Bioprospecting White-Rot Basidiomycete Irpex lacteus for Improved Extraction of Lignocellulose-Degrading Enzymes and Their Further Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungus and Culture Conditions
2.2. Lignocellulosic Substrates
2.3. Enzyme Production
2.4. Enzyme Extraction
2.5. Enzymatic Hydrolysis
2.6. Sugar Analysis
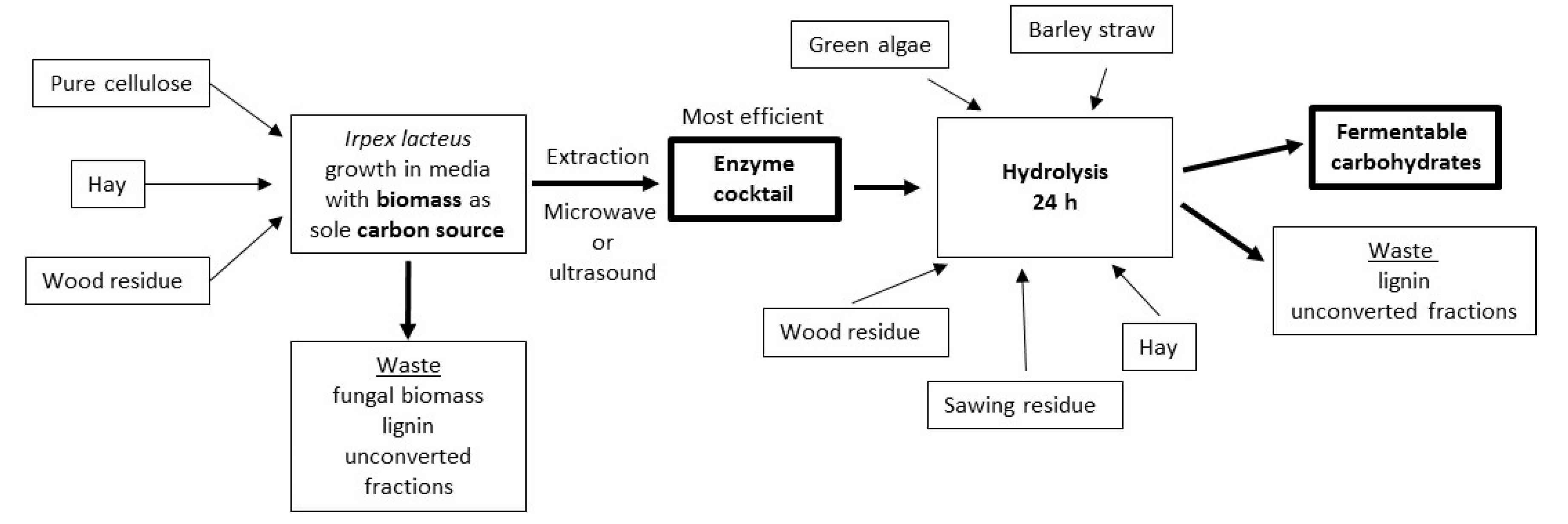
3. Results and Discussion
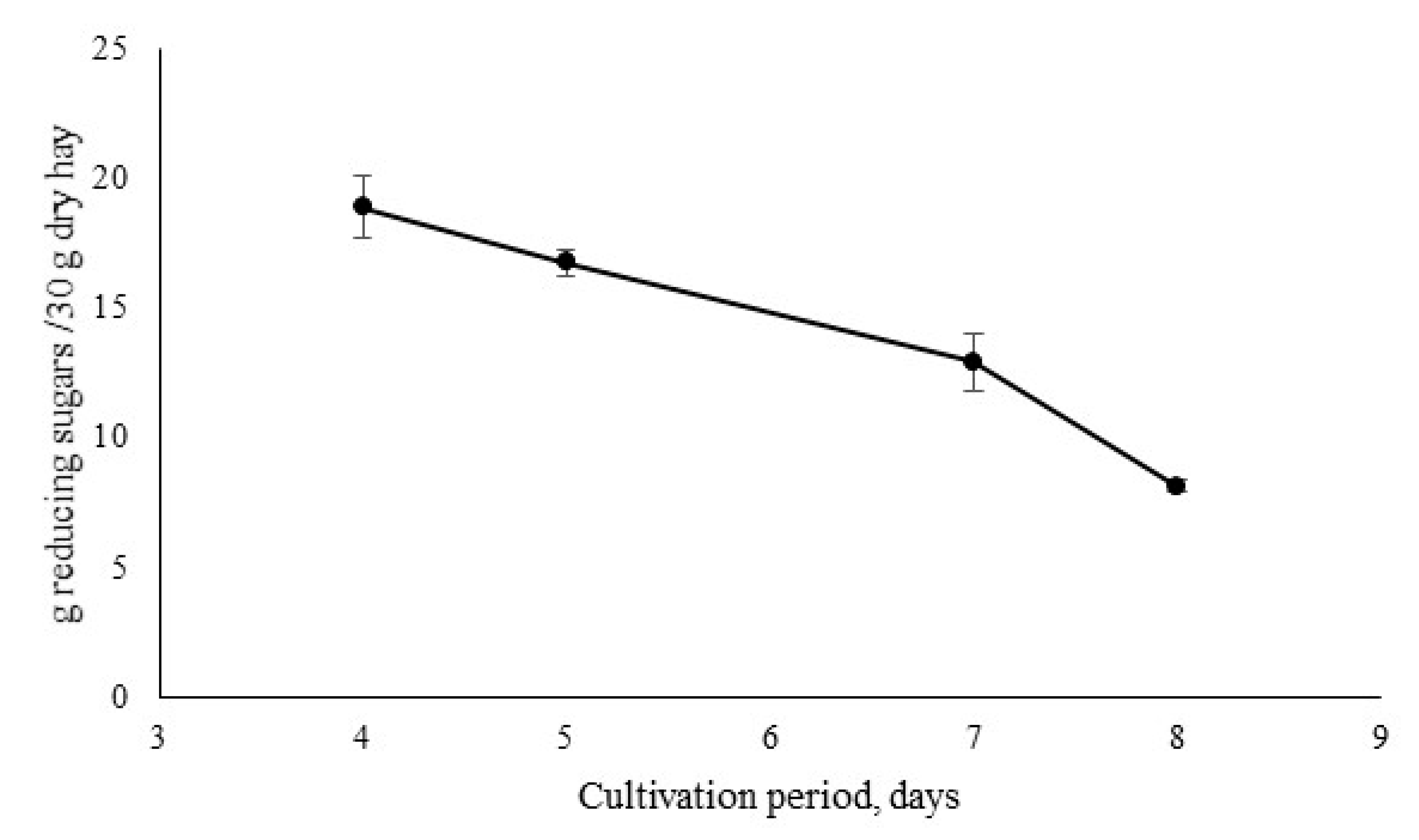
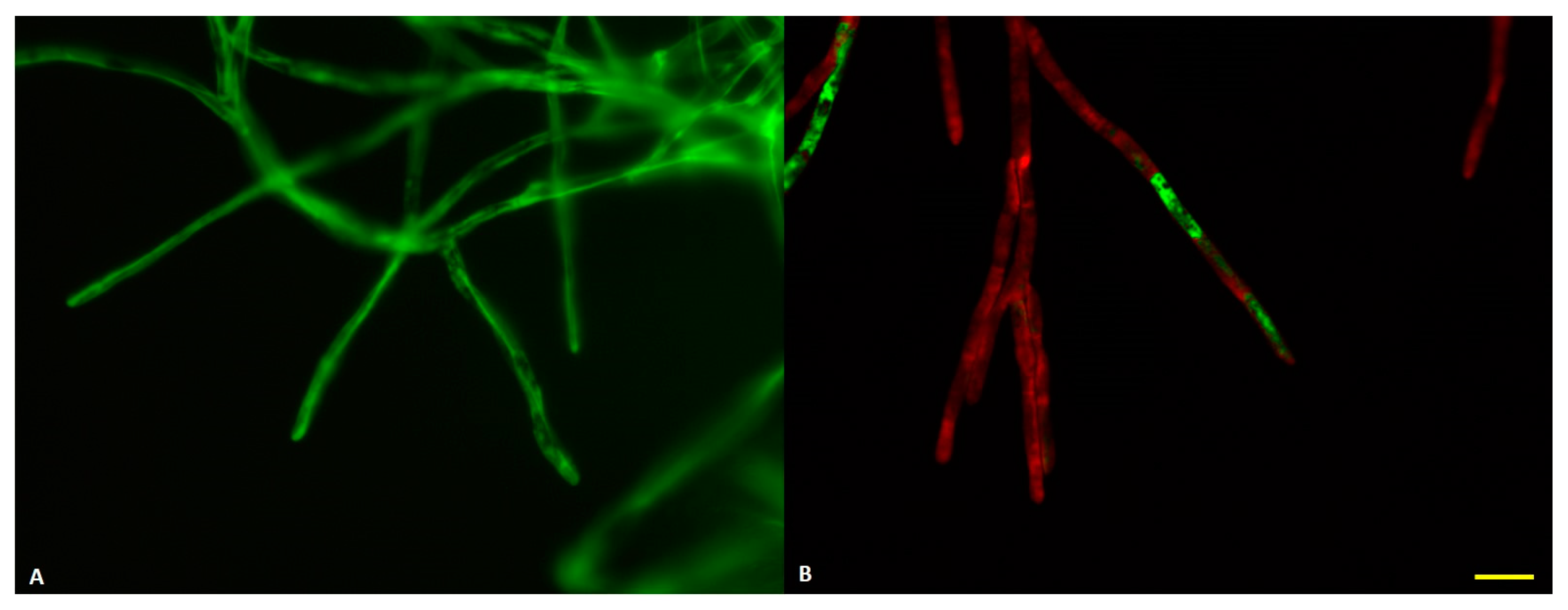
3.1. Fungal Cultivation Conditions in Biomass Substrate
3.2. Enzyme Extraction Efficiency
3.3. Production of Fermentable Sugars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Directive (EU). 2018/2001 on the promotion of the use of energy from renewable sources. Off. J. Eur. Union 2018, 5, 82–209. [Google Scholar]
- European Commission 11 February 2020 Eurostat. Renewable Energy for Heating and Cooling. Available online: https://ec.europa.eu/eurostat/en/web/products-eurostat-news/product/-/asset_publisher/VWJkHuaYvLIN (accessed on 25 February 2020).
- European Commission, 23 January 2020 Eurostat. Energy for Transport: 8% from Renewable Sources. Available online: https://ec.europa.eu/eurostat/en/web/products-eurostat-news/-/DDN-20200123-2 (accessed on 25 February 2020).
- Ahorsu, R.; Medina, F.; Constanti, M. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Laca, A.; Laca, A.; Diaz, M. Chapter 8. Hydrolysis: From cellulose and hemicellulose to simple sugars. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–240. [Google Scholar]
- Aslanzadeh, S.; Ishola, M.M.; Richards, T.; Tahersadeh, M.J. Chapter 1. An overview of existing individual unit operations. In Biorefineries. Integrated Biochemical Processes for Liquid Biofuels; Qureshi, N., Hodge, D.B., Vertes, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–36. [Google Scholar]
- Obeng, E.M.; Adam, S.N.N.; Budiman, C.; Ongkudon, C.M.; Maas, R.; Jose, J. Lignocellulases: A review of emerging and developing enzymes, systems, and practices. Biores. Bioproc. 2017, 4, 16. [Google Scholar] [CrossRef]
- Metreveli, E.; Kachlishvili, E.; Denchev, D.; Elisashvili, V. Improved cellulose and xylanase production by co-cultivation of white-rot basidiomycetes. EEEP 2014, 1, 5–11. [Google Scholar]
- Mishra, V.; Jana, A.K.; Jana, M.M.; Gupta, A. Fungal pretreatment of sweet sorghum bagasse with supplements: Improvement in lignin degradation, selectivity and enzymatic saccharification. 3 Biotech 2017, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Metreveli, E.; Kachlishvili, E.; Singer, S.W.; Elisashvili, V. Alteration of white-rot basidiomycetes cellulase and xylanase activities in the submerged co-cultivation and optimization of enzyme production by Irpex lacteus and Schizophyllum commune. Biores. Tech. 2017, 241, 652–660. [Google Scholar] [CrossRef]
- Rodriguez, M.D.; Paiva, I.M.A.; Castrillo, M.L.; Zapata, P.D.; Villalba, L.L. KH2PO4 improves cellulase production of Irpex lacteus and Pycnoporus sanguineus. J. King Saud Univ. Sci. 2019, 31, 434–444. [Google Scholar] [CrossRef]
- Oliver, C.M.; Mawson, R.; Melton, L.D.; Dumsday, G.; Welch, J.; Sanguansri, P.; Singh, T.K.; Augustin, M.A. Sequential low and medium frequency ultrasound assists biodegradation of wheat chaff by white rot fungal enzymes. Carbohydr. Polym. 2014, 111, 183–190. [Google Scholar] [CrossRef]
- Sabarez, H.; Oliver, C.M.; Mawson, R.; Dumsday, G.; Singh, T.; Bitto, N.; McSweeney, C.; Augustin, M.A. Synergism between ultrasonic pre-treatment and white rot fungal enzymes on biodegradation of wheat chaff. Ultrason. Sonoschem. 2014, 21, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Mezule, L.; Dalecka, B.; Juhna, T. Fermentable Sugar Production from Lignocellulosic waste. Chem. Eng. Trans. 2015, 43, 619–624. [Google Scholar]
- Qin, X.; Su, X.; Luo, H.; Ma, R.; Yao, B.; Ma, F. Deciphering lignocellulose deconstruction by the white rot fungus Irpex lacteus based on genomic and transcriptomic analyses. Biotechnol. Biofuels 2018, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Vail, A.; Sadowsky, M.J.; Schilling, S.J. Competition between two wood-degrading fungi with distinct influences on residues. FEMS Microbiol. Ecol. 2012, 1, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Media Recipes. Culture Collection of Algae and Protozoa. Available online: https://www.ccap.ac.uk/media/documents/3N_BBM_V.pdf (accessed on 27 October 2020).
- Adney, B.; Baker, J. Measurement of Cellulase Activities; Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory: Battelle, OH, USA, 2008; pp. 1–11. [Google Scholar]
- EloKIT vita™. Conelum. Available online: http://www.conelum.com/technology_and_products.html (accessed on 27 October 2020).
- Ghose, T.K. Measurement of cellulose activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Thiribhuvanamala, G.; Kalaiselvi, G.; Parthasarathy, S.; Madhavan, S.; Prakasam, V. Extracellular secretion of lignocellulolytic enzymes by diverse white rot basidiomycetes fungi. Ann. Phytomedicine 2017, 6, 20–29. [Google Scholar] [CrossRef]
- Steinberg, G. Hyphal growth: A tale of motors, lipids, and the Spitzenkörper. Eukaryot. Cell 2007, 6, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Kucharska, K.; Rybarczyk, P.; Holowacz, I.; Lukajtis, R.; Glinka, M.; Kaminski, M. Pretreatment of lignocellulosic materials as substrates for fermentation process. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [Green Version]
- De Souza Moretti, M.M.; Perrone, O.M.; Da Costa Carreira Nunes, C.; Taboga, S.; Boscolo, M.; da Silva, R.; Gomes, E. Effect of pretreatment and enzymatic hydrolysis on the physical-chemical composition and morphologic structure od sugarcane bagasse nad sugarcane straw. Biores. Tech. 2016, 219, 773–777. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Mezule, L.; Berzina, I.; Strods, M. The impact of substrate-enzyme proportion for efficient hydrolysis of hay. Energies 2019, 12, 3526. [Google Scholar] [CrossRef] [Green Version]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Malgas, S.; Dyk, J.S.; Pletschke, B.I. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase. World J. Microbiol. Biotechnol. 2015, 31, 1167–1175. [Google Scholar] [CrossRef] [PubMed]

| Substrate | Incubation Time | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Hay | 207.5 ± 7.2 | 215.3 ± 4.8 | 221.7 ± 8.0 |
| Wood residue chips | 50.3 ± 4.3 | 52.4 ± 4.2 | 59.6 ± 6.2 |
| Sawing residue chips | 24.8 ± 0.7 | 28.0 ± 1.2 | 33.4 ± 1.8 |
| Barley straw | 81.9 ± 6.6 | 104.3 ± 7.9 | 112.3 ± 10.3 |
| Green algae (dried) | 93.7 ± 13.2 | 110.2 ± 5.7 | 113.6 ± 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezule, L.; Civzele, A. Bioprospecting White-Rot Basidiomycete Irpex lacteus for Improved Extraction of Lignocellulose-Degrading Enzymes and Their Further Application. J. Fungi 2020, 6, 256. https://doi.org/10.3390/jof6040256
Mezule L, Civzele A. Bioprospecting White-Rot Basidiomycete Irpex lacteus for Improved Extraction of Lignocellulose-Degrading Enzymes and Their Further Application. Journal of Fungi. 2020; 6(4):256. https://doi.org/10.3390/jof6040256
Chicago/Turabian StyleMezule, Linda, and Anna Civzele. 2020. "Bioprospecting White-Rot Basidiomycete Irpex lacteus for Improved Extraction of Lignocellulose-Degrading Enzymes and Their Further Application" Journal of Fungi 6, no. 4: 256. https://doi.org/10.3390/jof6040256






