Basidiospores from Wood-Decay Fungi Transform Laccase Substrates in the Absence of Glucose and Nitrogen Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Spore Sources
2.2. Spore Sampling and Washing
2.3. Spore Incubation
2.4. Spore Cracking
2.5. Spectrophotometric Assays with Chromogenic Enzyme Substrates
2.6. Protein and Rest Glucose
2.7. Pentose and Hexose Sugars
2.8. Total Phenols
2.9. HPLC-MS Analysis of Guaiacol Transformation Products
2.10. HPLC-MS/MS Analysis of Carboxylic Acids
2.11. Spore Mineral Content
2.12. Statistical Treatments
3. Results
3.1. In- and Outside Concentrations of Major Organic Spore Constituents
3.2. Concentrations of Enzyme-Associated, Essential Minerals in Spores
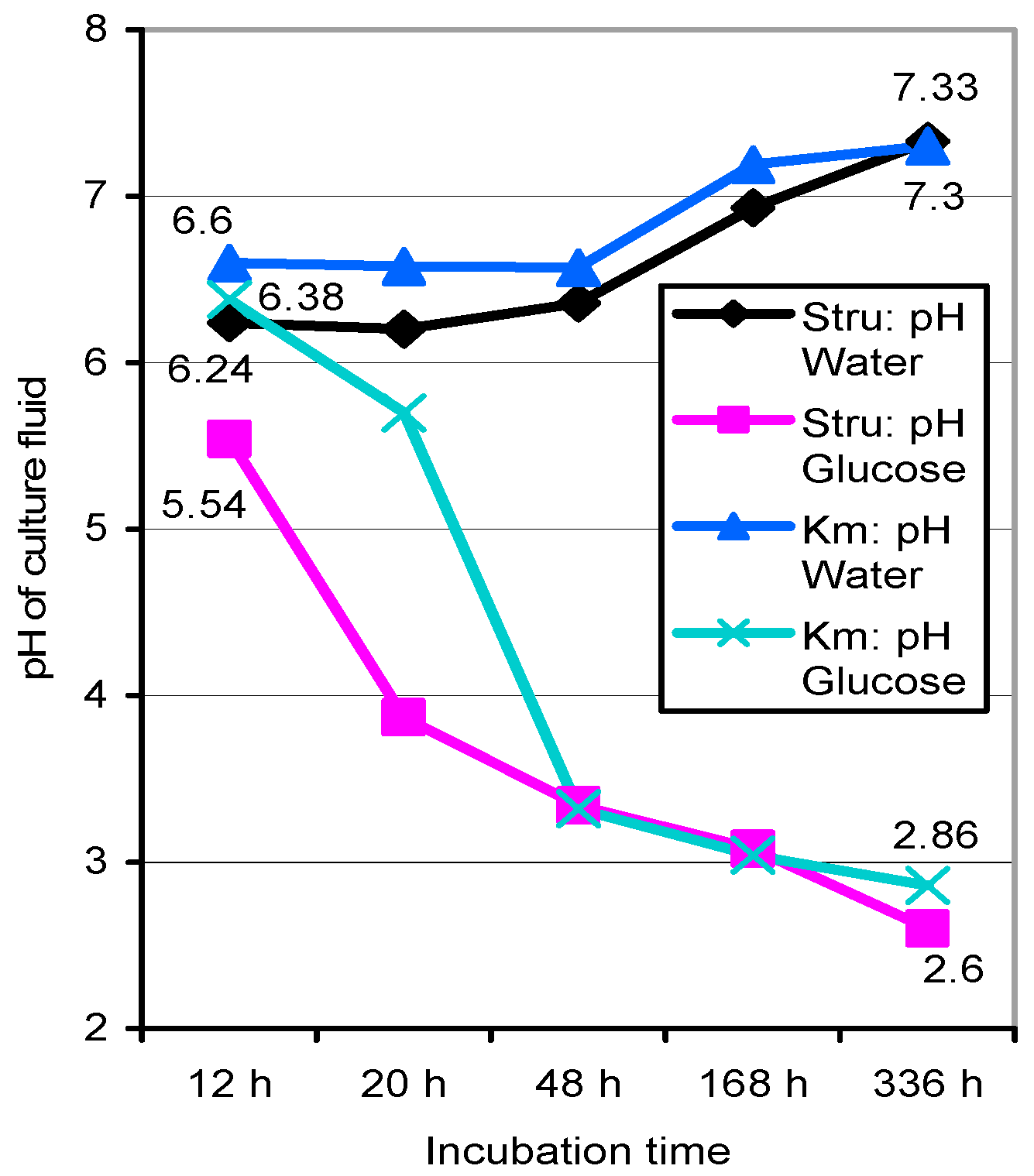
3.3. Basidiospore Germination under Nutrient-Limited Conditions in 1-cm Cuvettes
3.4. Release of Carboxylic Acids by Pre-Germination Spores in Cuvettes
3.5. Oxidation of Guaiacol by Basidiospore Suspensions of K. mutabilis in Erlenmeyer Flasks
3.6. Oxidation of ABTS by Basidiospore Suspensions of K. mutabilis in Erlenmeyer Flasks
3.7. HPLC-MS Analysis of Guaiacol Oligomers in Cuvette Cultures of K. mutabilis Spores
4. Discussion
4.1. Organic Resources and Protective Substances of Basidiospores
4.2. Polyphenoloxidases and Their Metal Co-Factors in Resting Spores
4.3. Early Metabolic Activities of Basidiospores and Their Partial Germination
4.4. Expression of Exo-Oxidoreductases Was Confined to the Germinating K. mutabilis Basidiospore
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zamoum, M.; Goudjal, Y.; Sabaou, N.; Mathieu, F.; Zitouni, A. Development of formulations based on Streptomyces rochei strain PTL2 spores for biocontrol of Rhizoctonia solani damping-off of tomato seedlings. Biocontrol Sci. Technol. 2017, 27, 723–738. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Larroche, C.; Pandey, A. Production of spores. In Current Developments in Solid-State Fermentation; Pandey, A., Soccol, C.R., Larroche, C., Eds.; Springer Asiatech Publishers, Inc.: New Delhi, India, 2008; pp. 230–252. [Google Scholar]
- Charudattan, R. Biological control of weeds by means of plant pathogens: Significance for integrated weed management in modern agro-ecology. BioControl 2001, 46, 229–260. [Google Scholar] [CrossRef]
- Virtanen, V.; Nyyssölä, A.; Vuolanto, A.; Leisola, M.; Seiskari, P. Bioreactor for solid-state cultivation of Phlebiopsis gigantea. Biotechnol. Lett. 2008, 30, 253–258. [Google Scholar] [CrossRef] [PubMed]
- El-Bendary, M.A. Production of mosquitocidal Bacillus sphaericus by solid state fermentation using agricultural wastes. World J. Microbiol. Biotechnol. 2010, 26, 153–159. [Google Scholar] [CrossRef]
- Sansinenea, E.; Ortiz, A. Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 2011, 33, 1523–1538. [Google Scholar] [CrossRef]
- Van Breukelen, F.R.; Haemers, S.; Wijffels, R.H.; Rinzema, A. Bioreactor and substrate selection for solid-state cultivation of the malaria mosquito control agent Metarhizium anisopliae. Process. Biochem. 2011, 46, 751–757. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Jambhulkar, H.P. Phytoremediation of coal mine spoil dump through integrated biotechnological approach. Biores. Technol. 2008, 99, 4732–4741. [Google Scholar] [CrossRef]
- Ram, L.C.; Srivastava, N.K.; Jha, S.K.; Sinha, A.K.; Masto, R.E.; Selvi, V.A. Management of lignite fly ash for improving soil fertility and crop productivity. Environ. Manag. 2007, 40, 438–452. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Teixeira, J.A. Lignocellulose as raw material in fermentation processes. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2010; pp. 897–909. [Google Scholar]
- Tengerdy, R.P.; Szakacs, G. Bioconversion of lignocellulose in solid substrate fermentation. Biochem. Eng. J. 2003, 13, 169–179. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.-T. Solid state fermentation and its applications. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 465–489. [Google Scholar]
- Larroche, C.; Gros, J.B. Special transformation processes using fungal spores and immobilized cells. Adv. Biochem. Eng. Biotechnol. 1997, 55, 179–220. [Google Scholar] [PubMed]
- Ruch, D.G.; Motta, J.J. Ultrastructure and cytochemistry of dormant basidiospores of Psilocybe cubensis. Mycologia 1987, 79, 387–398. [Google Scholar] [CrossRef]
- Ruch, D.G.; Burton, K.W.; Ingram, L.A. Occurrence of the glyoxylate cycle in basidiospores of homobasidiomycetes. Mycologia 1991, 83, 821–825. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Tereshina, V.M.; Garibova, L.V.; Zav’yalova, L.A.; Memorskaya, A.S.; Maryshova, N.S. Germination of basidiospores of Agaricus bisporus. Appl. Biochem. Microbiol. 2004, 40, 186–191. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferriera, I.C.F.R. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef]
- Narvaes da Rocha Campos, A.; Dutra Costa, M. Histochemistry and storage of organic compounds during basidiosporogenesis in the ectomycorrhizal fungus Pisolithus microcarpus. World J. Microbiol. Biotechnol. 2010, 26, 1745–1753. [Google Scholar] [CrossRef]
- Hayer, K.; Stratford, M.; Archer, D.B. Structural features of sugars that trigger or support conidial germination in the filamentous fungus Aspergillus niger. Appl. Environ. Microbiol. 2013, 79, 6924–6931. [Google Scholar] [CrossRef]
- Krijgsheld, P.; Bleichrodt, R.; Van Veluw, G.J.; Wang, F.; Muller, W.H.; Dijksterhuis, J.; Wösten, H.A.B. Development in Aspergillus. Stud. Mycol. 2013, 74, 1–29. [Google Scholar] [CrossRef]
- Mengel, K. Ernährung und Stoffwechsel der Pflanze, 7th ed.; Gustav Fischer: Jena, Germany, 1991. [Google Scholar]
- Bachofen, R.; Rast, D. Carboxylierungs-reaktionen in Agaricus bisporus. III. Pyruvat und Phosphoenolpyruvat als C02-Acceptoren. Arch. Microbiol. 1968, 60, 217–234. [Google Scholar]
- Mog, T.P.; Morton, H.L. Carbon dioxide stimulates germination of basidiospores of Polyporus dryophilus and Fomes rimosus. Phytopathology 1970, 60, 1305. [Google Scholar]
- Harman, G.E.; Mattick, L.R.; Nash, G.; Nedrow, B.L. Stimulation of fungal spore germination and inhibition of sporulation in fungal vegetative thalli by fatty acids and their volatile peroxidation products. Canad. J. Bot. 1980, 58, 1541–1547. [Google Scholar] [CrossRef]
- Brown, T.S., Jr.; Merrill, W. Germination of basidiospores of Fomes applanatus. Phytopathology 1973, 63, 547–550. [Google Scholar] [CrossRef]
- Deising, H.; Nicholson, R.L.; Haug, M.; Howard, R.J.; Mengden, K. Adhesion pad formation and the involvement of cutinase and esterases in the attachment of uredospores to the host cuticle. Plant. Cell 1992, 4, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Money, N.P. Mechanics of invasive fungal growth and the significance of turgor in plant infection. In Molecular Genetics of Host-Specific Toxins in Plant Disease; Kohmoto, K., Yoder, O.C., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1998; pp. 261–271. [Google Scholar]
- Moreau, R.A.; Seibles, T.S. Production of extracellular enzymes by germinating cysts of Phytophthora infestans. Canad. J. Bot. 1985, 63, 1811–1816. [Google Scholar] [CrossRef]
- Hyde, J.M.; Walkinshaw, C.H. Ultrastructure of basidiospores and mycelium of Lenzites saepiaria. J. Bacteriol. 1966, 92, 1218. [Google Scholar] [CrossRef]
- Scheld, H.W.; Perry, J.J. Basidiospore germination in the wooddestroying fungus Lenzites saepiaria. J. Gen. Microbiol. 1970, 60, 9–21. [Google Scholar] [CrossRef][Green Version]
- Passardi, F.; Bakalovic, N.; Teixeira, F.K.; Margis-Pinheiro, M.; Penel, C.; Dunand, C. Prokaryotic origins of the non-animal peroxidase superfamily and organelle-mediated transmission to eukaryotes. Genomics 2007, 89, 567–579. [Google Scholar] [CrossRef]
- Lu, Y.; Yeung, N.; Sieracki, N.; Marshall, N.M. Design of functional metalloproteins. Nature 2009, 460, 855–862. [Google Scholar] [CrossRef]
- Waldron, K.J.; Robinson, N.J. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 2009, 7, 25–35. [Google Scholar] [CrossRef]
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thornton, J.M. Metal ions in biological catalysis: From enzyme databases to general principles. J. Biol. Inorg. Chem. 2008, 13, 1205–1218. [Google Scholar] [CrossRef]
- Waldron, K.J.; Rutherford, J.C.; Ford, D.; Robinson, N.J. Metalloproteins and metal sensing. Nature 2009, 460, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G. Potential contributions of oxidoreductases from alfalfa plants to soil enzymology and biotechnology: A review. J. Nat. Sci. Sust. Technol. 2012, 6, 169–223. [Google Scholar]
- Hofrichter, M.; Ullrich, R.; Pecyna, M.J.; Liers, C.; Lundell, T. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. 2010, 87, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef] [PubMed]
- Nikolaivitis, E.; Dimarogona, M.; Karagiannaki, I.; Chalima, A.; Fishman, A.; Topakasa, E. Versatile fungal polyphenol oxidase with chlorophenol bioremediation potential: Characterization and protein engineering. Appl. Environ. Microbiol. 2018, 84, e01628-18. [Google Scholar] [CrossRef] [PubMed]
- Hölker, U.; Dohse, J.; Höfer, M. Extracellular laccases in ascomycetes Trichoderma atroviride and Trichoderma harzianum. Folia Microbiol. 2002, 47, 423–427. [Google Scholar] [CrossRef]
- Gramss, G. Kuehneromyces mutabilis. In The Biology and Cultivation of Edible Mushrooms; Chang, S.T., Hayes, W.A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 423–443. [Google Scholar]
- Gramss, G.; Voigt, K.-D. Clues for regulatory processes in fungal uptake and transfer of minerals to the basidiospore. Biol. Trace Elem. Res. 2013, 154, 140–149. [Google Scholar] [CrossRef]
- Günther, T.; Sack, U.; Hofrichter, M.; Lätz, M. Oxidation of PAH and PAH-derivatives by fungal and plant oxidoreductases. J. Basic Microbiol. 1998, 38, 113–122. [Google Scholar] [CrossRef]
- Sterjiades, R.; Dean, J.F.D.; Eriksson, K.-E.L. Laccase from sycamore maple (Acer pseudoplatanus) polymerizes monolignols. Plant. Physiol. 1992, 99, 1162–1168. [Google Scholar] [CrossRef]
- Givaudan, A.; Effosse, A.; Faure, D.; Potier, P.; Bouillant, M.-L.; Bally, R. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: Evidence for laccase activity in nonmotile strains of Azospirillum lipoferum. FEMS Microbiol. Lett. 1993, 108, 205–210. [Google Scholar] [CrossRef]
- Tremolieres, M.; Bieth, J.G. Isolation and characterization of the polyphenoloxidase from senescent leaves of black poplar. Phytochemistry 1984, 23, 501–505. [Google Scholar] [CrossRef]
- Périé, F.H.; Gold, M.H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl. Environ. Microbiol. 1991, 57, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Wariishi, H.; Valli, K.; Gold, M.H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J. Biol. Chem. 1992, 267, 23688–23695. [Google Scholar]
- Gramss, G. Activity of oxidative enzymes in fungal mycelia from grassland and forest soils. J. Basic Microbiol. 1997, 37, 407–423. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Wackett, L.P.; Gibson, D.T. Rapid method for detection and quantitation of hydroxylated aromatic intermediates produced by microorganisms. Appl. Environ. Microbiol. 1983, 45, 1144–1147. [Google Scholar] [CrossRef]
- Harkin, J.M.; Larsen, M.J.; Obst, J.R. Use of syringaldazine for detection of laccase in sporophores of wood rotting fungi. Mycologia 1974, 66, 469–476. [Google Scholar] [CrossRef]
- Gramss, G.; Voigt, K.-D. Regulation of heavy metal concentrations in cereal grains from uranium mine soils. Plant. Soil 2013, 364, 105–118. [Google Scholar] [CrossRef]
- Gramss, G.; Voigt, K.-D. Stability of the inherent target metallome in seed crops and a mushroom grown on soils of extreme mineral spans. Agronomy 2016, 6, 14. [Google Scholar] [CrossRef]
- Schachtschabel, P.; Blume, H.-P.; Brümmer, G.; Hartge, K.H.; Schwertmann, U. Lehrbuch der Bodenkunde, 14th ed.; Enke: Stuttgart, Germany, 1998. [Google Scholar]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim. Biophys. Acta 1998, 1379, 381–390. [Google Scholar] [CrossRef]
- Venkatasubramanian, L.; Maruthamuthu, P. Kinetics and mechanism of formation and decay of 2,2′-azinobis-(3-ethyl-benzothiazole-6-sulphonate) radical cation in aqueous solution by inorganic peroxides. Int. J. Chem. Kinet. 1989, 21, 399–421. [Google Scholar] [CrossRef]
- Gierer, J.; Opara, A.E. Studies on the enzymatic degradation of lignin. The action of peroxidase and laccase on monomeric and dimeric model compounds. Acta Chem. Scand. 1973, 27, 2909–2922. [Google Scholar] [CrossRef]
- Schmalzl, K.J.; Forsyth, C.M.; Evans, P.D. The reaction of guaiacol with iron(III) and chromium (VI) compounds as a model for wood surface modification. Wood Sci. Technol. 1995, 29, 307–319. [Google Scholar] [CrossRef]
- Schmalzl, K.J.; Forsyth, C.M.; Evans, P.D. Evidence for the formation of chromium (III) diphenoquinone complexes during oxidation of guaiacol and 2,6-dimethoxyphenol with chromic acid. Polymer Degrad. Stabil. 2003, 82, 399–407. [Google Scholar] [CrossRef]
- Simmons, K.E.; Minard, R.D.; Bollag, J.-M. Oxidative coupling and polymerization of guaiacol, a lignin derivative. Soil Sci. Soc. Am. J. 1988, 52, 1356–1360. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- López-Serrano, D.; Solano, F.; Sanchez-Amat, A. Identification of an operon involved in tyrosinase activity and melanin synthesis in Marinomonas mediterranea. Gene 2004, 342, 179–187. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 1–12. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Seguin, P.; Ahn, J.-K.; Kim, J.-J.; Chun, S.-C.; Kim, E.-H.; Seo, S.-H.; Kang, E.-Y.; Kim, S.-L.; Park, Y.-J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.L. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Ferreira, M.-J.; Queiros, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Faure, D.; Bouillant, M.L.; Bally, R. Isolation of Azospirillum lipoferum 4T Tn5 mutants affected in melanization and laccase activity. Appl. Environ. Microbiol. 1994, 60, 3413–3415. [Google Scholar] [CrossRef]
- Hullo, M.-F.; Moszer, I.; Danchin, A.; Martin-Verstraete, I. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 2001, 183, 5426–5430. [Google Scholar] [CrossRef]
- Clutterbuck, A.J. Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J. Gen. Microbiol. 1972, 70, 423–435. [Google Scholar] [CrossRef]
- Sugareva, V.; Härtl, A.; Brock, M.; Hübner, K.; Rohde, M.; Heinekamp, T.; Brakhage, A.A. Characterisation of the laccase-encoding gene abr2 of the dihydroxynaphthalene-like melanin gene cluster of Aspergillus fumigatus. Arch. Microbiol. 2006, 186, 345–355. [Google Scholar] [CrossRef]
- Martins, L.O.; Soares, C.M.; Pereira, M.M.; Teixeira, M.; Costa, T.; Jones, G.H.; Henriques, A.O. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 2002, 277, 18849–18859. [Google Scholar] [CrossRef]
- Barekzai, A.; Mengel, K. Effect of microbial decomposition of mature leaves on soil pH. J. Plant. Nutr. Soil Sci. 1993, 156, 93–94. [Google Scholar] [CrossRef]
- Perez, J.; Jeffries, T.W. Roles of manganese and organic acid chelators in regulating lignin degradation and biosynthesis of peroxidases by Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1992, 58, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Voigt, K.-D.; Bublitz, F.; Bergmann, H. Increased solubility of (heavy) metals in soil during microbial transformations of sucrose and casein amendments. J. Basic. Microbiol. 2003, 43, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, D.; Salzmann, M.; Stephan, D. Enzyme Handbook; Springer: Berlin, Germany, 1994; Volume 1–10. [Google Scholar]
- Bhat, T.K.; Singh, B.; Sharma, O.P. Microbial degradation of tannins—A current perspective. Biodegradation 1998, 9, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Field, J.A.; Lettinga, G. Biodegradation of tannins. In Metal Ions in Biological Systems. Degradation of Environmental Pollutants by Microorganisms and their Metalloenzymes; Sigel, H., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1992; Volume 28, pp. 61–97. [Google Scholar]
- Gramss, G.; Voigt, K.-D.; Bergmann, H. Mobilization of hazardous metals by plants growing in soils from uranium mining. In Uranium in the Aquatic Environment, Proceedings of the International Conference Uranium Mining and Hydrology III and the International Mine Water Association Symposium, Freiberg, Germany, 15–21 September 2002; Merkel, B.J., Planer-Friedrich, B., Wolkendorfer, C., Eds.; Springer: Berlin, Germany, 2002; pp. 521–528. [Google Scholar]
- Piccolo, A.; Conte, P.; Spaccini, R.; Chiarella, M. Effects of some dicarboxylic acids on the association of dissolved humic substances. Biol. Fertil. Soils 2003, 37, 255–259. [Google Scholar] [CrossRef]
- Belcarz, A.; Ginalska, G.; Kornillowicz-Kowalska, T. Extracellular enzyme activities of Bjerkandera adusta R59 soil strain, capable of daunomycin and humic acids degradation. Appl. Microbiol. Biotechnol. 2005, 68, 686–694. [Google Scholar] [CrossRef]
- Rabinovich, M.L.; Bolobova, A.V.; Vasil’chenko, L.G. Fungal decomposition of natural aromatic structures and xenobiotics: A review. Appl. Biochem. Microbiol. 2004, 40, 1–17. [Google Scholar] [CrossRef]
- Waites, M.J.; Morgan, N.L.; Rockey, J.S.; Higton, A.G. Industrial Microbiology. An Introduction; Blackwell Publishing: Oxford, UK, 2001. [Google Scholar]
- Gramss, G. Aspects determining the dominance of Fomitopsis pinicola in the colonization of deadwood and the role of the pathogenicity factor oxalate. Forests 2020, 11, 290. [Google Scholar] [CrossRef]
- Gramss, G.; Kirsche, B.; Voigt, K.-D.; Günther, T.; Fritsche, W. Conversion rates of five polycyclic aromatic hydrocarbons in liquid cultures of fifty-eight fungal species and the concomitant production of oxidative enzymes. Mycol. Res. 1999, 103, 1009–1018. [Google Scholar] [CrossRef]
- Umezawa, T.; Higuchi, T. Mechanism of aromatic ring cleavage of β-O-4 lignin substructure models by lignin peroxidase. FEBS Lett. 1987, 218, 255–260. [Google Scholar] [CrossRef]
- Jung, D. Immobilization of Enzymes on Mesoporous Supports and Their Application in Continuous-Flow Biotransformations. Ph.D. Thesis, University Erlangen-Nürnberg, Erlangen, Germany, 2012. [Google Scholar]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase–mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Gramss, G. Reappraising a controversy: Formation and role of the azodication (ABTS2+) in the laccase-ABTS catalyzed breakdown of lignin. Fermentation 2017, 3, 27. [Google Scholar] [CrossRef]



| Medium | Ca | Cu | Fe | Mn |
|---|---|---|---|---|
| Bideionized water | <10 | 0.39 ± 0.12 | 0.12 ± 0.04 | 0.01 ± 0.01 |
| Glucose 5 g L−1 | 34 ± 7 | 1.3 ± 0.32 | 0.27 ± 0.08 | 0.13 ± 0.04 |
| Guaiacol 133 mg L−1 | 34 ± 4 | 1.4 ± 0.12 | 0.45 ± 014 | 0.08 ± 0 |
| ABTS 224 mg L−1 | 64 ± 1 | 1.8 ± 0 | 0.61 ± 0.02 | 0.34 ± 0.06 |
| Spore suspension a | 111 ± 16 | 2.4 ± 0.92 | 3.8 ± 1.2 | 1.66 ± 0.12 |
| Species | Extract | Laccase | Protein | Carbohydrate | Total Phenol c |
|---|---|---|---|---|---|
| Stropharia | 1. Wash | 32.8 ± 3.90 | 21 ± 99 | 7270 ± 33 a | 18.5 ± 2.1 |
| 2. Wash | 7.38 ± 2.56 | ND | ND | 18.8 ± 3.6 | |
| 3. Wash | 1.34 ± 1.34 | ND | ND | 13.3 ± 1.8 | |
| 4. Wash | 0.28 ± 0.20 | ND | ND | 10.9 ± 0.1 | |
| Total 1–4 | 41.7 ± 4.9 | 21 ± 99 | 7270 ± 33 a | 61.5 ± 4.6 | |
| Inside concentr. | 688 ± 84 | 12,600 ± 405 | 160,000 ± 2670 a 96,600 ± 1200 b | 452 ± 53 | |
| Kuehneromyces | 1. Wash | 0.15 ± 0.15 | 168 ± 121 | 9730 a | 68.3 ± 6.3 |
| 2. Wash | ND | ND | ND | 73.6 ± 6.9 | |
| 3. Wash | ND | ND | ND | 36.9 ± 2.5 | |
| 4. Wash | ND | ND | ND | 40.2 ± 0.5 | |
| Total 1–4 | 0.15 ± 0.15 | 168 ± 121 | 9730 a | 219 ± 9.7 | |
| Inside concentr. | 0.30 ± 0.03 | 924 ± 49 | 15,130 ± 490 a 6750 ± 56 b | 767 ± 27 |
| Element | Stropharia | Kuehneromyces | Presence in Enzymes a | Concentration Ranges in Whole Wheat Grains b |
|---|---|---|---|---|
| Ca | 755 ± 44 | 277 ± 19 | 2 | 280–540 |
| Cd | 0.860 ± 0.012 | 0.850 ± 0.003 | (1 enzyme) | 0.02–3 (0.05–0.4) |
| Co | 0.094 ± 0.002 | 0.705 ± 0.006 | 1 | <0.03 |
| Cu | 20.3 ± 0.3 | 24 ± 0.6 | 1 | 11–14 (2–20) |
| Fe | 385 ± 5 | 199 ± 4 | 8 | 40–64 |
| K | 6200 ± 42 | 8160 ± 32 | 0.5 | 4100–6500 |
| Mg | 640 ± 4 | 1140 ± 3 | 16 | 1160–1700 |
| Mn | 28 ± 0.2 | 73.5 ± 1.2 | 6 | 18–34 (14–30) |
| Na | 304 ± 4 | 92 ± 6 | 0.5 | 30–50 |
| Ni | 3.43 ± 0.32 | 13 ± 0.07 | 0.5 | 0.18–1.4 (0.1–3) |
| P | 5470 ± 64 | 5150 ± 43 | 4000–5300 | |
| Zn | 79 ± 2 | 75 ± 0.8 | 9 | 35–190 (10–100) |
| Medium | Treatment | Stropharia | Kuehneromyces |
|---|---|---|---|
| Glucose solution | Unwashed | On spore deposits, vertical hyphae 1.5–3 mm, and floating mycelia up to 3 mm; Mrel = 1 | On spore deposits, vertical hyphae 1–1.5 mm, and floating mycelia up to 1.5 mm; Mrel = 1 |
| Washed | Vertical hyphae 0.7 mm, no floating mycelia; Mrel = 0.2. Both germination rates around 35%; rest glucose, 4.5 g L−1 | Vertical hyphae 0.5–1 mm and traces of floating mycelia; Mrel = 0.25. Both germination rates around 35%; rest glucose, 4.5 g L−1 | |
| Water | Unwashed | <1‰ of spores with germ tubes 2.5 (to 25) µm; Mrel = 1 | <<1‰ of spores with germ tubes 2.5 µm |
| Washed | <1‰ of spores with germ tubes 2.5 (to 25) µm; Mrel = 0.6 | No germinating spores |
| Carboxylic Acids | In Glucose Solution | In the First Wash |
|---|---|---|
| Aliphatic acids | ||
| Citric | 766 | ND |
| Fumaric | 1340 | ND |
| Glycolic | 150 | ND |
| Malic | 1800 | ND |
| Malonic | 1340 | ND |
| Succinic | 8415 | 230 |
| Tartaric | 105 | ND |
| Aromatic acids | ||
| Benzoic | <15 | 4.4 |
| Gallic | <15 | <5.6 |
| Spore Incubation Medium Amended with (L−1) | Relative Mycelial Quantity | Plateau Concentr. of Product µM | Enzymatic ABTS Oxidation in µM Per53 h b Per Min a | Protein Released g kg−1 Spores | Rest Glucose g L−1 | Mean Final pH of Spore Medium | |
|---|---|---|---|---|---|---|---|
| Spore load 3–4 mg per flask (0.5–0.67 g L−1) | |||||||
| Glucose 5 g | 1 | 3.4–35 | 0.6–1.7 2 | 2.5–3 2 | 2.6 | ||
| Guaiacol 133 mg | 1.3 | 64–133 1 | 0.7–1.3 1 | 4.4–5.3 1 | 0 1 | 2.8 | |
| Glucose/guaiacol | 0.8–1.7 | 16–25 2 | 6.6 | 0.14 2 | 1.8–3.0 2 | 3.5–3.8 2 | 2.7 |
| Spore load < 0.3 mg per flask (<0.05 g L−1) | |||||||
| ABTS 224 mg | <0.1 | 1.9–47 | ND | ND | ND | 0 | |
| Glucose/ABTS | <0.1 | 2.3–67 | ND | ND | ND | 4.5–5 | |
| Glucose 5 g | 2 c | 2.3–6.1 c | 3.8 c | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramss, G.; Voigt, K.-D. Basidiospores from Wood-Decay Fungi Transform Laccase Substrates in the Absence of Glucose and Nitrogen Supplements. J. Fungi 2020, 6, 62. https://doi.org/10.3390/jof6020062
Gramss G, Voigt K-D. Basidiospores from Wood-Decay Fungi Transform Laccase Substrates in the Absence of Glucose and Nitrogen Supplements. Journal of Fungi. 2020; 6(2):62. https://doi.org/10.3390/jof6020062
Chicago/Turabian StyleGramss, Gerhard, and Klaus-Dieter Voigt. 2020. "Basidiospores from Wood-Decay Fungi Transform Laccase Substrates in the Absence of Glucose and Nitrogen Supplements" Journal of Fungi 6, no. 2: 62. https://doi.org/10.3390/jof6020062
APA StyleGramss, G., & Voigt, K.-D. (2020). Basidiospores from Wood-Decay Fungi Transform Laccase Substrates in the Absence of Glucose and Nitrogen Supplements. Journal of Fungi, 6(2), 62. https://doi.org/10.3390/jof6020062





