N-Acetylglucosamine (GlcNAc) Sensing, Utilization, and Functions in Candida albicans
Abstract
:1. Introduction
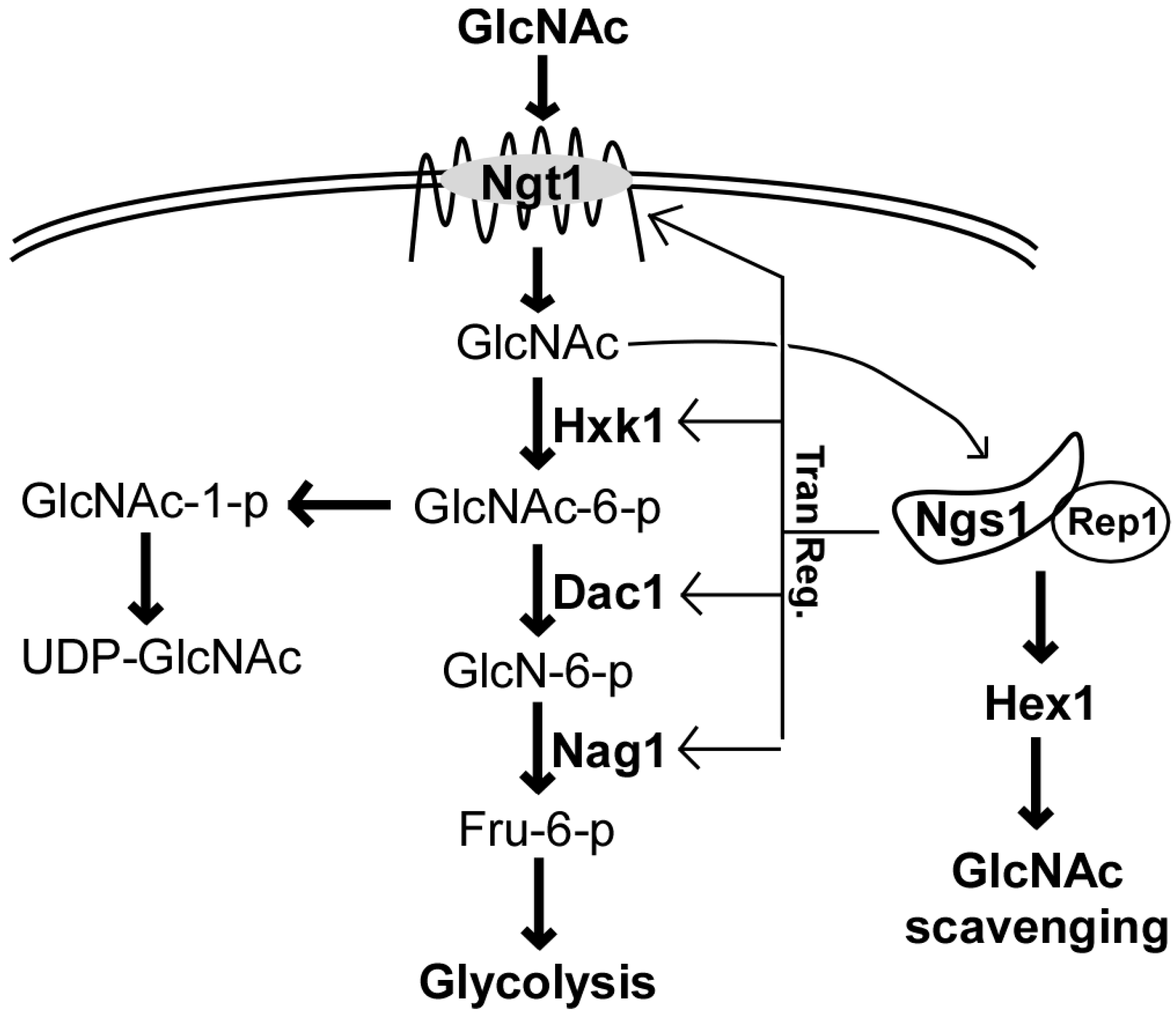
2. Ngt1, the GlcNAc-Specific Transporter
3. GlcNAc Catabolism
4. GlcNAc Regulates Morphological Transitions and Virulence in Fungi
5. GlcNAc Can Induce Morphological Transitions Independent of GlcNAc Catabolism
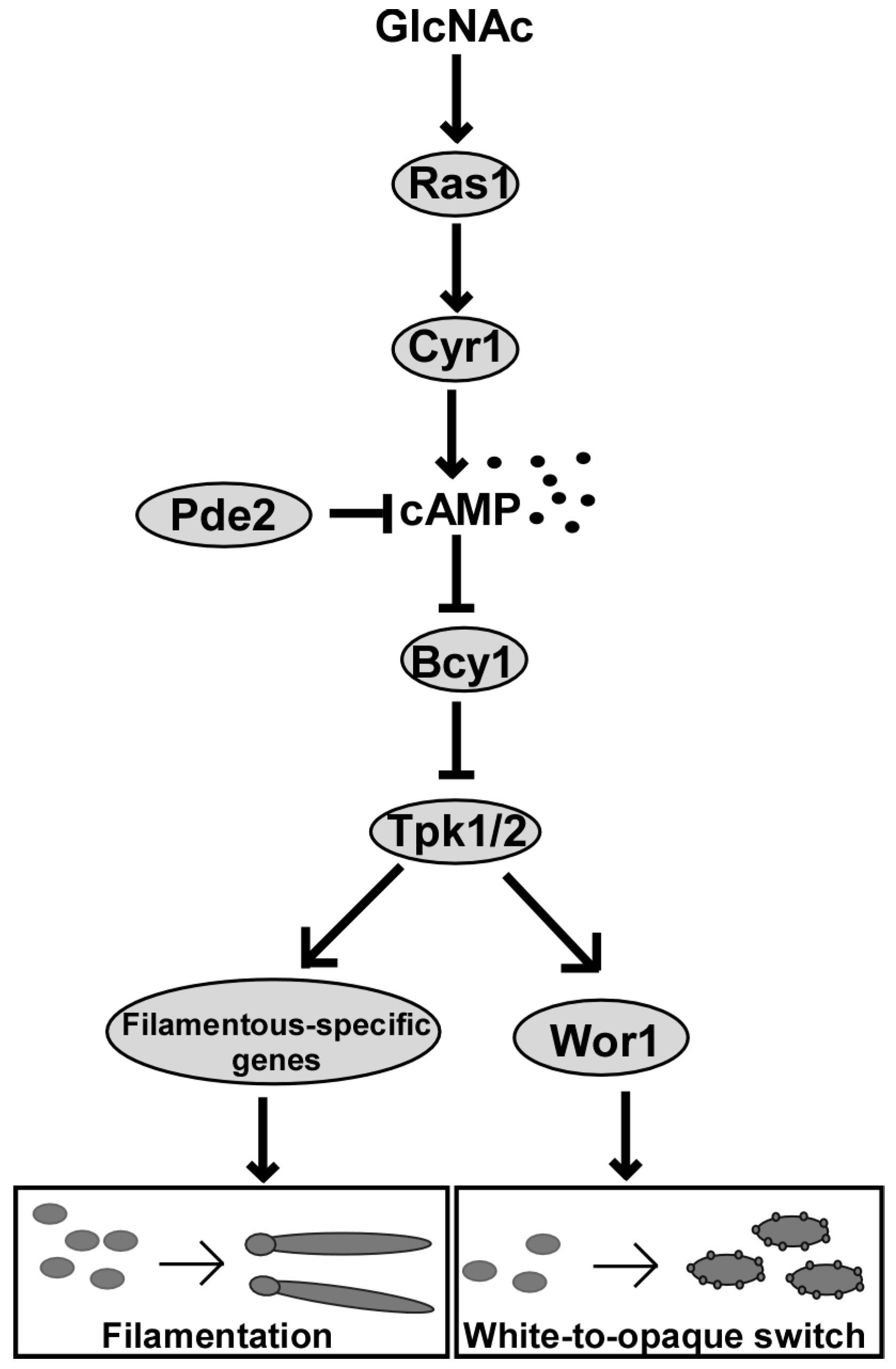
6. The Ras1-cAMP/PKA Signaling Pathway Functions in GlcNAc-Induced Filamentation
7. Ras1-cAMP/PKA Signaling Pathway Functions in GlcNAc-Induced White-Opaque Switching
8. GlcNAc-Induced Cell Death in C. albicans
9. Conclusions and Open Questions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Konopka, J.B. N-acetylglucosamine (glcnac) functions in cell signaling. Scientifica 2012, 2012, 489208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.; Naseem, S.; Konopka, J.B. N-acetylglucosamine regulates morphogenesis and virulence pathways in fungi. J. Fungi 2019, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Naseem, S.; Parrino, S.M.; Buenten, D.M.; Konopka, J.B. Novel roles for GlcNAc in cell signaling. Commun. Integr. Biol. 2012, 5, 156–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonetti, N.; Strippoli, V.; Cassone, A. Yeast-mycelial conversion induced by N-acetyl-d-glucosamine in Candida albicans. Nature 1974, 250, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yi, S.; Sahni, N.; Daniels, K.J.; Srikantha, T.; Soll, D.R. N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS Pathog. 2010, 6, e1000806. [Google Scholar] [CrossRef]
- Du, H.; Guan, G.; Li, X.; Gulati, M.; Tao, L.; Cao, C.; Johnson, A.D.; Nobile, C.J.; Huang, G. N-acetylglucosamine-induced cell death in Candida albicans and its implications for adaptive mechanisms of nutrient sensing in yeasts. mBio 2015, 6, e01376-15. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.B.; Lorenz, M.C. Multiple alternative carbon pathways combine to promote Candida albicans stress resistance, immune interactions, and virulence. mBio 2020, 11, e03070-19. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Zhang, Q.; Bing, J.; Ding, X.; Huang, G. Environmental and genetic regulation of white-opaque switching in Candida tropicalis. Mol. Microbiol. 2017, 106, 999–1017. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Tao, L.; Guan, G.; Yue, H.; Liang, W.; Cao, C.; Dai, Y.; Huang, G. Regulation of filamentation in the human fungal pathogen Candida tropicalis. Mol. Microbiol. 2016, 99, 528–545. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Du, H.; Guan, G.; Tong, Y.; Kourkoumpetis, T.K.; Zhang, L.; Bai, F.Y.; Huang, G. N-acetylglucosamine induces white-to-opaque switching and mating in Candida Tropicalis, providing new insights into adaptation and fungal sexual evolution. Eukaryot. Cell 2012, 11, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Perez-Campo, F.M.; Dominguez, A. Factors affecting the morphogenetic switch in Yarrowia lipolytica. Curr. Microbiol. 2001, 43, 429–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, S.A.; Naseem, S.; Konopka, J.B.; Sil, A. N-acetylglucosamine (glcnac) triggers a rapid, temperature-responsive morphogenetic program in thermally dimorphic fungi. PLoS Genet. 2013, 9, e1003799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty years of the sentry antifungal surveillance program: Results for Candida species from 1997-2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: Data from the prospective antifungal therapy (path) registry 2004-2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef] [Green Version]
- Odds, F.C. Molecular phylogenetics and epidemiology of Candida albicans. Future Microbiol. 2010, 5, 67–79. [Google Scholar] [CrossRef]
- Berman, J. Candida albicans. Curr. Biol. 2012, 22, R620–R622. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, F.J.; Konopka, J.B. Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans. Mol. Biol. Cell. 2007, 18, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.C.; Bender, J.A.; Fink, G.R. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 2004, 3, 1076–1087. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.J.; Jamaluddin, M.S.; Natarajan, K.; Kaur, D.; Datta, A. The inducible N-acetylglucosamine catabolic pathway gene cluster in Candida albicans: Discrete N-acetylglucosamine-inducible factors interact at the promoter of nag1. Proc. Natl. Acad. Sci. USA 2000, 97, 14218–14223. [Google Scholar] [CrossRef] [Green Version]
- Nadal, M.; Sawers, R.; Naseem, S.; Bassin, B.; Kulicke, C.; Sharman, A.; An, G.; An, K.; Ahern, K.R.; Romag, A.; et al. An N-acetylglucosamine transporter required for arbuscular mycorrhizal symbioses in rice and maize. Nat. Plants 2017, 3, 17073. [Google Scholar] [CrossRef] [Green Version]
- Kappel, L.; Gaderer, R.; Flipphi, M.; Seidl-Seiboth, V. The N-acetylglucosamine catabolic gene cluster in Trichoderma reesei is controlled by the ndt80-like transcription factor ron1. Mol. Microbiol. 2016, 99, 640–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada-Okabe, T.; Sakamori, Y.; Mio, T.; Yamada-Okabe, H. Identification and characterization of the genes for n-acetylglucosamine kinase and N-acetylglucosamine-phosphate deacetylase in the pathogenic fungus Candida albicans. Eur. J. Biochem. 2001, 268, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Datta, A. Molecular cloning and analysis of the nag1 cdna coding for glucosamine-6-phosphate deaminase from Candida albicans. J. Biol. Chem. 1993, 268, 9206–9214. [Google Scholar] [PubMed]
- Naseem, S.; Min, K.; Spitzer, D.; Gardin, J.; Konopka, J.B. Regulation of hyphal growth and N-acetylglucosamine catabolism by two transcription factors in Candida albicans. Genetics 2017, 206, 299–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; Lu, Y.; Liu, H. N-acetylglucosamine sensing by a gcn5-related n-acetyltransferase induces transcription via chromatin histone acetylation in fungi. Nat. Commun. 2016, 7, 12916. [Google Scholar] [CrossRef] [PubMed]
- Ruhela, D.; Kamthan, M.; Saha, P.; Majumdar, S.S.; Datta, K.; Abdin, M.Z.; Datta, A. In vivo role of Candida albicans beta-hexosaminidase (Hex1) in carbon scavenging. MicrobiologyOpen 2015, 4, 730–742. [Google Scholar] [CrossRef]
- Cannon, R.D.; Niimi, K.; Jenkinson, H.F.; Shepherd, M.G. Molecular cloning and expression of the Candida albicans beta-N-acetylglucosaminidase (hex1) gene. J. Bacteriol. 1994, 176, 2640–2647. [Google Scholar] [CrossRef] [Green Version]
- Min, K.; Biermann, A.; Hogan, D.A.; Konopka, J.B. Genetic analysis of Ndt80 family transcription factors in Candida albicans using new crispr-CAS9 approaches. mSphere 2018, 3, e00545-18. [Google Scholar] [CrossRef] [Green Version]
- Klengel, T.; Liang, W.J.; Chaloupka, J.; Ruoff, C.; Schroppel, K.; Naglik, J.R.; Eckert, S.E.; Mogensen, E.G.; Haynes, K.; Tuite, M.F.; et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr. Biol. 2005, 15, 2021–2026. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Srikantha, T.; Sahni, N.; Yi, S.; Soll, D.R. CO2 regulates white-to-opaque switching in Candida albicans. Curr. Biol. 2009, 19, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Davis, D. Adaptation to environmental ph in Candida albicans and its relation to pathogenesis. Curr. Genet. 2003, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.; Wilson, R.B.; Mitchell, A.P. Rim101-dependent and-independent pathways govern ph responses in Candida albicans. Mol. Cell. Biol. 2000, 20, 971–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G. Regulation of phenotypic transitions in the fungal pathogen Candida albicans. Virulence 2012, 3, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Van Dijck, P.; Datta, A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 2007, 71, 348–376. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Du, H.; Guan, G.; Dai, Y.; Nobile, C.J.; Liang, W.; Cao, C.; Zhang, Q.; Zhong, J.; Huang, G. Discovery of a “white-gray-opaque” tristable phenotypic switching system in Candida albicans: Roles of non-genetic diversity in host adaptation. PLoS Biol. 2014, 12, e1001830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pande, K.; Chen, C.; Noble, S.M. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat. Genet. 2013, 45, 1088–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, N.; Hasan, F.; Fries, B.C. Phenotypic switching in fungi. Curr. Fungal Inf. Rep. 2008, 2, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Slutsky, B.; Staebell, M.; Anderson, J.; Risen, L.; Pfaller, M.; Soll, D.R. White-opaque transition: A second high-frequency switching system in Candida albicans. J. Bacteriol. 1987, 169, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Slutsky, B.; Buffo, J.; Soll, D.R. High-frequency switching of colony morphology in Candida albicans. Science 1985, 230, 666–669. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Ishijima, S.A.; Hayama, K.; Takahashi, M.; Holmes, A.R.; Cannon, R.D.; Abe, S. N-acetylglucosamine increases symptoms and fungal burden in a murine model of oral candidiasis. Med. Mycol. 2012, 50, 252–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, C.Y.; Newport, G.; Murillo, L.A.; Jones, T.; Scherer, S.; Davis, R.W.; Agabian, N. Metabolic specialization associated with phenotypic switching in candidaalbicans. Proc. Natl. Acad. Sci. USA 2002, 99, 14907–14912. [Google Scholar] [CrossRef] [Green Version]
- Soll, D.R. Why does Candida albicans switch? FEMS Yeast Res. 2009, 9, 973–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Guan, G.; Li, C.; Nobile, C.J.; Tao, L.; Huang, G. Genetic regulation of the development of mating projections in Candida albicans. Emerg. Microbes Infect. 2020, 9, 413–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.G.; Johnson, A.D. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 2002, 110, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Soll, D.R. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J. Bacteriol. 1987, 169, 5579–5588. [Google Scholar] [CrossRef] [Green Version]
- Zordan, R.E.; Miller, M.G.; Galgoczy, D.J.; Tuch, B.B.; Johnson, A.D. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 2007, 5, e256. [Google Scholar] [CrossRef] [Green Version]
- Zordan, R.E.; Galgoczy, D.J.; Johnson, A.D. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc. Natl. Acad. Sci. USA 2006, 103, 12807–12812. [Google Scholar] [CrossRef] [Green Version]
- Srikantha, T.; Borneman, A.R.; Daniels, K.J.; Pujol, C.; Wu, W.; Seringhaus, M.R.; Gerstein, M.; Yi, S.; Snyder, M.; Soll, D.R. Tos9 regulates white-opaque switching in Candida albicans. Eukaryot. Cell 2006, 5, 1674–1687. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Wang, H.; Chou, S.; Nie, X.; Chen, J.; Liu, H. Bistable expression of Wor1, a master regulator of white-opaque switching in Candida albicans. Proc. Natl. Acad. Sci. USA 2006, 103, 12813–12818. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Tao, L.; Nobile, C.J.; Tong, Y.; Guan, G.; Sun, Y.; Cao, C.; Hernday, A.D.; Johnson, A.D.; Zhang, L.; et al. White-opaque switching in natural MTLa/alpha isolates of Candida albicans: Evolutionary implications for roles in host adaptation, pathogenesis, and sex. PLoS Biol. 2013, 11, e1001525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.N.; Conway, K.; Pujol, C.; Daniels, K.J.; Soll, D.R. Efg1 mutations, phenotypic switching, and colonization by clinical a/alpha strains of Candida albicans. mSphere 2020, 5, e00795-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.N.; Conway, K.; Conway, T.P.; Daniels, K.J.; Soll, D.R. Roles of the transcription factors Sfl2 and Efg1 in white-opaque switching in a/alpha strains of Candida albicans. mSphere 2019, 4, e00703-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, E.; Chrissian, C.; Cordero, R.J.B.; Liporagi-Lopes, L.; Stark, R.E.; Casadevall, A. N-acetylglucosamine affects Cryptococcus Neoformans cell-wall composition and melanin architecture. Microbiology 2017, 163, 1540–1556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, L.; Yuan, S.; Zhou, Q.; Wang, X.; Wang, L.; Hu, Z.; Yan, Y. Ngt1 is essential for n-acetylglucosamine-mediated filamentous growth inhibition and Hxk1 functions as a positive regulator of filamentous growth in candida tropicalis. Int. J. Mol. Sci. 2020, 21, 4036. [Google Scholar] [CrossRef]
- Naseem, S.; Gunasekera, A.; Araya, E.; Konopka, J.B. N-acetylglucosamine (GlcNAc) induction of hyphal morphogenesis and transcriptional responses in Candida albicans are not dependent on its metabolism. J. Biol. Chem. 2011, 286, 28671–28680. [Google Scholar] [CrossRef] [Green Version]
- Castilla, R.; Passeron, S.; Cantore, M.L. N-acetyl-d-glucosamine induces germination in Candida albicans through a mechanism sensitive to inhibitors of cAMP-dependent protein kinase. Cell. Signal. 1998, 10, 713–719. [Google Scholar] [CrossRef]
- Cao, C.; Guan, G.; Du, H.; Tao, L.; Huang, G. Role of the n-acetylglucosamine kinase (hxk1) in the regulation of white-gray-opaque tristable phenotypic transitions in c. Albicans. Fungal Genet. Biol. FG B 2016, 92, 26–32. [Google Scholar] [CrossRef]
- Rao, K.H.; Ruhela, D.; Ghosh, S.; Abdin, M.Z.; Datta, A. N-acetylglucosamine kinase, Hxk1 contributes to white-opaque morphological transition in Candida albicans. Biochem. Biophys. Res. Commun. 2014, 445, 138–144. [Google Scholar] [CrossRef]
- Rao, K.H.; Ghosh, S.; Natarajan, K.; Datta, A. N-acetylglucosamine kinase, Hxk1 is involved in morphogenetic transition and metabolic gene expression in Candida albicans. PLoS ONE 2013, 8, e53638. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Q.; Wei, Y.; Wang, Y.; Du, H. Multiple roles and diverse regulation of the r Ras/cAMP/protein kinase A pathway in Candida albicans. Mol. Microbiol. 2019, 111, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, D.A.; Sundstrom, P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. 2009, 4, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Fimia, G.M.; Sassone-Corsi, P. Cyclic AMP signalling. J. Cell Sci. 2001, 114, 1971–1972. [Google Scholar]
- Wang, Y. Fungal adenylyl cyclase acts as a signal sensor and integrator and plays a central role in interaction with bacteria. PLoS Pathog. 2013, 9, e1003612. [Google Scholar] [CrossRef] [Green Version]
- Piispanen, A.E.; Bonnefoi, O.; Carden, S.; Deveau, A.; Bassilana, M.; Hogan, D.A. Roles of ras1 membrane localization during Candida albicans hyphal growth and farnesol response. Eukaryot. Cell 2011, 10, 1473–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloutier, M.; Castilla, R.; Bolduc, N.; Zelada, A.; Martineau, P.; Bouillon, M.; Magee, B.B.; Passeron, S.; Giasson, L.; Cantore, M.L. The two isoforms of the cAMP-dependent protein kinase catalytic subunit are involved in the control of dimorphism in the human fungal pathogen Candida albicans. Fungal Gen. Biol. FG B 2003, 38, 133–141. [Google Scholar] [CrossRef]
- Inglis, D.O.; Sherlock, G. Ras signaling gets fine-tuned: Regulation of multiple pathogenic traits of Candida albicans. Eukaryot. Cell 2013, 12, 1316–1325. [Google Scholar] [CrossRef] [Green Version]
- Rocha, C.R.; Schroppel, K.; Harcus, D.; Marcil, A.; Dignard, D.; Taylor, B.N.; Thomas, D.Y.; Whiteway, M.; Leberer, E. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 2001, 12, 3631–3643. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.H.; Warn, P.; Ragni, E.; Popolo, L.; Nunn, C.D.; Turner, M.P.; Stateva, L. Deletion of PDE2, the gene encoding the high-affinity camp phosphodiesterase, results in changes of the cell wall and membrane in Candida albicans. Yeast 2005, 22, 285–294. [Google Scholar] [CrossRef]
- Giacometti, R.; Souto, G.; Silberstein, S.; Giasson, L.; Cantore, M.L.; Passeron, S. Expression levels and subcellular localization of bcy1p in Candida albicans mutant strains devoid of one BCY1 allele results in a defective morphogenetic behavior. Biochim. Biophys. Acta 2006, 1763, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Toda, T.; Cameron, S.; Sass, P.; Zoller, M.; Scott, J.D.; McMullen, B.; Hurwitz, M.; Krebs, E.G.; Wigler, M. Cloning and characterization of bcy1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol. Cell Biol. 1987, 7, 1371–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockmuhl, D.P.; Krishnamurthy, S.; Gerads, M.; Sonneborn, A.; Ernst, J.F. Distinct and redundant roles of the two protein kinase a isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol. Microbiol. 2001, 42, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cao, C.; Zheng, Q.; Huang, G. The regulatory subunit of protein kinase a (bcy1) in Candida albicans plays critical roles in filamentation and white-opaque switching but is not essential for cell growth. Front. Microbiol. 2016, 7, 2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrino, S.M.; Si, H.; Naseem, S.; Groudan, K.; Gardin, J.; Konopka, J.B. cAMP-independent signal pathways stimulate hyphal morphogenesis in Candida albicans. Mol. Microbiol. 2017, 103, 764–779. [Google Scholar] [CrossRef]
- Naseem, S.; Araya, E.; Konopka, J.B. Hyphal growth in Candida albicans does not require induction of hyphal-specific gene expression. Mol. Biol. Cell 2015, 26, 1174–1187. [Google Scholar] [CrossRef]
- Vesely, E.M.; Williams, R.B.; Konopka, J.B.; Lorenz, M.C. N-acetylglucosamine metabolism promotes survival of Candida albicans in the phagosome. mSphere 2017, 2, e00357-17. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Wu, M.; Bing, J.; Tao, L.; Ding, X.; Liu, X.; Huang, G. Global regulatory roles of the cAMP/PKA pathway revealed by phenotypic, transcriptomic and phosphoproteomic analyses in a null mutant of the PKA catalytic subunit in Candida albicans. Mol. Microbiol. 2017, 105, 46–64. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Cao, C.; Xie, J.; Ni, J.; Guan, G.; Tao, L.; Zhang, L.; Huang, G. N-acetylglucosamine-induced white-to-opaque switching in Candida albicans is independent of the Wor2 transcription factor. Fungal Gen. Biol. FG B 2014, 62, 71–77. [Google Scholar] [CrossRef]
- Du, H.; Guan, G.; Xie, J.; Cottier, F.; Sun, Y.; Jia, W.; Muhlschlegel, F.A.; Huang, G. The transcription factor Flo8 mediates CO2 sensing in the human fungal pathogen Candida albicans. Mol. Biol. Cell 2012, 23, 2692–2701. [Google Scholar] [CrossRef]
- Cao, F.; Lane, S.; Raniga, P.P.; Lu, Y.; Zhou, Z.; Ramon, K.; Chen, J.; Liu, H. The flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol. Biol. Cell 2006, 17, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Granot, D.; Snyder, M. Glucose induces cAMP-independent growth-related changes in stationary-phase cells of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1991, 88, 5724–5728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, M.W.; Hartwell, L.H. Control of cell division in Saccharomyces Cerevisiae by methionyl-trna. Proc. Natl. Acad. Sci. USA 1976, 73, 1664–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granot, D.; Dai, N. Sugar induced cell death in yeast is dependent on the rate of sugar phosphorylation as determined by Arabidopsis Thaliana hexokinase. Cell Death Differ. 1997, 4, 555–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granot, D.; Levine, A.; Dor-Hefetz, E. Sugar-induced apoptosis in yeast cells. FEMS Yeast Res. 2003, 4, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.E.; Smalley, D.J.; Tucker, D.L.; Leatham, M.P.; Norris, W.E.; Stevenson, S.J.; Anderson, A.B.; Grissom, J.E.; Laux, D.C.; Cohen, P.S.; et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA 2004, 101, 7427–7432. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.; Wang, H.; Liang, W.; Cao, C.; Tao, L.; Naseem, S.; Konopka, J.B.; Wang, Y.; Huang, G. The mitochondrial protein mcu1 plays important roles in carbon source utilization, filamentation, and virulence in Candida albicans. Fungal Gen. Biol. FG B 2015, 81, 150–159. [Google Scholar] [CrossRef]
- de Jonge, R.; Thomma, B.P. Fungal lysm effectors: Extinguishers of host immunity? Trends Microbiol. 2009, 17, 151–157. [Google Scholar] [CrossRef]
- Buist, G.; Steen, A.; Kok, J.; Kuipers, O.P. Lysm, a widely distributed protein motif for binding to (peptido) glycans. Mol. Microbiol. 2008, 68, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Kutty, S.N.; Philip, R. Marine yeasts-a review. Yeast 2008, 25, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Hagler, A.N.; Mendonca-Hagler, L.C. Yeasts from marine and estuarine waters with different levels of pollution in the state of rio de janeiro, brazil. Appl. Environ. Microbiol. 1981, 41, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Ennis, C.L.; Hernday, A.D.; Nobile, C.J.; Huang, G. N-Acetylglucosamine (GlcNAc) Sensing, Utilization, and Functions in Candida albicans. J. Fungi 2020, 6, 129. https://doi.org/10.3390/jof6030129
Du H, Ennis CL, Hernday AD, Nobile CJ, Huang G. N-Acetylglucosamine (GlcNAc) Sensing, Utilization, and Functions in Candida albicans. Journal of Fungi. 2020; 6(3):129. https://doi.org/10.3390/jof6030129
Chicago/Turabian StyleDu, Han, Craig L. Ennis, Aaron D. Hernday, Clarissa J. Nobile, and Guanghua Huang. 2020. "N-Acetylglucosamine (GlcNAc) Sensing, Utilization, and Functions in Candida albicans" Journal of Fungi 6, no. 3: 129. https://doi.org/10.3390/jof6030129




