Inactivation of Dermatophytes Causing Onychomycosis and Its Therapy Using Non-Thermal Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Experiments
2.1.1. Fungal Strains
2.1.2. Cultures
2.1.3. Arrangement of Experimental Plates
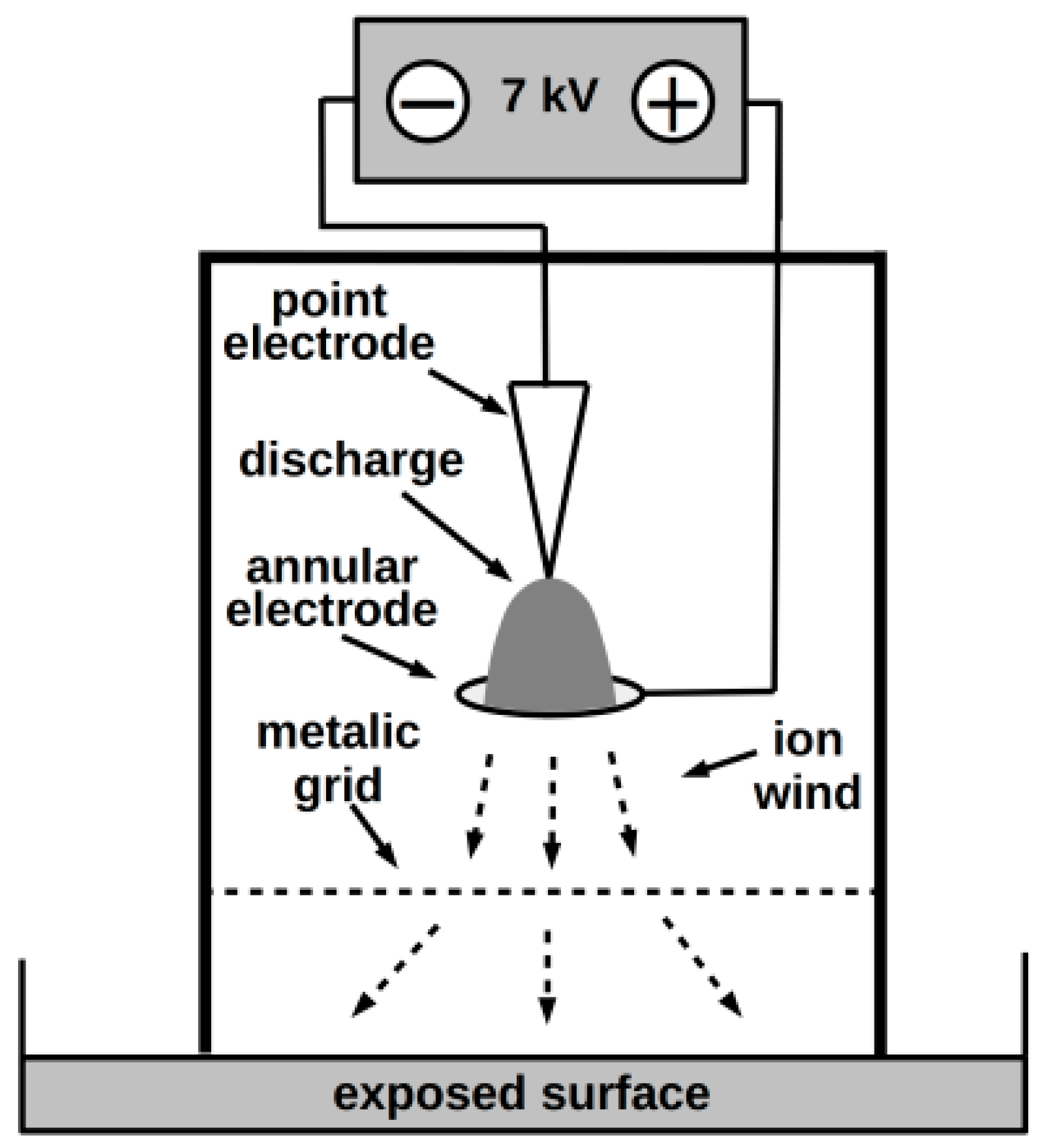
2.1.4. NTP Exposure of Plates
2.1.5. Evaluation
2.2. Onychomycosis Clinical Study
2.2.1. Clinical Sampling and Mycological Examination
2.2.2. PCR
2.2.3. Onychomycosis Therapy
2.2.4. Nail Plate Abrasion and Refreshment
2.2.5. Antimycotic Therapy
2.2.6. Therapeutic NTP Exposure of Nails
2.2.7. Evaluation of Therapy
3. Results
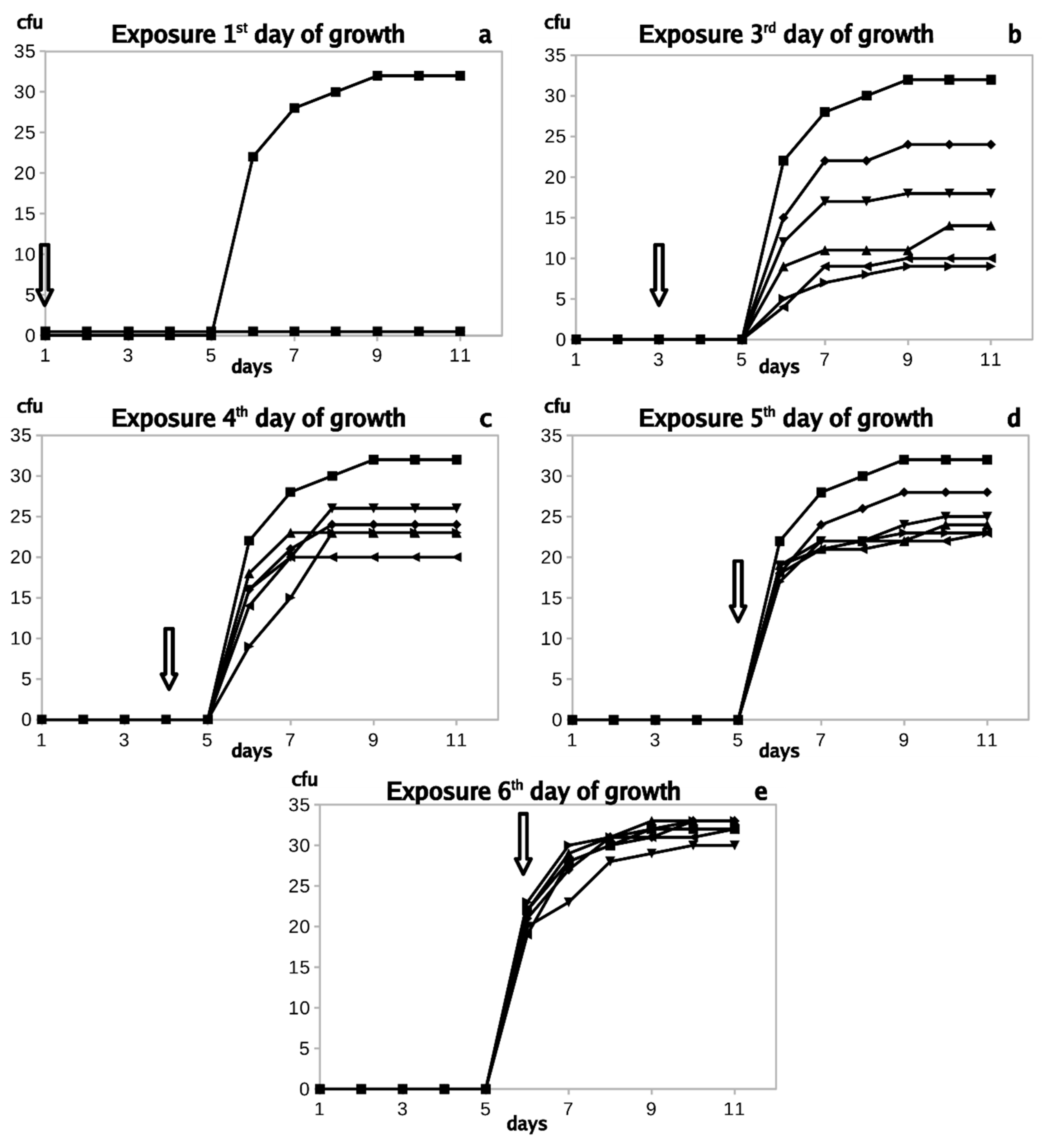
3.1. In Vitro Experiments
3.2. Onychomycosis Clinical Study
3.2.1. Participant Characteristics
3.2.2. Nail Plate Abrasion and Refreshment (Protocol 1)
3.2.3. Combination of Antimycotics with Non-Thermal Plasma Treatment (Protocol 2)
3.2.4. Combination of Nail Plate Abrasion and Refreshment with Non-Thermal Plasma Treatment (Protocol 3)
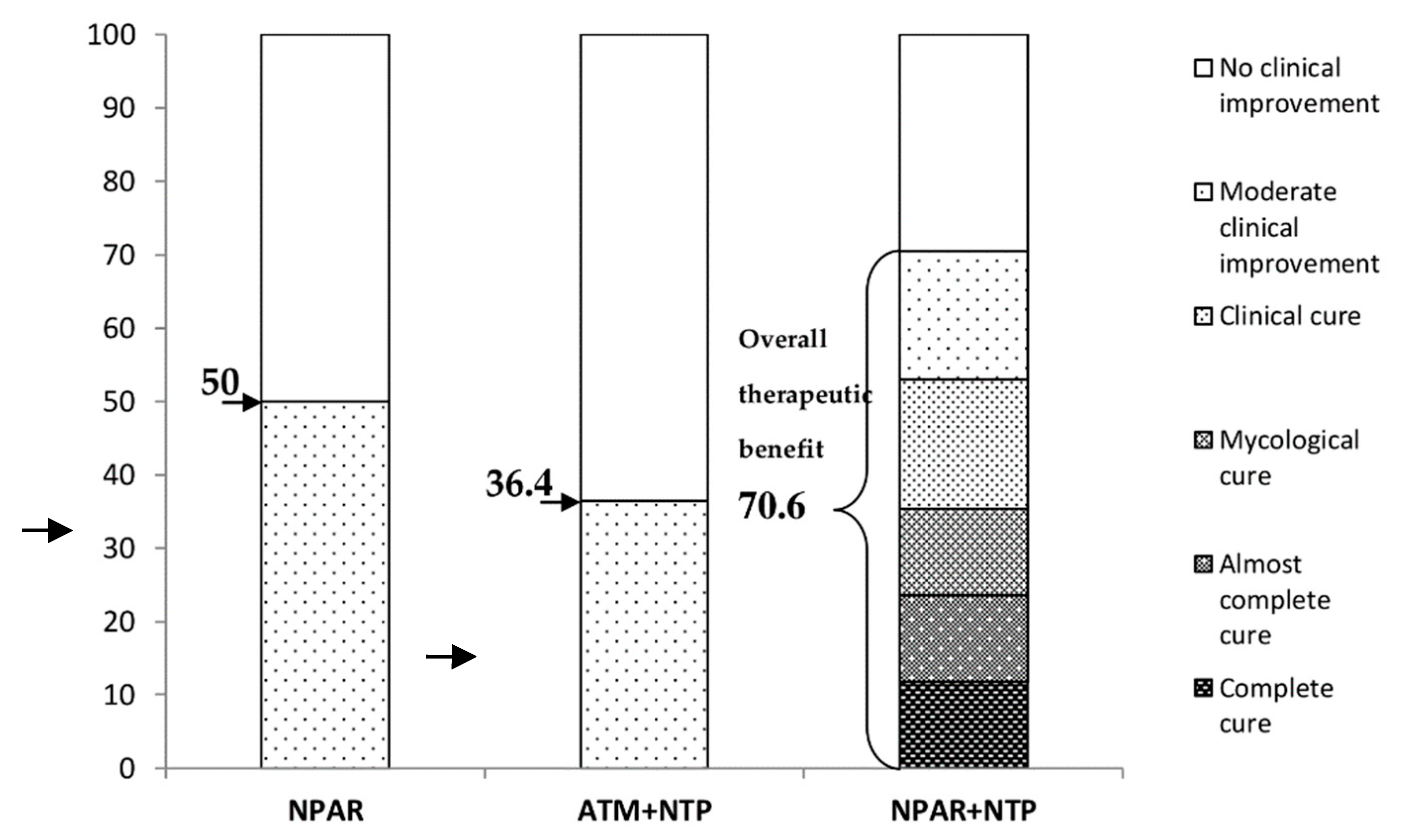
3.2.5. Evaluation of the Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elewski, B.E. Onychomycosis: Pathogenesis, diagnosis, and management. Clin. Microbiol. Rev. 1998, 11, 415–429. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.T.; Taylor, W.D.; Boyle, J. Guidelines for treatment of onychomycosis. Br. J. Dermatol. 2003, 148, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Foley, K.A.; Versteeg, S.G. New antifungal agents and new formulations against dermatophytes. Mycopathologia 2017, 182, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Asz-Sigall, D.; Tosti, A.; Arenas, R. Tinea unguium: Diagnosis and treatment in practice. Mycopathologia 2017, 182, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Bristow, I.R. The effectiveness of lasers in the treatment of onychomycosis: A systematic review. J. Foot Ankle Res. 2014, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francuzik, W.; Fritz, K.; Salavastru, C. Laser therapies for onychomycosis—Critical evaluation of methods and effectiveness. J. Eur. Acad. Dermatol. 2016, 30, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Aspiroz, C.; Martes, M.P.; Alcalde, V.; Espinel-Ingroff, A.; Rezusta, A. Treatment of refractory fingernail onychomycosis caused by nondermatophyte molds with methylaminolevulinate photodynamic therapy. J. Am. Acad. Dermatol. 2011, 65, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Morgado, L.F.; Trávolo, A.R.F.; Muehlmann, L.A.; Narcizo, P.S.; Nunes, R.B.; Pereira, P.A.G.; Py-Daniel, K.R.; Jiang, C.S.; Gu, J.; Azevedo, R.B.; et al. Photodynamic therapy treatment of onychomycosis with aluminium-phthalocyanine chloride nanoemulsions: A proof of concept clinical trial. J. Photochem. Photobiol. B Biol. 2017, 173, 266–270. [Google Scholar] [CrossRef]
- Ghannoum, M.; Isham, N. Fungal nail infections (onychomycosis): A never-ending story? PLoS Pathog. 2014, 10, e1004105. [Google Scholar] [CrossRef] [Green Version]
- Scholtz, V.; Julák, J. The “cometary” discharge, a possible new type of DC electric discharge in air at atmospheric pressure, and its bactericidal properties. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2010; p. 012005. [Google Scholar]
- Scholtz, V.; Julák, J. Plasma jetlike point-to-point electrical discharge in air and its bactericidal properties. IEEE Trans. Plasma Sci. 2010, 38, 1978–1980. [Google Scholar] [CrossRef]
- Yousfi, M.; Merbahi, N.; Sarrette, J.; Eichwald, O.; Ricard, A.; Gardou, J.; Ducasse, O.; Benhenni, M. Non thermal plasma sources of production of active species for biomedical uses: Analyses, optimization and prospect. In Biomedical Engineering—Frontiers and Challenges; IntechOpen: London, UK, 2011. [Google Scholar]
- Khun, J.; Scholtz, V.; Hozák, P.; Fitl, P.; Julák, J. Various DC-driven point-to-plain discharges as non-thermal plasma sources and their bactericidal effects. Plasma Sources Sci. Technol. 2018, 27, 065002. [Google Scholar] [CrossRef]
- Julák, J.; Soušková, H.; Scholtz, V.; Kvasničková, E.; Savická, D.; Kříha, V. Comparison of fungicidal properties of non-thermal plasma produced by corona discharge and dielectric barrier discharge. Folia Microbiol. 2018, 63, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Kelly, S.; Turner, M.M. Atomic oxygen patterning from a biomedical needle-plasma source. J. Appl. Phys. 2013, 114, 123301. [Google Scholar] [CrossRef] [Green Version]
- Sysolyatina, E.; Mukhachev, A.; Yurova, M.; Grushin, M.; Karalnik, V.; Petryakov, A.; Trushkin, N.; Ermolaeva, S.; Akishev, Y. Role of the charged particles in bacteria inactivation by plasma of a positive and negative corona in ambient air. Plasma Processes Polym. 2014, 11, 315–334. [Google Scholar] [CrossRef]
- Liu, D.X.; Liu, Z.C.; Chen, C.; Yang, A.J.; Li, D.; Rong, M.Z.; Chen, H.L.; Kong, M.G. Aqueous reactive species induced by a surface air discharge: Heterogeneous mass transfer and liquid chemistry pathways. Sci. Rep. 2016, 6, 23737. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jäger, A.; Polívka, L.; Syková, E.; Dejneka, A.; Kubinová, Š. The interplay between biological and physical scenarios of bacterial death induced by non-thermal plasma. Biomaterials 2016, 82, 71–83. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Julák, J.; Scholtz, V. The potential for use of non-thermal plasma in microbiology and medicine. Epidemiol. Microbiol. Im. Cas. Spol. Epidemiol. Mikrobiol. Ceske Lek. Spol. J.E. Purkyne 2020, 69, 29–37. [Google Scholar]
- Zhao, Y.M.; Ojha, S.; Burgess, C.M.; Sun, D.W.; Tiwari, B.K. Inactivation efficacy and mechanisms of plasma activated water on bacteria in planktonic state. J. Appl. Microbiol. 2020. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Von Woedtke, T.; Weltmann, K.-D. Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Gweon, B.; Kim, K.; Choe, W.; Shin, J.H. Therapeutic uses of atmospheric pressure plasma: Cancer and wound. In Biomedical Engineering: Frontier Research and Converging Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 357–385. [Google Scholar]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for treating cancer: Opportunities for adaptive and self-adaptive approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Hong, Y.J.; Park, J.; Lee, S.; Choi, E.; Kwon, G.C.; Park, B. A novel approach to inactivate the clinical isolates of Trichophyton mentagrophytes and Trichophyton rubrum by using non-thermal plasma. J. Microb. Biochem. Technol. 2014, 6, 314–319. [Google Scholar]
- Shapourzadeh, A.; Rahimi-Verki, N.; Atyabi, S.M.; Shams-Ghahfarokhi, M.; Jahanshiri, Z.; Irani, S.; Razzaghi-Abyaneh, M. Inhibitory effects of cold atmospheric plasma on the growth, ergosterol biosynthesis, and keratinase activity in Trichophyton rubrum. Arch. Biochem. Biophys. 2016, 608, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores. J. Food Eng. 2017, 196, 139–149. [Google Scholar] [CrossRef]
- Borges, A.C.; Lima, G.M.G.; Nishime, T.M.C.; Gontijo, A.V.L.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In vivo study. PLoS ONE 2018, 13, e0199832. [Google Scholar] [CrossRef] [PubMed]
- Hojnik, N.; Modic, M.; Ni, Y.; Filipič, G.; Cvelbar, U.; Walsh, J.L. Effective fungal spore inactivation with an environmentally friendly approach based on atmospheric pressure air plasma. Environ. Sci. Technol. 2019, 53, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Yadav, B.; Roopesh, M.; Jo, C. Cold plasma for effective fungal and mycotoxin control in foods: Mechanisms, inactivation effects, and applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Julák, J.; Scholtz, V.; Vaňková, E. Medically important biofilms and non-thermal plasma. World J. Microb Biot. 2018, 34, 178. [Google Scholar] [CrossRef]
- Soušková, H.; Scholtz, V.; Julák, J.; Kommová, L.; Savická, D.; Pazlarová, J. The survival of micromycetes and yeasts under the low-temperature plasma generated in electrical discharge. Folia Microbiol. 2011, 56, 77–79. [Google Scholar] [CrossRef]
- Scholtz, V.; Soušková, H.; Hubka, V.; Švarcová, M.; Julák, J. Inactivation of human pathogenic dermatophytes by non-thermal plasma. J. Microbiol. Methods 2015, 119, 53–58. [Google Scholar] [CrossRef]
- Xiong, Z.; Roe, J.; Grammer, T.C.; Graves, D.B. Plasma treatment of onychomycosis. Plasma Processes Polym. 2016, 13, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Daeschlein, G.; Scholz, S.; von Woedtke, T.; Niggemeier, M.; Kindel, E.; Brandenburg, R.; Weltmann, K.-D.; Junger, M. In vitro killing of clinical fungal strains by low-temperature atmospheric-pressure plasma jet. IEEE Trans. Plasma Sci. 2010, 39, 815–821. [Google Scholar] [CrossRef]
- Scholtz, V.; Soušková, H.; Švarcová, M.; Kríha, V.; Živná, H.; Julák, J. Inactivation of dermatophyte infection by nonthermal plasma on animal model. Med. Mycol. 2017, 55, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Švarcová, M.; Julák, J.; Hubka, V.; Soušková, H.; Scholtz, V. Treatment of a superficial mycosis by low-temperature plasma: A case report. Prague Med. Rep. 2014, 115, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. National Library of Medicine (Bethesda, MD, USA) (2013). Pilot Study to Evaluate Plasma Treatment of Onychomycosis; NCT01819051. Available online: https://clinicaltrials.gov/ct2/show/NCT01819051 (accessed on 1 October 2020).
- ClinicalTrials.gov. National Library of Medicine (Bethesda, MD, USA) (2016-2019). Evaluating the Safety, Tolerability and Preliminary Efficacy of Plasma in Improving the Appearance of Onychomycosis; NCT02724384. Available online: https://clinicaltrials.gov/ct2/show/NCT02724384 (accessed on 1 October 2020).
- ClinicalTrials.gov. National Library of Medicine (Bethesda, MD, USA) (2017-2018). Early Feasibility Study to Evaluate the Efficacy of the RenewalNail™ Plasma Treatment System in Patients With Onychomycosis (Fungal Nail); NCT03216200. Available online: https://clinicaltrials.gov/ct2/show/NCT03216200 (accessed on 1 October 2020).
- Lux, J.; Dobiáš, R.; Scholtz, V.; Khun, J.; Soušková, H.; Mrázková, E.; Julák, J. Možnosti terapie onychomykózy nízkoteplotním plazmatem. Cesk. Dermatol. 2018, 93, 266–271. [Google Scholar]
- Julák, J.; Scholtz, V. Decontamination of human skin by low-temperature plasma produced by cometary discharge. Clin. Plasma Med. 2013, 1, 31–34. [Google Scholar] [CrossRef]
- Haertel, B.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Heinlin, J.; Morfill, G.; Landthaler, M.; Stolz, W.; Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Karrer, S. Plasma medicine: Possible applications in dermatology. J. Dtsch. Dermatol. Ges. 2010, 8, 968–976. [Google Scholar] [CrossRef]
- Hubka, V.; Čmoková, A.; Peano, A.; Větrovský, T.; Dobiáš, R.; Mallátová, N.; Lysková, P.; Mencl, K.; Janouškovcová, H.; Stará, J. Zoonotické dermatofytózy: Klinický obraz, diagnostika, etiologie, léčba, epidemiologická situace u nás. Cesk. Dermatol. 2018, 93, 205–292. [Google Scholar]
- Hubka, V.; Větrovský, T.; Dobiášová, S.; Skořepová, M.; Lysková, P.; Mencl, K.; Mallátová, N.; Janouškovcová, H.; Hanzlíčková, J.; Dobiáš, R. Molekulární epidemiologie dermatofytóz v České republice—Výsledky dvouleté studie. Cesk. Dermatol. 2014, 89, 167–174. [Google Scholar]
- Scholtz, V.; Kvasničková, E.; Julák, J. Microbial inactivation by electric discharge with metallic grid. Acta Phys. Pol. A 2013, 124, 62–65. [Google Scholar] [CrossRef]
- Dobiáš, R.; Kantorová, M.; Jaworská, P.; Hamal, P.; Mrázek, J. Využití PCR-HRMA při přímé detekci a identifikaci původců dermatofytóz z klinických vzorků. Cesk. Dermatol. 2018, 93, 259–265. [Google Scholar]
- Didehdar, M.; Khansarinejad, B.; Amirrajab, N.; Shokohi, T. Development of a high-resolution melting analysis assay for rapid and high-throughput identification of clinically important dermatophyte species. Mycoses 2016, 59, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, A.M.; van der Ent, M.; Klaassen, A.; Bohm, N.; Andriesse, G.I.; Wintermans, R.G. Evaluation of a single-tube real-time PCR for detection and identification of 11 dermatophyte species in clinical material. Clin. Microbiol. Infect. 2010, 16, 704–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohst, T.; Kupsch, C.; Graser, Y. Detection of common dermatophytes in clinical specimens using a simple quantitative real-time TaqMan polymerase chain reaction assay. Br. J. Dermatol. 2016, 174, 602–609. [Google Scholar] [CrossRef]
- Gupta, A.K.; Studholme, C. How do we measure efficacy of therapy in onychomycosis: Patient, physician, and regulatory perspectives. J. Dermatol. Treat. 2016, 27, 498–504. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Treatment and prevention of recurrence. J. Am. Acad. Dermatol. 2019, 80, 853–867. [Google Scholar] [CrossRef]
- Lipner, S.R.; Friedman, G.; Scher, R.K. Pilot study to evaluate a plasma device for the treatment of onychomycosis. Clin. Exp. Dermatol. 2017, 42, 295–298. [Google Scholar] [CrossRef]
- Di Chiacchio, N.; Kadunc, B.V.; de Almeida, A.R.; Madeira, C.L. Nail abrasion. J. Cosmet. Dermatol. 2003, 2, 150–152. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Processes Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Friedman, P.C.; Miller, V.; Fridman, G.; Lin, A.; Fridman, A. Successful treatment of actinic keratoses using nonthermal atmospheric pressure plasma: A case series. J. Am. Acad. Dermatol. 2017, 76, 349–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Primer/Probe | Region | Sequence 5′-3′ | Reference |
|---|---|---|---|
| PanDerm_F1 | ITS1-5,8S | AGCGCYCGCCGRAGGA | [52] |
| PanDerm_R1 | ITS1-5,8S | GATTCACGGAATTCTGCAATTCAC | [52] |
| Derm_FAM | ITS1-5,8S | [6FAM]CGCATTTCGCTGCGTTCTTCATC[BHQ1] | [52] |
| PanDerm_HRM_F | ITS1-5,8S-ITS2 | TGCGGAAGGATCATTAACG | [50] |
| PanDerm_HRM_R | ITS1-5,8S-ITS2 | ACCAAGAGATCCGTTGTTG | [50] |
| Endpoint (Mycological/Clinical) | Definition |
|---|---|
| Mycological cure | Negative KOH microscopy, negative culture |
| Clinical cure | >90% clearance of the previously affected part of the nail plate |
| Moderate clinical improvement | 25–50% (or 30–60%) clinical improvement |
| No clinical improvement | 0–25% (or 0–30%) clinical improvement |
| Endpoint (Mycological + Clinical) | |
| Complete cure | Mycological cure, clinical cure (0% nail plate involvement) |
| Almost complete cure | Mycological cure, ≤10% nail plate involvement (or ≤5% nail plate involvement |
| Examination before Therapy | Examination after Therapy | ||||||
|---|---|---|---|---|---|---|---|
| Patient No. | Microscopy | Culture | DNA/PCR | Microscopy | Culture | DNA/PCR | Therapeutic Effect |
| 1 | positive | TI | TI | positive | TI | TI | MCI |
| 2 | positive | TR | TR | positive | TR | TR | MCI |
| 3 | positive | TI | TI | positive | TI | TI | MCI |
| 4 | positive | TR | TR | positive | TR | TR | MCI |
| 5 | positive | TR | TR | positive | TR | TR | MCI |
| 6 | positive | TR | TR | positive | TR | TR | MCI |
| 7 | positive | TR | TR | positive | TR | TR | NCI |
| 8 | positive | TR | TR | positive | TR | TR | NCI |
| 9 | positive | TR | TR | positive | TR | TR | NCI |
| 10 | positive | TR | TR | positive | TR | TR | NCI |
| 11 | positive | TI | TI | positive | TI | TI | NCI |
| 12 | positive | TR | TR | positive | TR | TR | NCI |
| Examination before Therapy | Examination after Therapy | ||||||
|---|---|---|---|---|---|---|---|
| Patient No. | Microscopy | Culture | DNA/PCR | Microscopy | Culture | Therapeutic Effect | ATM |
| 13 | positive | TR | NA | positive | TR | MCI | Amo |
| 14 | positive | TR | NA | positive | TR | MCI | Amo, Eco, Cic |
| 15 | positive | TR | NA | positive | TR | MCI | Amo |
| 16 | positive | TR | NA | positive | TR | MCI | Cic, 40% urea/vas |
| 17 | positive | TR | NA | positive | TR | NCI | Cic NP, Ter tbl |
| 18 | positive | TR | NA | positive | TR | NCI | Amo NP, Cic |
| 19 | positive | TR | NA | positive | TR | NCI | Amo NP, Cic |
| 20 | positive | TR | NA | positive | TR | NCI | Amo NP |
| 21 | positive | TR | NA | positive | TR | NCI | Cic, Amo NP |
| 22 | positive | TR | NA | positive | TR | NCI | Ter tbl, Cic NP |
| 23 | positive | negative | NA | positive | negative | NCI | Cic |
| Examination before Therapy | Examination after Therapy | ||||||
|---|---|---|---|---|---|---|---|
| Patient No. | Microscopy | Culture | DNA/PCR | Microscopy | Culture | DNA/PCR | Therapeutic Effect |
| 24 | positive | negative | negative | negative | negative | negative | Complete C |
| 25 | positive | TR | TR | negative | negative | negative | Complete C |
| 26 | positive | TI | TI | negative | negative | TI | ACC |
| 27 | positive | negative | negative | negative | negative | negative | ACC |
| 28 | positive | TI | TI | negative | negative | negative | MC, NCI |
| 29 | positive | TR | TR | negative | negative | TR | MC, NCI |
| 30 | positive | TR | TR | positive | TR | TR | NCI |
| Examination before Therapy | Examination after Therapy | ||||||
|---|---|---|---|---|---|---|---|
| Patient No. | Microscopy | Culture | DNA/PCR | Microscopy | Culture | DNA/PCR | Therapeutic Effect |
| 31 | negative | negative | negative | negative | negative | negative | Clinical C |
| 32 | negative | negative | negative | negative | negative | negative | Clinical C |
| 33 | negative | negative | negative | negative | negative | negative | Clinical C |
| 34 | negative | negative | negative | negative | negative | negative | MCI |
| 35 | negative | negative | negative | negative | negative | negative | MCI |
| 36 | negative | negative | negative | negative | negative | negative | MCI |
| 37 | negative | negative | negative | negative | negative | negative | NCI |
| 38 | negative | negative | negative | negative | negative | negative | NCI |
| 39 | negative | negative | negative | negative | negative | negative | NCI |
| 40 | negative | negative | negative | negative | negative | negative | NCI |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lux, J.; Dobiáš, R.; Kuklová, I.; Litvik, R.; Scholtz, V.; Soušková, H.; Khun, J.; Mrázek, J.; Kantorová, M.; Jaworská, P.; et al. Inactivation of Dermatophytes Causing Onychomycosis and Its Therapy Using Non-Thermal Plasma. J. Fungi 2020, 6, 214. https://doi.org/10.3390/jof6040214
Lux J, Dobiáš R, Kuklová I, Litvik R, Scholtz V, Soušková H, Khun J, Mrázek J, Kantorová M, Jaworská P, et al. Inactivation of Dermatophytes Causing Onychomycosis and Its Therapy Using Non-Thermal Plasma. Journal of Fungi. 2020; 6(4):214. https://doi.org/10.3390/jof6040214
Chicago/Turabian StyleLux, Jaroslav, Radim Dobiáš, Ivana Kuklová, Radek Litvik, Vladimír Scholtz, Hana Soušková, Josef Khun, Jakub Mrázek, Michaela Kantorová, Pavla Jaworská, and et al. 2020. "Inactivation of Dermatophytes Causing Onychomycosis and Its Therapy Using Non-Thermal Plasma" Journal of Fungi 6, no. 4: 214. https://doi.org/10.3390/jof6040214
APA StyleLux, J., Dobiáš, R., Kuklová, I., Litvik, R., Scholtz, V., Soušková, H., Khun, J., Mrázek, J., Kantorová, M., Jaworská, P., Prejdová, T., Šnupárková, J., Hamal, P., & Julák, J. (2020). Inactivation of Dermatophytes Causing Onychomycosis and Its Therapy Using Non-Thermal Plasma. Journal of Fungi, 6(4), 214. https://doi.org/10.3390/jof6040214






