018, 152, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Sabet, K.; Samra, A.; Mansour, I. Interaction between Fusarium oxysporum f. Vasinfectum and Cephalosporium maydis on cotton and maize. Ann. Appl. Biol. 1966, 58, 93–101. [Google Scholar] [CrossRef]
- Sabet, K.A.; Zaher, A.M.; Samra, A.S.; Mansour, I.M. Pathogenic behaviour of Cephalosporium maydis and c. Acremonium. Ann. Appl. Biol. 1970, 66, 257–263. [Google Scholar] [CrossRef]
- Degani, O.; Cernica, G. Diagnosis and control of Harpophora maydis, the cause of late wilt in maize. Adv. Microbiol. 2014, 4, 94–105. [Google Scholar] [CrossRef] [Green Version]
- Khokhar, M.K.; Hooda, K.S.; Sharma, S.S.; Singh, V. Post-flowering stalk rot complex of maize—Present status and future prospects. Maydica 2014, 59, 226–242. [Google Scholar]
- Degani, O.; Dor, S.; Abraham, D.; Cohen, R. Interactions between Magnaporthiopsis maydis and Macrophomina phaseolina, the causes of wilt diseases in maize and cotton. Microorganisms 2020, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Samra, A.S.; Sabet, K.A.; Hingorani, M.K. Late wilt disease of maize caused by Cephalosporium maydis. Phytopathology 1963, 53, 402–406. [Google Scholar]
- Gams, W. Phialophora and some similar morphologically little-differentiated anamorphs of divergent ascomycetes. Stud. Mycol. 2000, 187–200. [Google Scholar]
- Luo, J.; Zhang, N. Magnaporthiopsis, a new genus in magnaporthaceae (ascomycota). Mycologia 2013, 105, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Klaubauf, S.; Tharreau, D.; Fournier, E.; Groenewald, J.Z.; Crous, P.W.; de Vries, R.P.; Lebrun, M.H. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Stud. Mycol. 2014, 79, 85–120. [Google Scholar] [CrossRef]
- Saleh, A.A.; Leslie, J.F. Cephalosporium maydis is a distinct species in the gaeumannomyces-harpophora species complex. Mycologia 2004, 96, 1294–1305. [Google Scholar] [CrossRef]
- Abd El-Rahim, M.F.; Fahmy, G.M.; Fahmy, Z.M. Alterations in transpiration and stem vascular tissues of two maize cultivars under conditions of water stress and late wilt disease. Plant Pathol. 1998, 47, 216–223. [Google Scholar] [CrossRef]
- Michail, S.H.; Abou-Elseoud, M.S.; Nour Eldin, M.S. Seed health testing of corn for Cephalosporium maydis. Acta Phytopathol. Entomol. Hung. 1999, 34, 35–42. [Google Scholar]
- Drori, R.; Sharon, A.; Goldberg, D.; Rabinovitz, O.; Levy, M.; Degani, O. Molecular diagnosis for Harpophora maydis, the cause of maize late wilt in israel. Phytopathol. Mediterr. 2013, 52, 16–29. [Google Scholar]
- Shalaby, M.; El-Moghazy, S.; Mehesen, A.A. Biological control of maize late wilt disease caused by Cephalosporium maydis. J. Agric. Res. Kafrelsheikh Univ. 2009, 35, 1–19. [Google Scholar]
- Degani, O.; Goldblat, Y. Ambient stresses regulate the development of the maize late wilt causing agent, Harpophora maydis. Agric. Sci. 2014, 5, 571–582. [Google Scholar]
- El-Shafey, H.A.; El-Shorbagy, F.A.; Khalil, I.I.; El-Assiuty, E.M. Additional sources of resistance to the late-wilt disease of maize caused by Cephalosporium maydis. Agric. Res. Rev. Egypt 1988, 66, 221–230. [Google Scholar]
- Samra, A.S.; Sabet, K.A.; Abdel-Rahim, M.F. Effect of Soil Conditions and Cultural Practices on Infection with Stalk Rots; U.A.R. Ministry of Agric; Government Printing Offices: Cairo, Egypt, 1966; pp. 117–164. [Google Scholar]
- Singh, S.D.; Siradhana, B.S. Date of sowing in relation to late wilt disease of maize. Indian Phytopathol. 1988, 41, 489–491. [Google Scholar]
- Sabet, K.A.; Samra, A.S.; Mansour, I.M. Saprophytic behaviour of Cephalosporium maydis and c. Acremonium. Ann. Appl. Biol. 1970, 66, 265–271. [Google Scholar] [CrossRef]
- Dor, S.; Degani, O. Uncovering the host range for maize pathogen Magnaporthiopsis Maydis. Plants 2019, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Sahab, A.F.; Osman, A.R.; Soleman, N.K.; Mikhail, M.S. Studies on root-rot of lupin in Egypt and its control. Egypt. J. Phytopathol. 1985, 17, 23–35. [Google Scholar]
- Singh, S.; Siradhana, B. Effect of macro and micronutrients on the development of late wilt of maize induced by Cephalosporium maydis. Summa Phytopathol. 1990, 16, 140–145. [Google Scholar]
- Muhammad, S.; Amusa, N.A. In vitro inhibition of growth of some seedling blight inducing pathogens by compost-inhabiting microbes. Afr. J. Biotechnol. 2003, 2, 161–164. [Google Scholar]
- El-Assiuty, E.M.; El-Hamahmy, A.A.; El-Sharkawy, A.Y. Bacillus subtilis, pseudomonas fluorescens and verticillium tricorpus as biological agents against late-wilt of maize. Egypt. J. Appl. Sci. 1991, 6, 8245–8829. [Google Scholar]
- El-Mehalowy, A.A.; Hassanein, N.M.; Khater, H.M.; Daram El-Din, E.A.; Youssef, Y.A. Influence of maize root colonization by rhizosphere actinomycetes and yeast fungi on plant growth and on the biological control of late wilt disease. Int. J. Agric. Biol. 2004, 6, 599–605. [Google Scholar]
- Elshahawy, I.E.; El-Sayed, A.E.-K.B. Maximizing the efficacy of Trichoderma to control Cephalosporium maydis, causing maize late wilt disease, using freshwater microalgae extracts. Egypt. J. Biol. Pest Control 2018, 28, 48. [Google Scholar] [CrossRef] [Green Version]
- Fayzalla, E.; Sadik, E.; Elwakil, M.; Gomah, A. Soil solarization for controlling Cephalosporium maydis, the cause of late wilt disease of maize in Egypt. Egypt J. Phytopathol. 1994, 22, 171–178. [Google Scholar]
- Abd-el-Rahim, M.F.; Sabet, K.A.; El-Shafey, H.A.; El-Assiuty, E.M. Chemical control of the late-wilt disease of maize caused by Cephalosporium Maydis. Agric. Res. Rev. 1982, 60, 31–49. [Google Scholar]
- Degani, O.; Weinberg, T.; Graph, S. Chemical control of maize late wilt in the field. Phytoparasitica 2014, 42, 559–570. [Google Scholar] [CrossRef]
- Ortiz-Bustos, C.M.; Testi, L.; García-Carneros, A.B.; Molinero-Ruiz, L. Geographic distribution and aggressiveness of Harpophora maydis in the Iberian peninsula, and thermal detection of maize late wilt. Eur. J. Plant Pathol. 2016, 144, 383–397. [Google Scholar] [CrossRef]
- Zeller, K.A.; Abou-Serie, M.I.; El-Assuity, E.M.; Fahmy, Z.M.; Bekheet, F.M.; Leslie, J.F. Relative competitiveness and virulence of four clonal lineages of Cephalosporium maydis from Egypt toward greenhouse-grown maize. Plant Dis. 2002, 86, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Degani, O.; Dor, S.; Movshowitz, D.; Fraidman, E.; Rabinowitz, O.; Graph, S. Effective chemical protection against the maize late wilt causal agent, Harpophora maydis, in the field. PLoS ONE 2018, 13, e0208353. [Google Scholar] [CrossRef]
- Chen, A.; Orlov-Levin, V.; Meron, M. Applying high-resolution visible-channel aerial imaging of crop canopy to precision irrigation management. Agric. Water Manag. 2019, 216, 196–205. [Google Scholar] [CrossRef]
- Murray, M.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [Green Version]
- Weller, S.; Elphinstone, J.; Smith, N.; Boonham, N.; Stead, D. Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Appl. Environ. Microbiol. 2000, 66, 2853–2858. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, N.C. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S.; Movshovitz, D.; Rabinovitz, O. Methods for studying Magnaporthiopsis maydis, the maize late wilt causal agent. Agronomy 2019, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Johal, L.; Huber, D.M.; Martyn, R. Late wilt of corn (maize) pathway analysis: Intentional introduction of Cephalosporium maydis. In Pathways analysis for the introduction to the U.S. of plant pathogens of economic importance; USDA-APHIS: Riverdale Park, MD, USA, 2004. [Google Scholar]
- Fernández-Ortuño, D.; Torés, J.A.; De Vicente, A.; Pérez-García, A. Mechanisms of resistance to QOI fungicides in phytopathogenic fungi. Int. Microbiol. 2010, 11, 1–9. [Google Scholar]
- Avila-Adame, C.; Koller, W. Characterization of spontaneous mutants of Magnaporthe grisea expressing stable resistance to the qo-inhibiting fungicide azoxystrobin. Curr. Genet. 2003, 42, 332–338. [Google Scholar] [CrossRef]








| Experiment | Dates | Average Temp. | Min Temp. | Max Temp. | Average Humid. | Min Humid. | Max Humid. | Precipit-Ation |
|---|---|---|---|---|---|---|---|---|
| Assessing Azoxystrobin spraying during and after land tillage | 23 April–5 July 2018 | 25 °C | 18 °C | 36 °C | 56% | 21% | 88% | 27 mm |
| Assessing Azoxystrobin + Difenoconazole seed coating and various fungicides applied by drip irrigation | 21 June–5 September 2018 | 28 °C | 21 °C | 36 °C | 63% | 30% | 85% | 3 mm |
| Treatment | Spraying during Land Tillage (L/Hectare) | Spraying 13 DAS b (L/Hectare) | Spraying 29 DAS (L/Hectare) | Spraying 41 DAS (L/Hectare) |
|---|---|---|---|---|
| 1 – Control c | - | - | - | - |
| 2 | 5 | - | - | - |
| 3 | 10 | - | - | - |
| 4 | 15 | - | - | - |
| 5 | 20 | - | - | - |
| 6 | - | 2.5 | 2.5 | - |
| 7 | 5 | 2.5 | 2.5 | 2.5 |
| 8 | 7.5 | 2.5 | 2.5 | 2.5 |
| 9 | 12.5 | 2.5 | 2.5 | 2.5 |
| Fungicide Commercial Name and Abbreviations | Manufacturer, Supplier | Active Ingredient (Common Name) | Group Name | Chemical Group | Target Site of Action | Active Ingredient (g/L) | Applied in the Field |
|---|---|---|---|---|---|---|---|
| Amistar b (AS) | Syngenta (Basel, Switzerland), Adama Makhteshim (Airport City, Israel) | Azoxystrobin (CAS no. 131860-33-8) | QoI-fungicides (quinone outside inhibitors) | Methoxy-acrylates | Respiration C3: cytochrome bc1 (ubiquinol oxidase) at Qo site (cyt b gene) | 250 | Land tillage (5–20 L/hectare) Spraying (2.5 L/hectare × 2/3) Dripline protection (2.25 L/hectare × 3) |
| Dividend (DC) | Syngenta (Basel, Switzerland), Gadot Agro (Kidron, Israel) | Difenoconazole (CAS no. 119446-68-3) | DMI-fungicides (DeMethylation Inhibitors, SBI: Class I) | Triazoles | Sterol Biosynthesis in membranes G1: C14-demethylase in sterol biosynthesis (erg11/cyp51) | 30 | Dripline protection (2.25 L/hectare × 3) |
| Ortiva top b (AS + DC) | Syngenta (Basel, Switzerland), Adama Makhteshim (Airport City, Israel) | Azoxystrobin (CAS no. 131860-33-8) | QoI-fungicides (quinone outside inhibitors) | Methoxy-acrylates | Respiration C3: cytochrome bc1 (ubiquinol oxidase) at Qo site (cyt b gene) | 250 | Seed coating 0.002 (mL/seed) Dripline protection (2.25 L/hectare × 3), separately and in fungicide alternation |
| Difenoconazole (CAS no. 119446-68-3) | DMI-fungicides (DeMethylation Inhibitors, SBI: Class I) | Triazoles | Sterol Biosynthesis in membranes G1: C14-demethylase in sterol biosynthesis (erg11/cyp51) | 125 | |||
| Proline + Folicur b (PR + TE) | Bayer CropScience (Monheim am Rhein, Germany), Lidorr Chemicals Ltd. (Ramat Hasharon, Israel) | Prothioconazole (Proline) (CAS no. 178928-70-6) | DMI-fungicides (DeMethylation Inhibitors) (SBI: Class I) | Triazolinthiones | Sterol Biosynthesis in membranes G1: C14-demethylation in sterol biosynthesis (erg11/cyp51) | 275 | Dripline protection (2.25 L/hectare) in fungicide alternation |
| Tebuconazole (Folicur) (CAS no. 107534-96-3) | DMI-fungicides (DeMethylation Inhibitors) (SBI: Class I) | Triazoles | Sterol Biosynthesis in membranes G1: C14-demethylation in sterol biosynthesis (erg11/cyp51) | 200 | |||
| Velum + Flint b (FL + TR) | Bayer CropScience (Monheim am Rhein, Germany), Lidorr Chemicals Ltd. (Ramat Hasharon, Israel) | Fluopyram (Velum) (CAS no. 658066-35-4) | SDHI (Succinate dehydrogenase inhibitors) | Pyridinyl-ethyl-benzamides | Respiration C2: complex II: succinate-dehydrogenase | 200 | Dripline protection (2.25 L/hectare) in fungicide alternation |
| Trifloxystrobin (Flint) (CAS no. 141517-21-7) | QoI-fungicides (Quinone outside Inhibitors) | Oximino acetates | Respiration C3: complex III: cytochrome bc1 (ubiquinol oxidase) at Qo site (cyt b gene) | 500 | |||
| Signum b W.G. (BC + PS) | BASF (Ludwigshafen, Germany), Adama Agan (Ashdod, Israel) | 26.7% Boscalid (CAS no. 188425-85-6) | SDHI (Succinate dehydrogenase inhibitors) | Pyridine- carboxamides | Respiration C2: complex II: succinate-dehydrogenase | 267 | Dripline protection (2.25 L/hectare × 3) |
| 6.7% Pyraclostrobin (CAS No. 175013-18-0) | QoI-fungicides (Quinone outside Inhibitors) | Methoxy-carbamates | Respiration C3: cytochrome bc1 (ubiquinol oxidase) at Qo site (cyt b gene) | 67 |
| Pairs | Primer | Sequence | Uses | Amplifica-Tion | References |
|---|---|---|---|---|---|
| 1 | A200a-for A200a-rev | 5′-CCGACGCCTAAAATACAGGA-3′ 5′-GGGCTTTTTAGGGCCTTTTT-3′ | qPCR a | M. maydis AFLP b -derived species-specific fragment | [18] |
| 2 | Cox-F Cox-R | 5′-GTATGCCACGTCGCATTCCAGA-3′ 5′-CAACTACGGATATATAAGRRCCRRAACTG -3′ c | qPCR control | Cytochrome c oxidase (COX) gene | [40] [42] |
| Treatment (L/Hectare) | Emergence (no./m2, 17 DAS) | Yield (kg/m2, 74 DAS) | Class A (kg/m2, 74 DAS) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean c | S.E. | Mean | S.E. | Mean | S.E. | Percent | ||
| 1 | Control b | 10.8 AB | 0.58 | 2.58 A | 0.07 | 2.26 A | 0.05 | 88% |
| 2 | 5 Tillage | 11.4 AB | 1.08 | 2.63 A | 0.09 | 2.39 A | 0.08 | 91% |
| 3 | 10 Tillage | 12.4 A | 0.51 | 2.61 A | 0.16 | 2.19 A | 0.13 | 84% |
| 4 | 15 Tillage | 10.2 B | 0.73 | 2.57 A | 0.15 | 2.32 A | 0.10 | 90% |
| 5 | 20 Tillage | 11.2 AB | 0.58 | 2.57 A | 0.18 | 2.22 A | 0.15 | 86% |
| 6 | 5 Spraying | 10.4 B | 0.51 | 2.32 A | 0.09 | 2.13 A | 0.13 | 92% |
| 7 | 5 Tillage + 7.5 Spraying | 10.4 B | 0.75 | 2.67 A | 0.06 | 2.37 A | 0.12 | 89% |
| 8 | 7.5 Tillage + 7.5 Spraying | 11.8 AB | 0.58 | 2.58 A | 0.15 | 2.15 A | 0.09 | 83% |
| 9 | 12.5 Tillage + 7.5 Spraying | 11.6 AB | 0.68 | 2.73 A | 0.19 | 2.34 A | 0.17 | 86% |
| Treatment (L/Hectare) | 29 DAS (Root) | 58 DAS (Stem) | 73 DAS (Stem) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean c | S.E. | Infect. d | Mean | S.E. | Infect. | Mean | S.E. | Infect. | |
| Control b | 2.1 × 10−5 B | 1.4 × 10−5 | 20% | 1.5 × 10−4 B | 5.3 × 10−5 | 40% | 6.5 × 10−5 A | 7.1 × 10−5 | 20% |
| 5 Tillage | 1.2 × 10−3 A | 1.1 × 10−3 | 40% | 1.6 × 10−5 B | 7.1 × 10−6 | 20% | 0 A | 0 | 0% |
| 10 Tillage | 0 B | 0 | 0% | 3.1 × 10−5 B | 1.3 × 10−5 | 40% | 2.8 × 10−1 A | 2.8 × 10−1 | 60% |
| 15 Tillage | 0 B | 0 | 0% | 1.6 × 10−4 B | 5.3 × 10−5 | 40% | 9.4 × 10−4 A | 6.7 × 10−4 | 40% |
| 20 Tillage | 0 B | 0 | 0% | 7.6 × 10−5 B | 2.3 × 10−5 | 40% | 2.7 × 10−3 A | 1.1 × 10−3 | 100% |
| 5 Spraying | 0 B | 0 | 0% | 4.1 × 10−5 B | 1.8 × 10−5 | 40% | 4.6 × 10−2 A | 4.6 × 10−2 | 40% |
| 5 Tillage + 7.5 Spraying | 0 B | 0 | 0% | 1.6 × 10−4 B | 3.3 × 10−5 | 60% | 4.1 × 10−1 A | 4.1 × 10−1 | 20% |
| 7.5 Tillage + 7.5 Spraying | 0 B | 0 | 0% | 6.0 × 10−5 B | 2.7 × 10−5 | 20% | 2.2 × 10−3 A | 1.2 × 10−3 | 100% |
| 12.5 Tillage + 7.5 Spraying | 0 B | 0 | 0% | 4.6 × 10−4 A | 9.9 × 10−5 | 100% | 3.0 × 10−3 A | 2.0 × 10−3 | 60% |
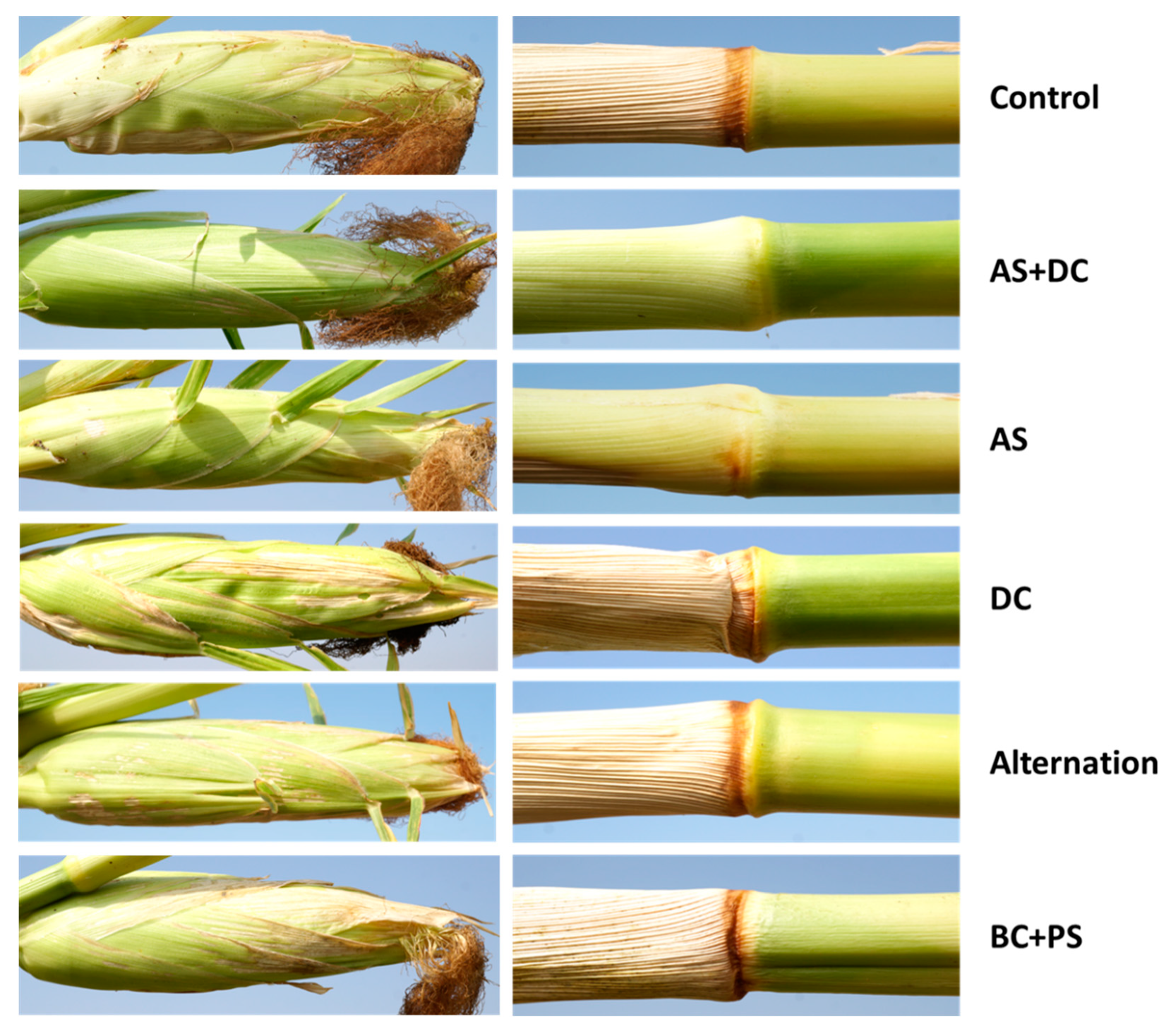
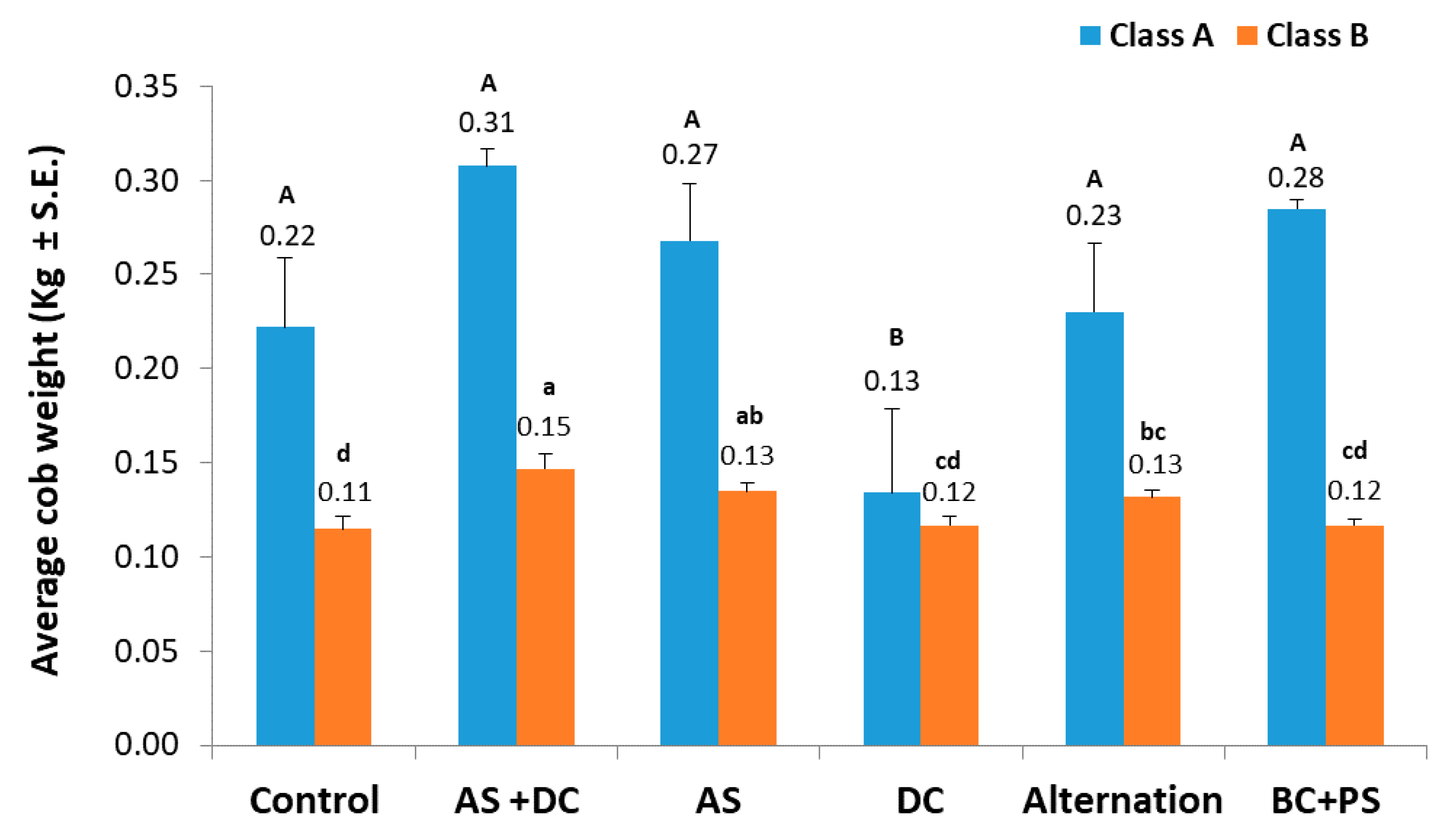
| Treatment c (L/Hectare) | Emergence (no./m2, 15 DAS) | Root Biomass (mg, 30 DAS) | Shoot Biomass (mg, 30 DAS) | |||
|---|---|---|---|---|---|---|
| Mean | S.E. | Mean | S.E. | Mean | S.E. | |
| Control b | 9.3 | 0.54 | 4.73 | 0.70 | 94.41 | 12.55 |
| AS + DC | 9.6 | 0.54 | 4.66 | 0.78 | 100.50 | 11.69 |
| AS | 9.0 | 0.56 | 5.23 | 0.81 | 105.85 | 10.21 |
| DC | 9.1 | 0.28 | 6.47 | 1.01 | 105.66 | 9.07 |
| Alternation | 9.6 | 0.40 | 5.09 | 1.25 | 89.94 | 13.18 |
| BC + PS | 8.9 | 0.41 | 5.96 | 1.00 | 97.46 | 15.15 |
| Treatment c (L/Hectare) | 31 DAS (Root) | 58 DAS (Stem) | 71 DAS (Stem) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | S.E. | Infect. | Mean | S.E. | Infect. | Mean | S.E. | Infect. | |
| Control | 0 | 0 | 0% | 3.5 × 10−5 | 2.2 × 10−5 | 30% | 0.024 | 0.013 | 80% |
| AS + DC | 1.7 × 10−4 | 1.7 × 10−4 | 20% | 2.0 × 10−3 | 2.0 × 10−3 | 20% | 0.053 | 0.042 | 40% |
| AS | 1.4 × 10−6 | 1.4 × 10−6 | 10% | 9.7 × 10−5 | 3.9 × 10−5 | 70% | 0.016 | 0.011 | 70% |
| DC | 0 | 0 | 0% | 3.2 × 10−5 | 1.4 × 10−5 | 50% | 2.079 | 2.064 | 80% |
| Alternation | 0 | 0 | 0% | 3.3 × 10−5 | 1.3 × 10−5 | 70% | 0.001 | 0.001 | 60% |
| BC + PS | 2.0 × 10−4 | 1.4 × 10−4 | 40% | 9.4 × 10−4 | 8.5 × 10−4 | 70% | 0.589 | 0.780 | 100% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degani, O.; Dor, S.; Chen, A.; Orlov-Levin, V.; Stolov-Yosef, A.; Regev, D.; Rabinovitz, O. Molecular Tracking and Remote Sensing to Evaluate New Chemical Treatments Against the Maize Late Wilt Disease Causal Agent, Magnaporthiopsis maydis. J. Fungi 2020, 6, 54. https://doi.org/10.3390/jof6020054
Degani O, Dor S, Chen A, Orlov-Levin V, Stolov-Yosef A, Regev D, Rabinovitz O. Molecular Tracking and Remote Sensing to Evaluate New Chemical Treatments Against the Maize Late Wilt Disease Causal Agent, Magnaporthiopsis maydis. Journal of Fungi. 2020; 6(2):54. https://doi.org/10.3390/jof6020054
Chicago/Turabian StyleDegani, Ofir, Shlomit Dor, Assaf Chen, Valerie Orlov-Levin, Avital Stolov-Yosef, Danielle Regev, and Onn Rabinovitz. 2020. "Molecular Tracking and Remote Sensing to Evaluate New Chemical Treatments Against the Maize Late Wilt Disease Causal Agent, Magnaporthiopsis maydis" Journal of Fungi 6, no. 2: 54. https://doi.org/10.3390/jof6020054
APA StyleDegani, O., Dor, S., Chen, A., Orlov-Levin, V., Stolov-Yosef, A., Regev, D., & Rabinovitz, O. (2020). Molecular Tracking and Remote Sensing to Evaluate New Chemical Treatments Against the Maize Late Wilt Disease Causal Agent, Magnaporthiopsis maydis. Journal of Fungi, 6(2), 54. https://doi.org/10.3390/jof6020054






