Comparison Effects of Ruminal Crabtree-Negative Yeasts and Crabtree-Positive Yeasts for Improving Ensiled Rice Straw Quality and Ruminal Digestion Using In Vitro Gas Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast-Fermented Rice Straw, and Experimental Design
2.2. Fermentation Products and Chemical Composition
2.3. Yeast Counts and Microorganism Analysis of Ensiled RS
2.4. In Vitro Gas Production and Digestibility
2.5. In Vitro Ruminal Fermentation and Ruminal Microbial Counts
2.6. Statistical Analysis and Calculation
3. Results
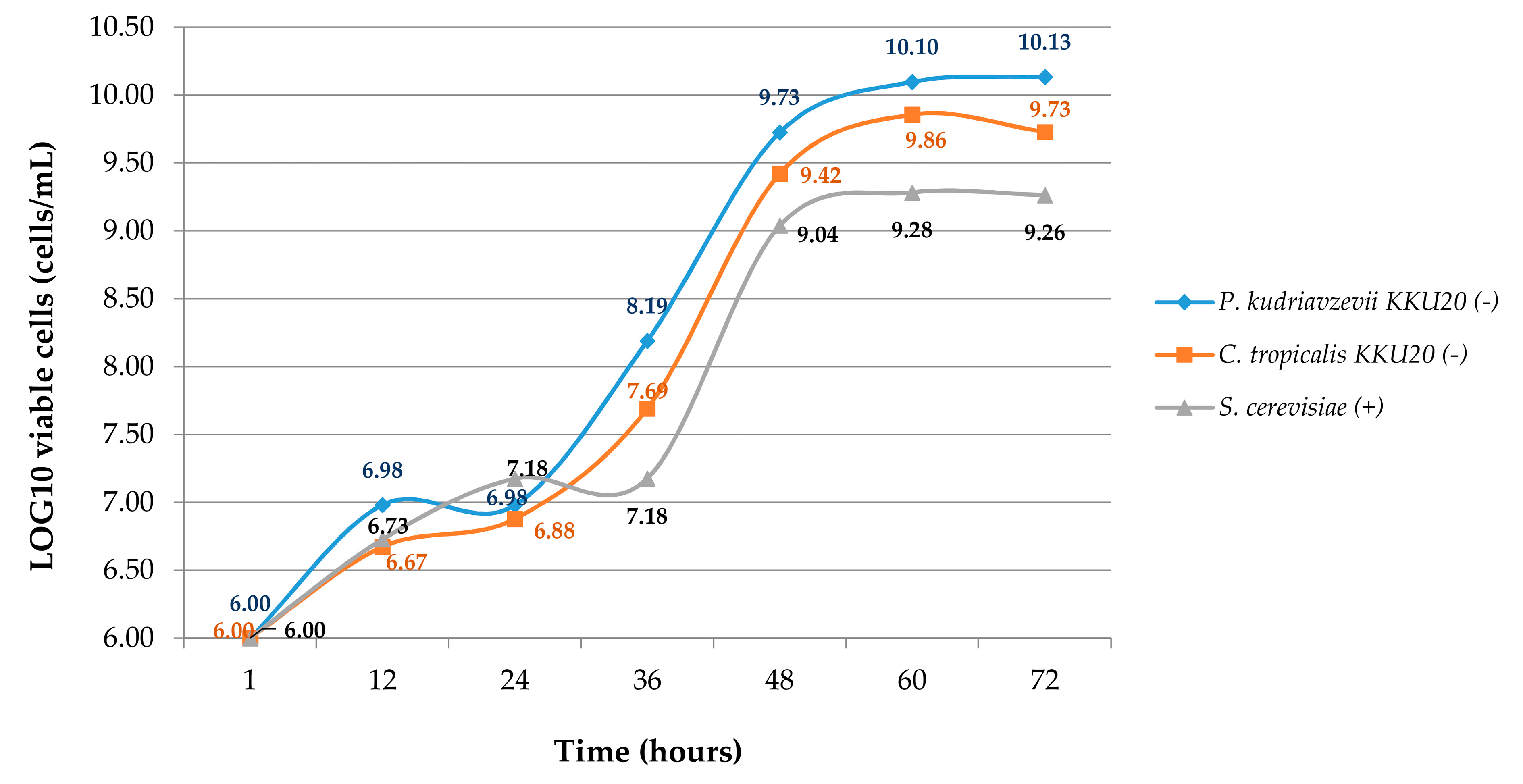
3.1. Viable Cell Counts in Medium Solution of Crabtree-Negative Ruminal Yeast and Positive Yeasts
3.2. Chemical Compositions of Rice Straw Ensiled with Yeast
3.3. Microbiological Analysis of Ensiled RS
3.4. Ensilage Quality of Ensiled RS
3.5. In Vitro Gas Production and Degradability
3.6. In Vitro Ruminal Volatile Fatty Acids
3.7. Microbial Populations in Rumen Fluids
4. Discussion
4.1. Chemical Compositions Changed in Ensiled RS
4.2. Microorganism Populations after Ensiled RS
4.3. Fermentation Characteristics of Ensiled RS
4.4. Gas Production, In Vitro Digestibility, and Microbial Population
4.5. Ruminal Fermentation Products
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; He, Z.; Beauchemin, K.A.; Tang, S.; Zhou, C.; Han, X.; Wang, M.; Kang, J.; Odongo, N.E.; Tan, Z. Evaluation of different yeast species for improving in vitro fermentation of cereal straws. Asian Australas. J. Anim. Sci. 2016, 29, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Khampa, S.; Chaowarat, P.; Singhalert, R.; Wanapat, M. Supplementation of yeast fermented cassava chip as a replacement concentrate on rumen fermentation efficiency and digestibility on nutrients in cattle. Asian Australas. J. Anim. Sci. 2009, 3, 18–24. [Google Scholar] [CrossRef][Green Version]
- Polyorach, S.; Wanapat, M. Improving the quality of rice straw by urea and calcium hydroxide on rumen ecology, microbial protein synthesis in beef cattle. J. Anim. Physiol. Anim. Nutr. 2015, 99, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Foiklang, S.; Upama, P.; Kolyanee, W.; Japanya, R.; Ounpon, P.; Pengsiri, K.; Wanapat, M.; Yammuen-art, S. In vitro gas kinetics and digestibility as influenced by yeast media solution ratios and physical forms of rice straw. Khon. Kaen. Agric. J. 2017, 45, 74–79. [Google Scholar]
- Beev, G.; Todorova, P.; Tchobanova, S. Yeast cultures in ruminant nutrition. Bulg. J. Agric. Sci. 2007, 13, 357–374. [Google Scholar]
- Mohammed, S.F.; Mahmood, F.A.; Abas, E.R. A review on effects of yeast (Saccharomyces cerevisiae) as feed additives in ruminants performance. J. Entomol. Zool. Stud. 2018, 6, 629–635. [Google Scholar]
- De Deken, R. The Crabtree effect: A regulatory system in yeast. Microbiology 1966, 44, 149–156. [Google Scholar] [CrossRef]
- Dashko, S.; Zhou, N.; Compagno, C.; Piškur, J. Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 2014, 14, 826–832. [Google Scholar] [CrossRef]
- Van Urk, H.; Voll, W.L.; Scheffers, W.A.; Van Dijken, J.P. Transient-state analysis of metabolic fluxes in Crabtree-positive and Crabtree-negative yeasts. Appl. Environ. Microbiol. 1990, 56, 281–287. [Google Scholar] [CrossRef]
- Wardrop, F.; Liti, G.; Cardinali, G.; Walker, G. Physiological responses of Crabtree- positive and Crabtree-negativeyeasts to glucose upshifts in a chemostat. Ann. Microbiol. 2004, 54, 103–114. [Google Scholar]
- Habeeb, A.A.M. Importance of yeast in ruminants feeding on production and reproduction. Evol. Ecol. Res. 2017, 2, 49. [Google Scholar]
- Suntara, C.; Cherdthong, A. Screening and isolation of potential yeast from rumen fluids and optimization of biomass and cellulase production. In Seminar in Animal Science, Department of Animal Science; Khon Kaen University: Khon Kaen, Thailand, 2019. [Google Scholar]
- Association of Official Analytical Chemist (AOAC). The Official Methods of Analysis of the Association of Official Analytical Chemist, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1998. [Google Scholar]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. 2. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Anal. Chem. 1963, 46, 829–835. [Google Scholar]
- Cai, Y. Analysis method for silage. In Field and Laboratory Methods for Grassland Science; Japanese Society of Grassland Science, Ed.; Tosho Printing Co., Ltd.: Tokyo, Japan, 2004; p. 279. [Google Scholar]
- Cai, Y.; Benno, Y.; Ogawa, M.; Kumai, S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 1999, 82, 520–526. [Google Scholar] [CrossRef]
- Prueksatrakul, T.; Phoopra-in, P.; Jaiyen, P.; Vilairat, P.; Jantivas, R. Application of 2 step extraction method for determination of lactic acid in waste water. In Proceedings of the National and International Conference & Research Presentation 2015 “Create and Development to Approach ASEAN Community II”, Bangkok, Thailand, 18–19 June 2015; pp. 155–162. [Google Scholar]
- Fiala, J.; Lloyd, D.; Rychtera, M.; Kent, C.; Al-Rubeai, M. Evaluation of cell numbers and viability of Saccharomyces cerevisiae by different counting methods. Biotechnol. Tech. 1999, 13, 787–795. [Google Scholar] [CrossRef]
- Kozaki, M.; Uchimura, T.; Okada, S. Experimental Manual of Lactic Acid Bacteria; Asakurasyoten: Tokyo, Japan, 1992; pp. 34–37. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle; National Research Council: Seventh Revised Edition; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Blümmel, M.; Makkar, H.; Becker, K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. Nutr. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Tilley, J.; Terry, R. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Samuel, M.; Sagathevan, S.; Thomas, J.; Mathen, G. An HPLC method for estimation of volatile fatty acids in ruminal fluid. Indian J. Anim. Sci. 1997, 67, 805–807. [Google Scholar]
- Ørskov, E.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Statistical Analysis Systems (SAS). SAS/STAT User’s Guide; Statistical Analysis Systems Institute. Version 9.2th ed.; SAS Institute Inc: Cary, NC, USA, 2002. [Google Scholar]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics; McGraw-Hill Book Co. Inc.: New York, NY, USA, 1980; p. 633. [Google Scholar]
- Pronk, J.T.; Yde Steensma, H.; van Dijken, J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 1996, 12, 1607–1633. [Google Scholar] [CrossRef]
- Piškur, J.; Compagno, C. Molecular Mechanisms in Yeast Carbon Metabolism; Piškur, J., Compagno, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Verduyn, C.; Zomerdijk, T.P.; van Dijken, J.P.; Scheffers, W.A. Continuous measurement of ethanol production by aerobic yeast suspensions with an enzyme electrode. Appl. Microbiol. Biotechnol. 1984, 19, 181–185. [Google Scholar] [CrossRef]
- Clarke, M. Syrups. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: New York, NY, USA, 2003; p. 6000. [Google Scholar]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Elferink, S.J.O.; Spoelstra, S.F. Microbiology of ensiling. Silage Sci. Technol. 2003, 42, 31–93. [Google Scholar]
- Kung, L., Jr.; Stanley, R. Effect of stage of maturity on the nutritive value of whole-plant sugarcane preserved as silage. J. Anim. Sci. 1982, 54, 689–696. [Google Scholar] [CrossRef]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Zimmer, E. Efficient silage systems. In Proceedings of the Forage Conservation in the 80’s (Occasional Symposium No. 11), Brighton, UK, 27–30 November 1979; pp. 186–197. [Google Scholar]
- Pholsen, S.; Khota, W.; Pang, H.; Higgs, D.; Cai, Y. Characterization and application of lactic acid bacteria for tropical silage preparation. Anim. Sci. J. 2016, 87, 1202–1211. [Google Scholar] [CrossRef]
- Do Carmo-Sousa, L. Distribution of yeasts in nature. In The Yeasts; Rose, A.H., Harrison, J.S., Eds.; Academic Press: New York, NY, USA, 1969; pp. 79–105. [Google Scholar]
- Lynd, L.R.; Weimer, P.J.; Van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Sarawan, S.; Mahakhan, P.; Jindamorakot, S.; Vichitphan, K.; Vichitphan, S.; Sawaengkaew, J. Candida konsanensis sp. nov., a new yeast species isolated from Jasminum adenophyllum in Thailand with potentially carboxymethyl cellulase-producing capability. World J. Microb. Biot. 2013, 29, 1481–1486. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.; Heron, S. The Biochemistry of Silage; Chalcombe Publications: Southampton, UK, 1991. [Google Scholar]
- Van Dijken, J.P.; Van Den Bosch, E.; Hermans, J.J.; De Miranda, L.R.; Scheffers, W.A. Alcoholic fermentation by ‘non-fermentative’ yeasts. Yeast 1986, 2, 123–127. [Google Scholar] [CrossRef]
- Auclair, E. Yeast as an example of the mode of action of probiotics in monogastric and ruminant species. Feed Manufacturing in the Mediterranean Region. Reus Spain 2001, 45–53. [Google Scholar]
- Ávila, C.; Pinto, J.; Figueiredo, H.; Schwan, R. Effects of an indigenous and a commercial Lactobacillus buchneri strain on quality of sugar cane silage. Grass Forage Sci. 2009, 64, 384–394. [Google Scholar] [CrossRef]
- Piltz, J.; Kaiser, A. Principles of silage preservation. In Successful Silage’; Kaiser, A.G., Piltz, J.W., Burns, H.M., Griffiths, N.W., Eds.; Dairy Australia and Newsouth Wales Department of Primary Industries published, NSW DPI: Orange, Australia, 2004; pp. 25–56. [Google Scholar]
- Jianxin, L.; Jun, G. Ensiling Crop Residues. In FAO Animal Production and Health Paper (FAO); Publishing and Multimedia Service, Information Division, Food and Agriculture Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Sommart, K.; Parker, D.; Rowlinson, P.; Wanapat, M. Fermentation characteristics and microbial protein synthesis in an in vitro system using cassava, rice straw and dried ruzi grass as substrates. Asian Australas. J. Anim. Sci. 2000, 13, 1084–1093. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Koatdoke, U.; Cherdthong, A.; Khampa, S. Supplementation levels of palm oil in yeast (Saccharomyces cerevisiae) culture fermented cassava pulp on rumen fermentation and average daily gain in crossbred native cattle. Pak. J. Nutr. 2011, 10, 1115–1120. [Google Scholar]
- Chaucheyras-Durand, F.; Walker, N.; Bach, A. Effects of active dry yeasts on the rumen microbial ecosystem: Past, present and future. Anim. Feed Sci. Technol. 2008, 145, 5–26. [Google Scholar] [CrossRef]
- Galvão, K.N.; Santos, J.E.; Coscioni, A.; Villaseñor, M.; Sischo, W.M.; Berge, A.C.B. Effect of feeding live yeast products to calves with failure of passive transfer on performance and patterns of antibiotic resistance in fecal Escherichia coli. Reprod. Nutr. Dev. 2005, 45, 427–440. [Google Scholar] [CrossRef]
- Gang, G.; Chen, S.; Qiang, L.; Zhang, S.L.; Tao, S.; Cong, W.; Wang, Y.X.; Xu, Q.F.; Huo, W.J. The effect of lactic acid bacteria inoculums on in vitro rumen fermentation, methane production, ruminal cellulolytic bacteria populations and cellulase activities of corn stover silage. J. Integr. Agric. 2020, 19, 838–847. [Google Scholar]
- Castillo-González, A.; Burrola-Barraza, M.; Domínguez-Viveros, J.; Chávez-Martínez, A. Rumen microorganisms and fermentation. Arch. Med. Vet. 2014, 46, 349–361. [Google Scholar] [CrossRef]
- Mutsvangwa, T.; Edwards, I.; Topps, J.; Paterson, G. The effect of dietary inclusion of yeast culture (Yea-Sacc) on patterns of rumen fermentation, food intake and growth of intensively fed bulls. Anim. Sci. J. 1992, 55, 35–40. [Google Scholar] [CrossRef]
- Williams, P.; Tait, C.; Innes, G.; Newbold, C. Effects of the inclusion of yeast culture (Saccharomyces cerevisiae plus growth medium) in the diet of dairy cows on milk yield and forage degradation and fermentation patterns in the rumen of steers. J. Anim. Sci. 1991, 69, 3016–3026. [Google Scholar] [CrossRef]
- Dawson, K.; Rasmussen, M.; Allison, M. Digestive disorders and nutritional toxicity. In The Rumen Microbial Ecosystem; Hobson, P.N., Stewart, C.S., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 633–660. [Google Scholar]
- Chung, Y.-H.; Walker, N.; McGinn, S.; Beauchemin, K. Differing effects of 2 active dried yeast (Saccharomyces cerevisiae) strains on ruminal acidosis and methane production in nonlactating dairy cows. J. Dairy. Sci. 2011, 94, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.; Wallace, R. Energy-yielding and energy-consuming reactions. In The Rumen Microbial Ecosystem; Hobson, P.N., Stewart, C.S., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 246–282. [Google Scholar]
- Boadi, D.; Benchaar, C.; Chiquette, J.; Massé, D. Mitigation strategies to reduce enteric methane emissions from dairy cows: Update review. Can. J. Anim. Sci. 2004, 84, 319–335. [Google Scholar] [CrossRef]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef] [PubMed]
| Yeasts | Fermentation Days | Dry Matter g/kg | g/kg Dry Matter | |||||
|---|---|---|---|---|---|---|---|---|
| Organic Matter | Ether Extract | Crude Protein | Neutral Detergent Fiber | Acid Detergent Fiber | Acid Detergent Lignin | |||
| Control | 7 | 296.6 | 828.0 | 6.57 | 61.46 | 735.2 | 481.3 | 63.41 |
| 14 | 289.7 | 859.0 | 6.79 | 60.43 | 722.1 | 480.3 | 62.88 | |
| 21 | 280.2 | 860.8 | 6.77 | 59.90 | 727.5 | 452.1 | 64.99 | |
| P. kudriavzevii KKU20 | 7 | 287.0 | 870.7 | 7.09 | 60.03 | 694.7 | 452.4 | 63.28 |
| 14 | 283.3 | 880.2 | 5.97 | 61.37 | 704.7 | 493.6 | 66.00 | |
| 21 | 263.8 | 879.9 | 6.23 | 60.93 | 721.5 | 446.9 | 68.10 | |
| C. tropicalis KKEU20 | 7 | 286.7 | 848.9 | 6.35 | 60.10 | 697.6 | 473.5 | 65.60 |
| 14 | 281.0 | 865.3 | 6.49 | 60.63 | 701.5 | 462.5 | 65.23 | |
| 21 | 259.3 | 862.2 | 7.13 | 60.97 | 711.4 | 469.8 | 62.60 | |
| S. cerevisiae KKU20 | 7 | 272.1 | 869.7 | 6.87 | 60.63 | 698.9 | 483.4 | 62.50 |
| 14 | 268.0 | 867.1 | 6.67 | 60.90 | 716.0 | 460.1 | 65.63 | |
| 21 | 249.8 | 872.3 | 6.80 | 60.00 | 695.8 | 451.0 | 64.43 | |
| SEM | 9.65 | 7.12 | 0.67 | 0.46 | 10.2 | 1.58 | 0.29 | |
| Comparison | ||||||||
| Yeast | Control | 289.0 a | 849.1 c | 6.71 | 60.90 | 728.3 a | 4671.2 | 64.87 |
| P. kudriavzevii KKU20 | 277.8 ab | 872.3 a | 6.43 | 60.00 | 706.9 b | 464.3 | 65.79 | |
| C. tropicalis KKU20 | 275.5 ab | 859.5 bc | 6.66 | 59.10 | 703.5 b | 468.6 | 64.48 | |
| S. cerevisiae | 263.2 b | 870.7 ab | 6.78 | 58.20 | 703.5 b | 464.8 | 64.19 | |
| Day | 7 | 285.0 a | 854.9 b | 6.72 | 57.30 | 706.6 | 472.6 | 64.53 |
| 14 | 280.7 a | 868.1 a | 6.48 | 56.40 | 711.1 | 474.1 | 64.94 | |
| 21 | 262.4 b | 869.3 a | 6.73 | 55.50 | 714.0 | 454.9 | 65.03 | |
| Interaction | Yeast × Day | ns | ns | ns | ns | ns | ns | ns |
| Orthogonal contrast | ||||||||
| Contrast 1 | Control | 289.0 a | 849.27 | 6.71 | 60.60 | 728.3 a | 471.2 | 63.76 |
| Yeast | 272.9 b | 868.47 | 6.62 | 60.62 | 704.7 b | 465.9 | 64.82 | |
| Contrast 2 | Yeast (−) | 276.3 | 867.87 | 6.54 | 60.51 | 705.2 | 466.4 | 64.19 |
| Yeast (+) | 263.2 | 869.67 | 6.78 | 60.67 | 703.6 | 464.8 | 65.14 | |
| Strain | Ensilage Times (Day) | Microorganisms Log10 (cfu/g FM) | ||||
|---|---|---|---|---|---|---|
| Yeast | Lactic Acid Bacteria | Coliform | Aerobic Bacteria | Mold | ||
| Control | 7 | 5.89 d | 6.72 | ND | 6.52 ab | ND |
| 14 | 4.53 e | 6.86 | ND | 5.53 abcd | ND | |
| 21 | 4.11 e | 7.15 | ND | 6.77 a | ND | |
| P. kudriavzevii KKU20 | 7 | 10.3 a | 5.12 | ND | 2.98 g | ND |
| 14 | 9.44 a | 5.29 | ND | 3.54 fg | ND | |
| 21 | 7.89 bc | 5.22 | ND | 6.14 abc | ND | |
| C. tropicalis KKU20 | 7 | 9.85 a | 5.33 | ND | 3.77 efg | ND |
| 14 | 9.05 ab | 5.37 | ND | 4.45 def | ND | |
| 21 | 7.34 c | 5.33 | ND | 5.01 cde | ND | |
| S. cerevisiae | 7 | 10.1 a | 5.28 | ND | 2.90 g | ND |
| 14 | 9.56 a | 5.13 | ND | 5.16 bcd | ND | |
| 21 | 7.65 c | 5.25 | ND | 5.52 abcd | ND | |
| SEM | 0.21 | 0.15 | ND | 0.23 | ||
| Comparison | ||||||
| Yeast | Control | 4.84 | 6.90 a | ND | 6.27 | ND |
| P. kudriavzevii KKU20 | 9.01 | 5.21 b | ND | 4.22 | ND | |
| C. tropicalis KKU20 | 8.57 | 5.34 b | ND | 4.41 | ND | |
| S. cerevisiae | 8.90 | 5.22 b | ND | 4.53 | ND | |
| Day | 7 | 8.69 | 5.61 | ND | 4.04 | ND |
| 14 | 7.73 | 5.66 | ND | 4.67 | ND | |
| 21 | 6.76 | 5.74 | ND | 5.86 | ND | |
| Interaction | Yeasts × Day | * | Ns | ND | * | ND |
| Orthogonal Contrast | ||||||
| Contrast 1 | Control | 4.85 b | 6.90 a | ND | 6.27 a | ND |
| Yeast | 9.03 a | 5.26 b | ND | 4.39 b | ND | |
| Contrast 2 | Yeast (−) | 8.98 | 5.28 | ND | 4.31 | ND |
| Yeast (+) | 9.11 | 5.22 | ND | 4.53 | ND | |
| Yeast | Ensilage Times (day) | pH | Ammonia Nitrogen | Organic Acid (g/kg Dry Matter) | |||
|---|---|---|---|---|---|---|---|
| (g/kgDM) | Lactic Acid | Acetic Acid | Propionic Acid | Butyric Acid | |||
| Control | 7 | 4.09 | 1.60 | 26.1 | 4.36 | ND | 0.78 |
| 14 | 4.03 | 1.82 | 23.9 | 4.11 | ND | 0.75 | |
| 21 | 4.08 | 3.01 | 25.9 | 4.32 | ND | 0.81 | |
| P. kudriavzevii KKU20 | 7 | 4.18 | 1.17 | 23.5 | 5.59 | ND | 0.80 |
| 14 | 4.23 | 1.95 | 20.9 | 5.45 | ND | 0.84 | |
| 21 | 4.47 | 2.56 | 21.4 | 5.12 | ND | 0.79 | |
| C. tropicalis KKU20 | 7 | 4.02 | 1.66 | 22.7 | 5.25 | ND | 0.81 |
| 14 | 4.14 | 1.78 | 20.5 | 5.16 | ND | 0.82 | |
| 21 | 4.35 | 2.21 | 23.2 | 5.03 | ND | 0.80 | |
| S. cerevisiae | 7 | 4.04 | 1.59 | 22.4 | 5.32 | ND | 0.80 |
| 14 | 4.17 | 1.99 | 24.1 | 5.40 | ND | 0.83 | |
| 21 | 4.31 | 2.15 | 21.9 | 5.35 | ND | 0.83 | |
| SEM | 0.10 | 0.69 | 1.37 | 0.36 | 0.04 | ||
| Comparison | |||||||
| Yeast | Control | 4.07 | 2.14 | 25.3 a | 4.26 b | ND | 0.78 |
| P. kudriavzevii KKU20 | 4.30 | 1.89 | 21.9 b | 5.38 a | ND | 0.81 | |
| C. tropicalis KKU20 | 4.17 | 1.88 | 22.1 b | 5.14 a | ND | 0.81 | |
| S. cerevisiae | 4.17 | 1.91 | 22.8 b | 5.36 a | ND | 0.82 | |
| Day | 7 | 4.08 b | 1.50 | 23.7 | 5.13 | ND | 0.80 |
| 14 | 4.15 b | 1.88 | 22.4 | 5.03 | ND | 0.81 | |
| 21 | 4.30 a | 2.49 | 23.1 | 4.96 | ND | 0.81 | |
| Interaction | Yeasts × Day | Ns | ns | ns | ns | ns | |
| Orthogonal Contrast | |||||||
| Contrast 1 | Control | 4.07 | 2.14 | 25.3 a | 4.26 b | ND | 0.78 |
| Yeast | 4.21 | 1.90 | 22.3 b | 5.29 a | ND | 0.81 | |
| Contrast 2 | Yeast (+) | 4.23 | 1.89 | 22.0 | 5.27 | ND | 0.81 |
| Yeast (−) | 4.17 | 1.91 | 22.8 | 5.36 | ND | 0.82 | |
| Strain | Ensilage Times (Day) | Gas Kinetics | Gas Volume | Degradability (g/kg Dry Matter) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | a + b | 96 h | IVDMD | IVOMD | IVNDFD | IVADFD | ||
| Control | 7 | 4.44 | 103.6 | 0.052 a | 108.1 | 100.1 | 437.3 d | 607.5 | 699.3 b | 484.8 |
| 14 | 4.90 | 110.0 | 0.050 ab | 114.9 | 112.8 | 571.5 c | 591.5 | 699.7 b | 465.5 | |
| 21 | 3.83 | 104.3 | 0.051 ab | 108.1 | 101.1 | 602.3 abc | 593.0 | 699.9 b | 463.3 | |
| P. kudriavzevii KKU20 | 7 | 5.55 | 114.1 | 0.048 b | 119.7 | 117.8 | 623.1 abc | 715.9 | 706.1 b | 516.6 |
| 14 | 4.43 | 130.5 | 0.060 a | 134.9 | 130.5 | 639.8 a | 674.7 | 774.0 a | 493.2 | |
| 21 | 3.91 | 123.7 | 0.059 a | 127.6 | 122.5 | 608.4 abc | 676.5 | 696.3 b | 494.4 | |
| C. tropicalis KKU20 | 7 | 5.30 | 116.9 | 0.061 a | 122.2 | 115.4 | 619.3 abc | 670.2 | 715.0 b | 503.4 |
| 14 | 4.87 | 119.4 | 0.060 a | 124.3 | 119.9 | 631.8 ab | 691.0 | 718.5 b | 505.4 | |
| 21 | 2.94 | 117.6 | 0.057 a | 120.5 | 118.2 | 579.7 bc | 675.2 | 732.4 b | 496.4 | |
| S. cerevisiae | 7 | 0.96 | 105.7 | 0.051 ab | 106.6 | 106.3 | 630.7 ab | 696.2 | 716.5 b | 501.8 |
| 14 | 2.40 | 120.0 | 0.048 ab | 122.4 | 120.3 | 609.8 abc | 681.7 | 725.7 b | 498.3 | |
| 21 | 3.22 | 115.3 | 0.049 ab | 118.5 | 114.8 | 617.0 abc | 679.0 | 727.3 b | 500.3 | |
| SEM | 1.26 | 3.16 | 0.02 | 3.51 | 3.49 | 1.63 | 1.60 | 1.29 | 1.27 | |
| Comparison | ||||||||||
| Yeast | Control | 4.39 | 105.9 c | 0.051 | 110.3 b | 104.7 c | 537.0 | 597.3 b | 699.6 | 471.2 b |
| P.kudriavzevii KKU20 | 4.63 | 122.8 a | 0.055 | 127.4 a | 123.6 a | 623.8 | 689.1 a | 725.5 | 501.4 a | |
| C. tropicalis KKU20 | 4.37 | 117.9 ab | 0.060 | 122.3 a | 117.8 ab | 610.2 | 678.8 a | 722.0 | 501.7 a | |
| S. cerevisiae | 2.20 | 113.6 b | 0.049 | 115.8 b | 113.8 b | 619.2 | 685.7 a | 723.2 | 500.1 a | |
| Day | 7 | 4.06 | 110.1 c | 0.053 | 114.1 b | 109.9 b | 577.6 | 672.5 | 709.2 | 501.7 |
| 14 | 4.15 | 119.9 a | 0.054 | 124.1 a | 120.9 a | 613.2 | 659.7 | 729.5 | 490.6 | |
| 21 | 3.48 | 115.2 b | 0.054 | 118.7 b | 114.1 b | 601.8 | 655.9 | 714.0 | 488.6 | |
| Interaction | Yeast × Day | ns | ns | ** | Ns | ns | ** | ns | * | ns |
| Orthogonal Contrast | ||||||||||
| Contrast 1 | Control | 4.39 | 105.9 b | 0.051 | 110.3 b | 104.7 b | 53.7 b | 537.0 b | 597.3 b | 699.6 b |
| Yeast | 3.73 | 118.1a | 0.055 | 121.9 a | 118.4 a | 61.8 a | 617.7 a | 684.5 a | 723.5 a | |
| Contrast 2 | Yeast (−) | 4.50 | 120.4 a | 0.058 a | 124.9 a | 120.7 a | 61.7 | 617.0 | 683.9 | 723.7 |
| Yeast (+) | 2.20 | 113.6 b | 0.049 b | 115.8 b | 113.8 b | 61.9 | 619.2 | 685.7 | 723.2 | |
| Yeasts | Ensilage Times (Day) | Total Volatile Fatty Acids (mmol/L) | Acetic Acid (mol/100 mol) | Propionic Acid (mol/100 mol) | Butyric Acid (mol/100 mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | 8 h | Mean | 4 h | 8 h | Mean | 4 h | 8 h | Mean | 4 h | 8 h | Mean | ||
| Control | 7 | 68.8 | 80.2 | 74.5 | 72.6 | 70.9 | 72.1 ab | 16.7 | 17.6 | 16.7 | 10.7 | 11.5 a | 11.1 a |
| 14 | 72.8 | 84.4 | 78.6 | 71.0 | 70.2 | 69.9 bc | 18.3 | 18.3 | 19.0 | 10.7 | 11.6 a | 11.1 a | |
| 21 | 70.4 | 81.7 | 76.0 | 73.4 | 75.6 | 74.9 a | 15.8 | 16.0 | 14.8 | 10.9 | 8.4 ed | 9.65 bc | |
| P. kudriavzevii KKU20 | 7 | 88.6 | 91.4 | 90.0 | 70.8 | 66.0 | 68.8 bc | 20.0 | 25.0 | 22.2 | 9.20 | 8.9 cd | 9.07 cd |
| 14 | 89.7 | 89.3 | 89.5 | 68.6 | 68.8 | 68.7 bc | 21.5 | 24.2 | 22.9 | 9.85 | 7.08 e | 8.46 d | |
| 21 | 87.2 | 88.6 | 87.9 | 63.9 | 64.3 | 64.2 d | 26.3 | 24.8 | 25.3 | 9.86 | 10.9 ab | 10.4 ab | |
| C. tropicalis KKU20 | 7 | 88.6 | 90.5 | 84.4 | 71.8 | 65.3 | 70.2 bc | 18.4 | 24.7 | 21.0 | 9.85 | 9.6 cd | 9.88 bc |
| 14 | 80.7 | 82.6 | 81.7 | 68.1 | 65.3 | 66.6 cd | 23.0 | 25.5 | 24.3 | 9.10 | 9.7 bcd | 9.16 cd | |
| 21 | 78.4 | 83.0 | 85.8 | 68.9 | 65.4 | 67.1 cd | 21.2 | 26.3 | 23.9 | 9.89 | 8.3 ed | 9.11 cd | |
| S. cerevisiae | 7 | 86.3 | 90.5 | 88.4 | 68.4 | 65.0 | 66.8 cd | 22.0 | 26.0 | 23.3 | 9.53 | 9.4 cd | 9.61 bc |
| 14 | 85.8 | 86.8 | 86.7 | 68.9 | 67.0 | 68.6 bc | 21.8 | 22.2 | 21.0 | 9.33 | 9.9 bc | 9.53 bc | |
| 21 | 83.9 | 88.5 | 86.2 | 69.0 | 66.7 | 67.8 cd | 21.3 | 24.9 | 22.3 | 9.63 | 9.3 cd | 9.49 bc | |
| SEM | 1.96 | 2.55 | 1.58 | 2.47 | 1.46 | 1.27 | 2.67 | 1.76 | 1.2 | 0.26 | 0.40 | 0.29 | |
| Comparison | |||||||||||||
| Yeast | Control | 70.64 c | 82.1 b | 76.4 c | 72.3 | 72.2 a | 72.3 | 16.9 | 17.3 b | 16.9 b | 10.8 a | 10.50 | 10.6 |
| P. kudriavzevii KKU20 | 88.52 a | 89.7 a | 89.1 a | 67.8 | 66.4 b | 67.2 | 22.6 | 24.6 a | 23.5 a | 9.64 b | 9.00 | 9.32 | |
| C. tropicalis KKU20 | 82.6 b | 85.4 ab | 83.9 b | 69.6 | 65.3 b | 68.0 | 20.9 | 25.5 a | 23.0 a | 9.61 b | 9.19 | 9.38 | |
| S. cerevisiae | 85.3 ab | 88.6 a | 87.1 a | 68.8 | 66.3 b | 67.7 | 21.7 | 24.4 a | 22.2 a | 9.50 b | 9.55 | 9.55 | |
| Day | 7 | 83.0 | 88.1 | 84.3 | 70.9 | 66.8 | 69.5 | 19.3 | 23.3 | 20.8 | 9.83 | 9.85 | 9.92 |
| 14 | 82.3 | 85.8 | 84.1 | 69.2 | 67.8 | 68.4 | 21.2 | 22.5 | 21.8 | 9.74 | 9.57 | 9.57 | |
| 21 | 80.0 | 85.5 | 84.0 | 68.8 | 68.0 | 68.5 | 21.2 | 23.0 | 21.6 | 10.1 | 9.26 | 9.67 | |
| Interaction | Yeast × Day | ns | ns | ns | ns | ns | * | ns | ns | ns | ns | ** | ** |
| Orthogonal Contrast | |||||||||||||
| Contrast 1 | Control | 70.6 b | 82.06 b | 76.4 b | 72.3 a | 72.2 a | 72.3 a | 16.9 b | 17.3 b | 16.8 b | 10.8 a | 10.5 a | 10.6 a |
| Yeast | 85.5 a | 87.91 a | 86.7 a | 68.7 b | 65.9 b | 67.6 b | 21.7 a | 24.8 a | 22.9 a | 9.58 b | 9.25 b | 9.42 b | |
| Contrast 2 | Yeast (−) | 85.5 | 87.6 | 86.55 | 68.7 | 65.8 | 67.6 | 21.7 | 25.1 | 23.3 | 9.63 | 9.10 | 9.35 |
| Yeast (+) | 85.3 | 88.6 | 87.11 | 68.8 | 66.3 | 67.7 | 21.7 | 24.4 | 22.2 | 9.50 | 9.55 | 9.55 | |
| Yeasts | Fermentation Days | Bacteria (cell/mL) | Protozoa (cell/mL) | Fungi Zoospore (cell/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | 8 h | Mean | 4 h | 8 h | Mean | 4 h | 8 h | Mean | ||
| Control | 7 | 7.32 | 7.34 d | 7.33 e | 5.86 | 6.09 | 5.97 | 5.80 | 6.06 | 5.93 |
| 14 | 7.09 | 7.38 d | 7.23 ef | 5.80 | 6.29 | 6.05 | 5.86 | 6.16 | 6.01 | |
| 21 | 6.87 | 7.35 d | 7.11 f | 5.70 | 6.06 | 5.88 | 5.90 | 5.96 | 5.93 | |
| P. kudriavzevii KKU20 | 7 | 8.16 | 8.29 a | 8.22 a | 5.80 | 6.06 | 5.93 | 6.50 | 6.84 | 6.67 |
| 14 | 8.12 | 8.27 a | 8.19 a | 5.58 | 5.73 | 5.66 | 6.36 | 6.72 | 6.54 | |
| 21 | 7.93 | 8.22 a | 8.08 ab | 5.63 | 6.16 | 5.90 | 6.30 | 6.84 | 6.57 | |
| C. tropicalis KKU20 | 7 | 8.08 | 8.24 a | 8.16 ab | 5.83 | 6.00 | 5.92 | 6.56 | 6.88 | 6.72 |
| 14 | 8.00 | 8.26 a | 8.13 ab | 5.70 | 6.09 | 5.90 | 6.52 | 6.71 | 6.61 | |
| 21 | 7.80 | 8.12 a | 7.95 bc | 5.80 | 5.60 | 5.70 | 6.40 | 6.54 | 6.47 | |
| S. cerevisiae | 7 | 8.13 | 8.28 a | 8.21 a | 5.90 | 5.60 | 5.75 | 6.46 | 6.49 | 6.48 |
| 14 | 7.76 | 7.89 b | 7.82 c | 5.60 | 5.57 | 5.58 | 6.50 | 6.60 | 6.55 | |
| 21 | 7.43 | 7.61 c | 7.52 d | 5.33 | 5.37 | 5.35 | 6.47 | 6.41 | 6.44 | |
| SEM | 0.12 | 0.06 | 0.06 | 0.14 | 0.16 | 0.13 | 0.15 | 0.12 | 0.10 | |
| Comparison | ||||||||||
| Yeast | Control | 7.09 c | 7.36 | 7.23 | 5.79 | 6.15 a | 5.97 | 5.85 b | 6.06 c | 5.96 b |
| P. kudriavzevii KKU20 | 8.07 a | 8.26 | 8.17 | 5.67 | 5.98 a | 5.83 | 6.38 a | 6.79 a | 6.59 a | |
| C. tropicalis KKU20 | 7.96 ab | 8.21 | 8.08 | 5.78 | 5.89 a | 5.84 | 6.49 a | 6.70 a | 6.60 a | |
| S. cerevisiae | 7.77 b | 7.93 | 7.85 | 5.61 | 5.51 b | 5.56 | 6.47 a | 6.50 b | 6.49 a | |
| Day | 7 | 7.92 a | 8.04 | 7.98 | 5.85 | 5.94 | 5.89 | 6.33 | 6.57 | 6.45 |
| 14 | 7.74 a | 7.95 | 7.85 | 5.67 | 5.92 | 5.80 | 6.31 | 6.55 | 6.43 | |
| 21 | 7.50 b | 7.83 | 7.67 | 5.62 | 5.80 | 5.71 | 6.27 | 6.44 | 6.35 | |
| Interaction | Yeast × Day | ns | ** | ** | ns | ns | ns | ns | ns | ns |
| Orthogonal contrast | ||||||||||
| Contrast 1 | Control | 7.09 b | 7.16 b | 7.23 b | 5.79 | 6.15 a | 5.97 a | 5.85 b | 6.06 b | 5.96 b |
| Yeast | 7.93 a | 7.93 a | 8.03 a | 5.69 | 5.79 b | 5.74 b | 6.45 a | 6.67 a | 6.56 a | |
| Contrast 2 | Yeast (−) | 8.01 a | 8.03 a | 8.12 a | 5.72 | 5.94 a | 5.83 a | 6.44 | 6.75 a | 6.60 |
| Yeast (+) | 7.77 b | 7.73 b | 7.85 b | 5.61 | 5.51 b | 5.56 b | 6.47 | 6.50b | 6.49 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suntara, C.; Cherdthong, A.; Uriyapongson, S.; Wanapat, M.; Chanjula, P. Comparison Effects of Ruminal Crabtree-Negative Yeasts and Crabtree-Positive Yeasts for Improving Ensiled Rice Straw Quality and Ruminal Digestion Using In Vitro Gas Production. J. Fungi 2020, 6, 109. https://doi.org/10.3390/jof6030109
Suntara C, Cherdthong A, Uriyapongson S, Wanapat M, Chanjula P. Comparison Effects of Ruminal Crabtree-Negative Yeasts and Crabtree-Positive Yeasts for Improving Ensiled Rice Straw Quality and Ruminal Digestion Using In Vitro Gas Production. Journal of Fungi. 2020; 6(3):109. https://doi.org/10.3390/jof6030109
Chicago/Turabian StyleSuntara, Chanon, Anusorn Cherdthong, Suthipong Uriyapongson, Metha Wanapat, and Pin Chanjula. 2020. "Comparison Effects of Ruminal Crabtree-Negative Yeasts and Crabtree-Positive Yeasts for Improving Ensiled Rice Straw Quality and Ruminal Digestion Using In Vitro Gas Production" Journal of Fungi 6, no. 3: 109. https://doi.org/10.3390/jof6030109
APA StyleSuntara, C., Cherdthong, A., Uriyapongson, S., Wanapat, M., & Chanjula, P. (2020). Comparison Effects of Ruminal Crabtree-Negative Yeasts and Crabtree-Positive Yeasts for Improving Ensiled Rice Straw Quality and Ruminal Digestion Using In Vitro Gas Production. Journal of Fungi, 6(3), 109. https://doi.org/10.3390/jof6030109







