Systemic Colonization by Metarhizium robertsii Enhances Cover Crop Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Inoculum
2.2. Seed Surface Sterilization and Inoculation
2.3. Plant Growth and Experimental Design
2.4. Evaluation of Plant Endophytic Colonization
2.5. Plant Response to Inoculation with M. robertsii
2.6. Statistical Analyses
3. Results
3.1. Endophytic Colonization of Plants and Tissue Location
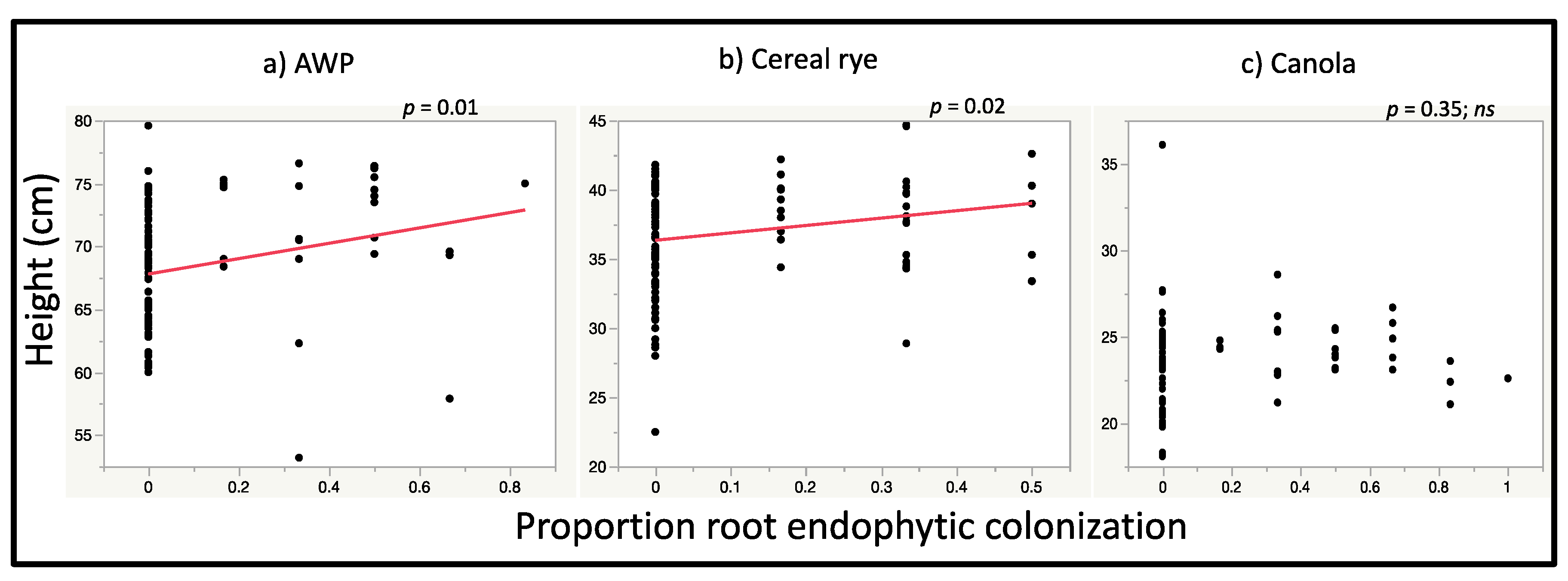
3.2. Plant Growth Response to Seed Inoculation with M. robertsii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- St. Leger, R.J. Studies on adaptations of Metarhizium anisopliae to life in the soil. J. Invertebr. Pathol. 2008, 98, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Pava-Ripoll, M.; Angelini, C.; Fang, W.; Wang, S.; Posada, F.J.; St. Leger, R.J. The rhizosphere-competent entomopathogen Metarhizium anisopliae expresses a specific subset of genes in plant root exudate. Microbiology 2011, 157, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raya-Diaz, S.; Sanchez-Rodriguez, A.R.; Segura-Fernandez, J.M.; del Campillo, M.D.; Quesada-Moraga, E. Entomopathogenic fungi-based mechanisms for improved Fe nutrition in sorghum plants grown on calcareous substrates. PLoS ONE 2017, 12, e0185903. [Google Scholar] [CrossRef] [Green Version]
- Behie, S.W.; Moreira, C.C.; Sementchoukova, I.; Barelli, L.; Zelisko, P.M.; Bidochka, M.J. Carbon translocation from a plant to an insect-pathogenic endophytic fungus. Nat. Commun. 2017, 8, 14245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behie, S.W.; Zelisko, P.M.; Bidochka, M.J. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 2012, 336, 1576–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaber, L.R. Grapevine leaf tissue colonization by the fungal entomopathogen Beauveria bassiana s.l. and its effect against downy mildew. BioControl 2015, 60, 103–112. [Google Scholar] [CrossRef]
- Jaber, L.R.; Enkerli, J. Fungal entomopathogens as endophytes: Can they promote plant growth? Biocontrol Sci. Technol. 2017, 27, 28–41. [Google Scholar] [CrossRef]
- Sasan, R.K.; Bidochka, M.J. Antagonism of the endophytic insect pathogenic fungus Metarhizium robertsii against the bean plant pathogen Fusarium solani f. sp. phaseoli. Can. J. Plant Pathol. 2013, 35, 288–293. [Google Scholar] [CrossRef]
- Ahmad, I.; Jiménez-Gasco, M.d.M.; Luthe, D.S.; Shakeel, S.N.; Barbercheck, M.E. Endophytic Metarhizium robertsii promotes maize growth, suppresses insect growth, and alters plant defense gene expression. Biol. Control 2020, 144, 104167. [Google Scholar] [CrossRef]
- Batta, Y.A. Efficacy of endophytic and applied Metarhizium anisopliae (Metch.) Sorokin (Ascomycota: Hypocreales) against larvae of Plutella xylostella L. (Yponomeutidae: Lepidoptera) infesting Brassica napus plants. Crop Protect. 2013, 44, 128–134. [Google Scholar] [CrossRef]
- Lopez, D.C.; Sword, G.A. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Steinwender, B.M.; Enkerli, J.; Widmer, F.; Eilenberg, J.; Meyling, N.V. Molecular diversity of the Metarhizium anisopliae lineage in an agricultural field. IOBC/wprs Bulletin 2011, 66, 113–115. [Google Scholar]
- Tiago, P.V.; de Oliveira, N.T.; Lima, E.A.D.A. Biological insect control using Metarhizium anisopliae: Morphological, molecular, and ecological aspects. Cienc. Rural 2014, 44, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Akello, J.; Sikora, R. Systemic acropedal influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biol. Control 2012, 61, 215–221. [Google Scholar] [CrossRef]
- Behie, S.W.; Jones, S.J.; Bidochka, M.J. Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol. 2015, 13, 112–119. [Google Scholar] [CrossRef]
- Elena, G.J.; Beatriz, P.J.; Alejandro, P.; Lecuona, R. Metarhizium anisopliae (Metschnikoff) Sorokin promotes growth and has endophytic activity in tomato plants. Adv. Biol. Res. 2011, 5, 22–27. [Google Scholar]
- Greenfield, M.; Gomez-Jimenez, M.I.; Ortiz, V.; Vega, F.E.; Kramer, M.; Parsa, S. Beauveria bassiana and Metarhizium anisopliae endophytically colonize cassava roots following soil drench inoculation. Biol. Control 2016, 95, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Sasan, R.K.; Bidochka, M.J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 2012, 99, 101–107. [Google Scholar] [CrossRef]
- Barelli, L.; Moreira, C.C.; Bidochka, M.J. Initial stages of endophytic colonization by Metarhizium involves rhizoplane colonization. Microbiology 2018, 164, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.G.; O’Brien, T.R.; Fang, W.G.; St. Leger, R.J. The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl. Microbiol. Biotechnol. 2014, 98, 7089–7096. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.L.; Pelizza, S.; Vianna, M.; Allegrucci, N.; Cabello, M.N.; Toledo, A.V.; Mourelos, C.; Scorsetti, A.C. Effect of endophytic entomopathogenic fungi on soybean Glycine max (L.) Merr. growth and yield. J. King Saud Univ. Sci. 2018, 31, 728–736. [Google Scholar] [CrossRef]
- Krell, V.; Jakobs-Schoenwandt, D.; Vidal, S.; Patel, A.V. Encapsulation of Metarhizium brunneum enhances endophytism in tomato plants. Biol. Control 2018, 116, 62–73. [Google Scholar] [CrossRef]
- Jaber, L.R.; Alananbeh, K.M. Fungal entomopathogens as endophytes reduce several species of Fusarium causing crown and root rot in sweet pepper (Capsicum annuum L.). Biol. Control 2018, 126, 117–126. [Google Scholar] [CrossRef]
- Liu, S.F.; Wang, G.J.; Nong, X.Q.; Liu, B.; Wang, M.M.; Li, S.L.; Cao, G.C.; Zhang, Z.H. Entomopathogen Metarhizium anisopliae promotes the early development of peanut root. Plant Protect. Sci. 2017, 53, 101–107. [Google Scholar]
- Liao, X.G.; Lovett, B.; Fang, W.G.; St. Leger, R.J. Metarhizium robertsii produces indole-3-acetic acid, which promotes root growth in Arabidopsis and enhances virulence to insects. Microbiology 2017, 163, 980–991. [Google Scholar] [CrossRef]
- Jaber, L.R.; Araj, S.E. Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol. Control 2018, 116, 53–61. [Google Scholar] [CrossRef]
- Kabaluk, J.T.; Ericsson, J.D. Metarhizium anisopliae seed treatment increases yield of field corn when applied for wireworm control. Agron. J. 2007, 99, 1377–1381. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.L.; Sheaffer, C.C.; Wyse, D.L.; Staley, C.; Gould, T.J.; Sadowsky, M.J. Structure of bacterial communities in soil following cover crop and organic fertilizer incorporation. Appl. Microbiol. Biotechnol. 2016, 100, 9331–9341. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.; St. Leger, R.J. Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveal that it is rhizosphere competent. Appl. Environ. Microbiol. 2002, 68, 6383–6387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyrebek, M.; Huber, C.; Sasan, R.K.; Bidochka, M.J. Three sympatrically occurring species of Metarhizium show plant rhizosphere specificity. Microbiology 2011, 157, 2904–2911. [Google Scholar] [CrossRef] [Green Version]
- Steinwender, B.M.; Enkerli, J.; Widmer, F.; Eilenberg, J.; Kristensen, H.L.; Bidochka, M.J.; Meyling, N.V. Root isolations of Metarhizium spp. from crops reflect diversity in the soil and indicate no plant specificity. J. Invertebr. Pathol. 2015, 132, 142–148. [Google Scholar] [CrossRef]
- Alvarez, R.; Steinbach, H.S.; De Paepe, J.L. Cover crop effects on soils and subsequent crops in the pampas: A meta-analysis. Soil Tillage Res. 2017, 170, 53–65. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover crops and ecosystem services: Insights from studies in temperate soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef] [Green Version]
- Finney, D.M.; White, C.M.; Kaye, J.P. Biomass production and carbon/nitrogen ratio influence ecosystem services from cover crop mixtures. Agron. J. 2016, 108, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Schipanski, M.E.; Barbercheck, M.; Douglas, M.R.; Finney, D.M.; Haider, K.; Kaye, J.P.; Kemanian, A.R.; Mortensen, D.A.; Ryan, M.R.; Tooker, J.; et al. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agr. Syst. 2014, 125, 12–22. [Google Scholar] [CrossRef]
- White, C.M.; DuPont, S.T.; Hautau, M.; Hartman, D.; Finney, D.M.; Bradley, B.; LaChance, J.C.; Kaye, J.P. Managing the trade off between nitrogen supply and retention with cover crop mixtures. Agr. Ecosyst. Environ. 2017, 237, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Murrell, E.G.; Schipanski, M.E.; Finney, D.M.; Hunter, M.C.; Burgess, M.; LaChance, J.C.; Baraibar, B.; White, C.M.; Mortensen, D.A.; Kaye, J.P. Achieving diverse cover crop mixtures: Effects of planting date and seeding rate. Agron. J. 2017, 109, 259–271. [Google Scholar] [CrossRef]
- Jabbour, R.; Barbercheck, M.E. Soil management effects on entomopathogenic fungi during the transition to organic agriculture in a feed grain rotation. Biol. Control 2009, 51, 435–443. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Mullen, C.; Barbercheck, M. Plant identity, but not diversity, and agroecosystem characteristics affect the occurrence of M. robertsii in an organic cropping system. Biol. Control 2018, 124, 18–29. [Google Scholar] [CrossRef]
- Boetel, M.A.; Majumdar, A.; Jaronski, S.T.; Horsley, R.D. Cover crop and conidia delivery system impacts on soil persistence of Metarhizium anisopliae (Hypocreales: Clavicipitaceae) in sugarbeet. Biocontrol Sci. Technol. 2012, 22, 1284–1304. [Google Scholar] [CrossRef]
- Pell, J.K.; Hannam, J.J.; Steinkraus, D.C. Conservation biological control using fungal entomopathogens. BioControl 2010, 55, 187–198. [Google Scholar] [CrossRef]
- USDA. National Organic Standards. Available online: https://www.ams.usda.gov/rules-regulations/organic (accessed on 24 March 2020).
- Zimmermann, G. The ‘Galleria bait method’ for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Fernandes, E.K.K.; Keyser, C.A.; Rangel, D.E.N.; Foster, R.N.; Roberts, D.W. CTC medium: A novel dodine-free selective medium for isolating entomopathogenic fungi, especially Metarhizium acridum, from soil. Biol. Control 2010, 54, 197–205. [Google Scholar] [CrossRef]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 101, 512–530. [Google Scholar] [CrossRef] [Green Version]
- Kepler, R.M.; Ugine, T.A.; Maul, J.E.; Cavigelli, M.A.; Rehner, S.A. Community composition and population genetics of insect pathogenic fungi in the genus Metarhizium from soils of a long-term agricultural research system. Environ. Microbiol. 2015, 17, 2791–2804. [Google Scholar] [CrossRef]
- Parsa, S.; Ortiz, V.; Vega, F.E. Establishing fungal entomopathogens as endophytes: Towards endophytic biological control. Jove-J. Vis. Exp. 2013, 74, e50360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cenis, J.L. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res. 1992, 20, 2380. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.R. For testing the significance of regression coefficients, go ahead and log-transform count data. Methods Ecol. Evol. 2015, 6, 828–835. [Google Scholar] [CrossRef]
- Ahmad, I.; Zaib, S.; Alves, P.C.M.S.; Luthe, D.S.; Bano, A.; Shakeel, S.N. Molecular and physiological analysis of drought stress responses in Zea mays treated with plant growth promoting rhizobacteria. Biol. Plant. 2019, 63, 536–547. [Google Scholar] [CrossRef]
- Sergaki, C.; Lagunas, B.; Lidbury, I.; Gifford, M.L.; Schafer, P. Challenges and approaches in microbiome research: From fundamental to applied. Front. Plant Sci. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Bing, L.A.; Lewis, L.C. Suppression of Ostrinia nubilalis (Hübner)(Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ. Entomol. 1991, 20, 1207–1211. [Google Scholar] [CrossRef]
- Golo, P.S.; Gardner, D.R.; Grilley, M.M.; Takemoto, J.Y.; Krasnoff, S.B.; Pires, M.S.; Fernandes, E.K.; Bittencourt, V.R.; Roberts, D.W. Production of destruxins from Metarhizium spp. fungi in artificial medium and in endophytically colonized cowpea plants. PLoS ONE 2014, 9, e104946. [Google Scholar] [CrossRef]
- Dutta, P.; Kaushik, H.; Bhawmick, P.; Puzari, K.; Hazarika, G. Metarhizium anisopliae as endophyte has the ability of plant growth enhancement. Int. J. Curr. Res. 2015, 7, 14300–14304. [Google Scholar]
- Mantzoukas, S.; Chondrogiannis, C.; Grammatikopoulos, G. Effects of three endophytic entomopathogens on sweet sorghum and on the larvae of the stalk borer Sesamia nonagrioides. Entomol. Exp. Appl. 2015, 154, 78–87. [Google Scholar] [CrossRef]
- Kaushik, H.; Dutta, P. Establishment of Metarhizium anisopliae, an entomopathogen as endophyte for biological control in tea. Res. Crop 2016, 17, 375–387. [Google Scholar] [CrossRef]
- Razinger, J.; Lutz, M.; Schroers, H.J.; Urek, G.; Grunder, J. Evaluation of insect associated and plant growth promoting fungi in the control of cabbage root flies. J. Econ. Entomol. 2014, 107, 1348–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyser, C.A.; Thorup-Kristensen, K.; Meyling, N.V. Metarhizium seed treatment mediates fungal dispersal via roots and induces infections in insects. Fungal Ecol. 2014, 11, 122–131. [Google Scholar] [CrossRef]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, 163–173. [Google Scholar]
- Saikkonen, K.; Wali, P.R.; Helander, M. Genetic compatibility determines endophyte-grass combinations. PLoS ONE 2010, 5, e11395. [Google Scholar] [CrossRef] [Green Version]
- Jaber, L.R.; Enkerli, J. Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol. Control 2016, 103, 187–195. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Khan, S.A.; Kang, S.M.; Shinwari, Z.K.; Kamran, M.; Ur Rehman, S.; Kim, J.G.; Lee, I.J. Pure culture of Metarhizium anisopliae LHL07 reprograms soybean to higher growth and mitigates salt stress. World J. Microbiol. Biotechnol. 2012, 28, 1483–1494. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Urbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 48. [Google Scholar] [CrossRef] [Green Version]
- Biate, D.L.; Kumari, A.; Annapurna, K.; Kumar, L.V.; Ramadoss, D.; Reddy, K.K.; Naik, S. Legume root exudates: Their role in symbiotic interactions. In Plant Microbes Symbiosis: Applied Facets; Springer: Berlin, Germany, 2015; pp. 259–271. [Google Scholar]
- von Roepenack-Lahaye, E.; Degenkolb, T.; Zerjeski, M.; Franz, M.; Roth, U.; Wessjohann, L.; Schmidt, J.; Scheel, D.; Clemens, S. Profiling of Arabidopsis secondary metabolites by capillary liquid chromatography coupled to electrospray ionization quadrupole time-of-flight mass spectrometry. Plant Physiol. 2004, 134, 548–559. [Google Scholar] [CrossRef] [Green Version]
- Raya-Diaz, S.; Quesada-Moraga, E.; Barron, V.; del Campillo, M.C.; Sanchez-Rodriguez, A. Redefining the dose of the entomopathogenic fungus Metarhizium brunneum (Ascomycota, Hypocreales) to increase Fe bioavailability and promote plant growth in calcareous and sandy soils. Plant Soil 2017, 418, 387–404. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; Jiménez-Gasco, M.d.M.; Luthe, D.S.; Barbercheck, M.E. Systemic Colonization by Metarhizium robertsii Enhances Cover Crop Growth. J. Fungi 2020, 6, 64. https://doi.org/10.3390/jof6020064
Ahmad I, Jiménez-Gasco MdM, Luthe DS, Barbercheck ME. Systemic Colonization by Metarhizium robertsii Enhances Cover Crop Growth. Journal of Fungi. 2020; 6(2):64. https://doi.org/10.3390/jof6020064
Chicago/Turabian StyleAhmad, Imtiaz, María del Mar Jiménez-Gasco, Dawn S. Luthe, and Mary E. Barbercheck. 2020. "Systemic Colonization by Metarhizium robertsii Enhances Cover Crop Growth" Journal of Fungi 6, no. 2: 64. https://doi.org/10.3390/jof6020064
APA StyleAhmad, I., Jiménez-Gasco, M. d. M., Luthe, D. S., & Barbercheck, M. E. (2020). Systemic Colonization by Metarhizium robertsii Enhances Cover Crop Growth. Journal of Fungi, 6(2), 64. https://doi.org/10.3390/jof6020064




