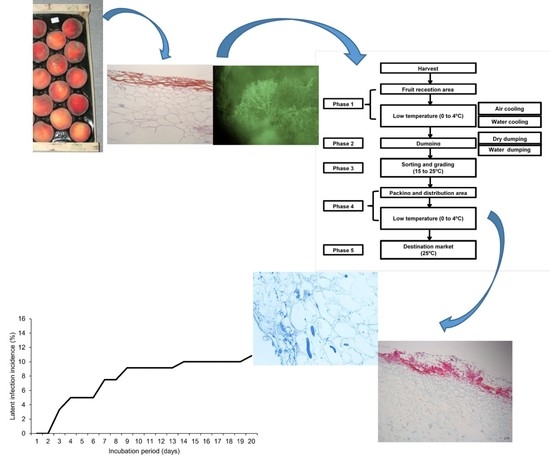
Impact of Postharvest Handling on Preharvest Latent Infections Caused by Monilinia spp. in Nectarines
Abstract
:1. Introduction
2. Materials and Methods
- DD: Degree-days above 0 °C.
- Trc: Temperature on the pre-cooling chamber (°C).
- trc: time on the pre-cooling chamber (h).
- Twd: Temperature on water dump (°C).
- twd: time on water dump (h).
- Tec: Temperature on the cold-storage chamber (°C).
- tec: time on the cold-storage chamber (h).
- y: percentage of latent infection.
- t: degree-days above 0 °C (DD).
- A: maximum latent infection reached.
- μm: maximum fungal growth rate.
- λ: lag phase duration before the beginning of latent infection growth.
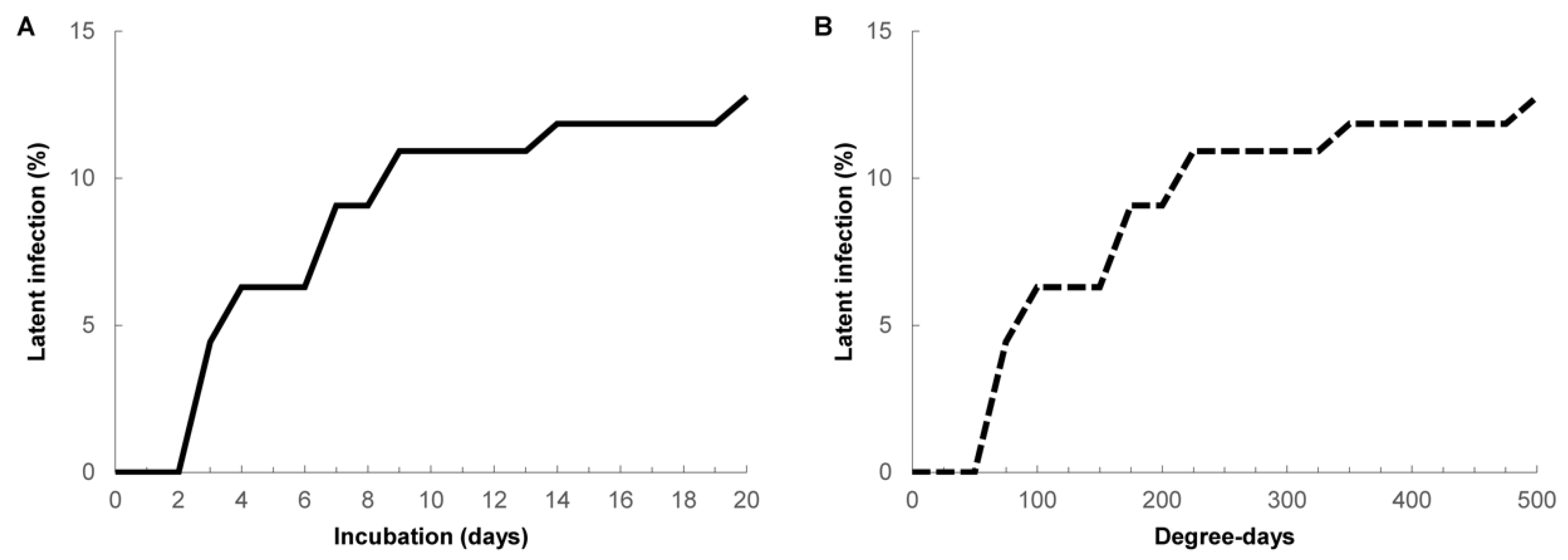
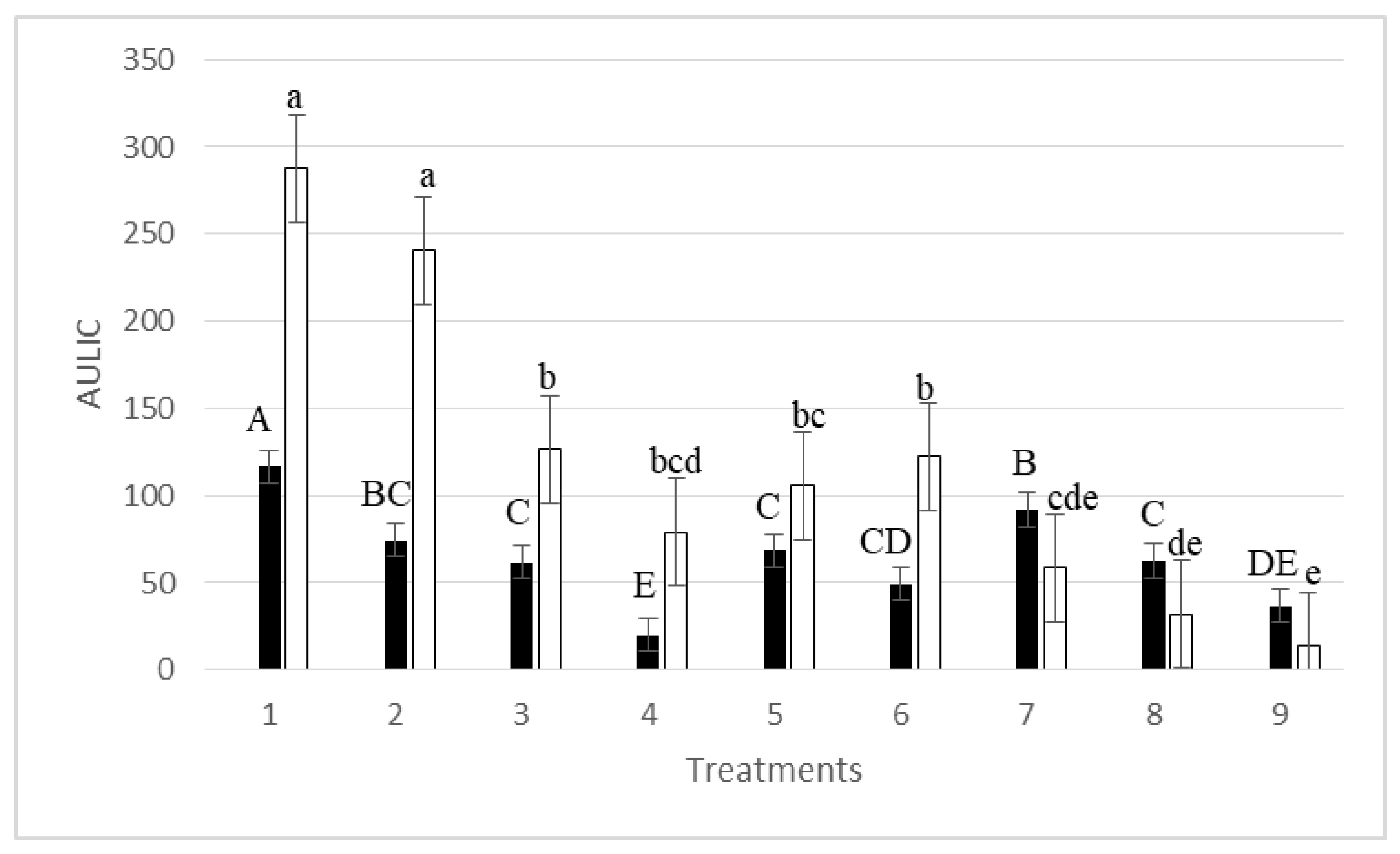
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villarino, M.; Egüen, B.; Lamarca, N.; Segarra, J.; Usall, J.; Melgarejo, P.; De Cal, A. Occurrence of Monilinia laxa and M. fructigena after introduction of M. fructicola in peach orchards in Spain. Eur. J. Plant Pathol. 2013, 137, 835–845. [Google Scholar] [CrossRef]
- Villarino, M.; Melgarejo, P.; Usall, J.; Segarra, J.; Lamarca, N.; De Cal, A. Secondary inoculum dynamics of Monilinia spp. and relationship to the incidence of postharvest brown rot in peaches and the weather conditions during the growing season. Eur. J. Plant Pathol. 2012, 133, 585–598. [Google Scholar] [CrossRef]
- Biggs, A.R.; Northover, J. Early and late-season susceptibility of peach fruits to Monilinia fructicola. Plant Dis. 1988, 72, 1070–1074. [Google Scholar] [CrossRef]
- Phillips, D.J. Effect of temperature on Monilinia fructicola conidia produced on fresh stone fruits. Plant Dis. 1984, 68, 610–612. [Google Scholar] [CrossRef]
- Garcia-Benitez, C.; Melgarejo, P.; De Cal, A. Fruit maturity and postharvest environmental conditions influence the pre-penetration stages of Monilinia infections in peaches. Int. J. Food Microbiol. 2017, 241, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Benitez, C.; Melgarejo, P.; De Cal, A.; Fontaniella, B. Microscopic analyses of latent and visible Monilinia fructicola infections in nectarines. PLoS ONE 2016, 11, e0160675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrde, R.J.; Willetts, H.J. The Brown Rot Fungi of Fruit: Their Biology and Control; Pergamon Press: Oxford, UK, 1977. [Google Scholar]
- Cruickshank, R.H.; Wade, G.C. Production of appressoria by Monilinia fructicola. Mycol. Res. 1992, 96, 425–428. [Google Scholar] [CrossRef]
- Gell, I.; De Cal, A.; Torres, R.; Usall, J.; Melgarejo, P. Relationship between the incidence of latent infections caused by Monilinia spp. and the incidence of brown rot of peach fruit: Factors affecting latent infection. Eur. J. Plant Pathol. 2008, 121, 487–498. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, Z.; Michailides, T.J. Analysis of factors affecting latent infection and sporulation of Monilinia fructicola on prune fruit. Plant Dis. 2001, 85, 999–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, K.M.; Michailides, T.J.; Scherm, H. Incidence of latent infection of immature peach fruit by Monilinia fructicola and relationship to brown rot in Georgia. Plant Dis. 2000, 84, 853–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourie, P.H.; Holz, G. Germination of dry, airborne conidia of Monilinia laxa and disease expression on nectarine fruit. Australas. Plant Pathol. 2003, 32, 9–18. [Google Scholar] [CrossRef]
- Fourie, P.H.; Holz, G. Germination of dry, airborne conidia of Monilinia laxa and disease expression on plum fruit. Australas. Plant Pathol. 2003, 32, 19–25. [Google Scholar] [CrossRef]
- Northover, J.; Cerkauskas, R.F. Detection and significance of symptomless latent infections of Monilinia fructicola in plums. Can. J. Plant Pathol. 1994, 16, 30–36. [Google Scholar] [CrossRef]
- Thomidis, T. Influence of relative virulence and latent infections on the development of Monilinia to Greek peach orchards. Crop Prot. 2017, 94, 159–165. [Google Scholar] [CrossRef]
- Luo, Y.; Michailides, T.J. Factors affecting latent infection of prune fruit by Monilinia fructicola. Phytopathology 2001, 91, 864–872. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Michailides, T.J. Threshold conditions that lead latent infection to prune fruit rot caused by Monilinia fructicola. Phytopathology 2003, 93, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Bernat, M.; Segarra, J.; Casals, C.; Teixido, N.; Torres, R.; Usall, J. Relevance of the main postharvest handling operations on the development of brown rot disease on stone fruits. J. Sci. Food Agric. 2017, 97, 5319–5326. [Google Scholar] [CrossRef] [Green Version]
- Meier, U.; Graf, H.; Hack, H.; Hess, M.; Kennel, W.; Klose, R.; Mappes, D.; Seipp, D.; Stauss, R.; Streif, J.; et al. Phänologische entwick-lungsstadien des kernobstes (Malus domestica Borkh. und Pyrus communis L.), des steinobstes (Prunus arten), der johannisbeere (Ribes arten) und der erdbeere (Fragaria × ananassa Duch.). Nachr. Dtsch. Pflanzenschutzd. 1994, 46, 141–153. [Google Scholar]
- De Cal, A.; Melgarejo, P. Effects of long-wave UV light on Monilinia growth and identification of species. Plant Dis. 1999, 83, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Gell, I.; Cubero, J.; Melgarejo, P. Two different PCR approaches for universal diagnosis of brown rot and identification of Monilinia spp. in stone fruit trees. J. Appl. Microbiol. 2007, 103, 2629–2637. [Google Scholar] [CrossRef]
- Yates, F. Contingency table involving small numbers and the χ2 test. Suppl. J. R. Stat. Soc. 1934, 1, 217–235. [Google Scholar] [CrossRef]
- Sauer, D.B.; Burroughs, R. Disinfection of seed surfaces with sodium-hypochlorite. Phytopathology 1986, 76, 745–749. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; Wiley Interscience: New York, NY, USA, 1990; 532p. [Google Scholar]
- Snedecor, G.W.; Cochram, W.G. Statistical Methods, 7th ed.; Iowa State University, University Press: Ames, IA, USA, 1980. [Google Scholar]
- Bernat, M.; Casals, C.; Torres, R.; Teixidó, N.; Usall, J. Infection risk of Monilinia fructicola on stone fruit during cold storage and immersion in the dump tank. Sci. Hortic. 2019, 256, 108589. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K.J.A.E.M. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 216–1231. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Goonewardene, L.A. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can. J. Anim. Sci. 2004, 84, 1–11. [Google Scholar] [CrossRef]
- Yang, S.; Logan, J.; Coffey, D.L. Mathematical formulae for calculating the base temperature for growing degree days. Agric. For. Meteorol. 1995, 74, 61–74. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Kader, A.A. Peach. In the Commercial Storage of Fruit, Vegetables, and Florist and Nursery Stocks. 2014. Available online: http://www.ba.ars.usda.gov/hb2066/peach.pdf (accessed on 5 October 2015).
- Fraser, H.W. Sizing and Layout of a Short-Term Refrigerated Storage for Fruits and Vegetables. Factsheet. Ontario Ministry of Agriculture, Food and Rural Affairs, 1 Stone RoadWest, Guelp; 1992. Available online: http://www.omafra.gov.on.ca/english/engineer/facts/92-124.htm (accessed on 5 October 2015).
- Brosnan, T.; Sun, D.W. Precooling techniques and applications for horticultural products—A review. Int. J. Refrig. 2001, 24, 154–170. [Google Scholar] [CrossRef]
- Dennis, C. Effect of storage and distribution conditions on the quality of vegetables. Symp. Q. Veg. 1984, 163, 85–104. [Google Scholar] [CrossRef]
- Robertson, J.A.; Meredith, F.I.; Horvat, R.J.; Senter, S.D. Effect of cold-storage and maturity on the physical and chemical characteristics and volatile constituents of peaches (cv. Cresthaven). J. Agric. Food Chem. 1990, 38, 620–624. [Google Scholar] [CrossRef]
- Kenneth, C.G.; Chien, Y.W.; Mikal, S. Agriculture Handbook Number 66: The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks, 5th ed.; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2016.
- Bernat, M.; Segarra, J.; Xu, X.M.; Casals, C.; Usall, J. Influence of temperature on decay, mycelium development and sporodochia production caused by Monilinia fructicola and M. laxa on stone fruits. Food Microbiol. 2017, 64, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Papavasileiou, A.; Testempasis, S.; Michailides, T.J.; Karaoglanidis, G.S. Frequency of brown rot fungi on blossoms and fruit in stone fruit orchards in Greece. Plant Pathol. 2015, 64, 416–424. [Google Scholar] [CrossRef]
- Tamm, L.; Flückiger, W. Influence of temperature and moisture on growth, spore production, and conidial germination of Monilinia laxa. Phytopathology 1993, 83, 1321–1326. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q. A new nonlinear method for calculating growing degree days. Sci. Rep. 2018, 8, 10149. [Google Scholar] [CrossRef]
- Xu, X.M.; Guerin, L.; Robinson, J.D. Effects of temperature and relative humidity on conidial germination and viability, colonization and sporulation of Monilinia fructigena. Plant Pathol. 2001, 50, 561–568. [Google Scholar] [CrossRef]
- Wagner, T.L.; Wu, H.; Sharpe, P.J.H.; Schoolfield, R.M.; Coulson, R.N. Modeling insect development rates: A literature review and application of a biophysical model. Ann. Entomol. Soc. Am. 1984, 77, 208–225. [Google Scholar] [CrossRef]
- Moore, J.L.; Remais, J.V. Developmental models for estimating ecological responses to environmental variability: Structural, parametric, and experimental issues. Acta Biotheor. 2014, 62, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, A.M.; Hocking, A.D. Advances in the predictive modelling of fungal growth in food. Trends Food Sci. Technol. 1997, 8, 353–358. [Google Scholar] [CrossRef]
- Parra-Marquez, J.C. Análisis del comportamiento del Modelo de Crecimiento de Gompertz en la predicción del crecimiento de la economía de Argentina, Bolivia, Chile y Perú. Estud. Econ. Apl. 2017, 35, 443–464. [Google Scholar] [CrossRef]
- Lill, R.E.; O’Doneghue, E.M.; King, G.A. Postharvest physiology of peaches and nectarines. Hortic. Rev. 1989, 11, 413–452. [Google Scholar]
- Khan, A.S.; Hussain, K.; Shah, H.M.S.; Malik, A.U.; Anwar, R.; Rehman, R.N.; Bakhsh, A. Cold storage influences postharvest chilling injury and quality of peach fruits. J. Hortic. Sci. Technol. 2018, 1, 28–34. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Mitchell, F.G.; Ju, Z. Susceptibility to chilling injury of peach, nectarine, and plum cultivars grown in California. Hortscience 1999, 34, 1116–1118. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, P.; Saulie-Carter, J. Postharvest Decay Control of Apples and Pears after Immersion Dumping; Agricultural Experiment Station, Oregon State University: Corvallis, OR, USA, 1979. [Google Scholar]
- Bernat, M.; Segarra, J.; Navas-Cortés, J.A.; Casals, C.; Teixido, N.; Torres, R.; Usall, J. Influence of temperature and humidity on the survival of Monilinia fructicola conidia on stone fruits and inert surfaces. Ann. Appl. Biol. 2018, 173, 63–70. [Google Scholar] [CrossRef]

| Treatment | Days on Pre-Cooling Chamber | Water Dumping | Days on Cold-Storage Chamber | Total Days at 4 °C | Total Days at 25 °C |
|---|---|---|---|---|---|
| 1 | 0 | No | 0 | 0 | 20 |
| 2 | 0 | Yes | 0 | 0 | 20 |
| 3 | 0 | Yes | 3 | 3 | 17 |
| 4 | 3 | Yes | 0 | 3 | 17 |
| 5 | 1 | Yes | 3 | 4 | 16 |
| 6 | 3 | Yes | 3 | 6 | 14 |
| 7 | 0 | Yes | 10 | 10 | 10 |
| 8 | 1 | Yes | 10 | 11 | 9 |
| 9 | 3 | Yes | 10 | 13 | 7 |
| Experiment | Nectarine Cultivar | Orchard Location | Brown Rot Incidence (%) | Incidence of Monilinia Latent Infection (%) | Frequency of M. fructicola Isolates (%) | Frequency of M. laxa Isolates (%) |
|---|---|---|---|---|---|---|
| 1 | Red Jim | Sudanell | 26.6a | 10.0a | 16.7a | 1.7b |
| 2 | Alba Red | Ivars de Noguera | 43.3a | 6.7a | 3.3b | 21.7a |
| χ2 | 1.172 | 0.000 | 4.537 | 9.784 | ||
| p-value | 0.279 | 1.000 | 0.033 | 0.002 |
| Incubation (Days) | Postharvest Handling Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 25.00 | 24.93 | 4.08 | 4.00 | 4.00 | 4.00 | 4.08 | 4.00 | 4.00 |
| 2 | 50.00 | 49.93 | 8.08 | 8.00 | 8.08 | 8.00 | 8.08 | 8.08 | 8.00 |
| 3 | 75.00 | 74.93 | 12.08 | 12.00 | 12.08 | 12.00 | 12.08 | 12.08 | 12.00 |
| 4 | 100.00 | 99.93 | 37.01 | 36.93 | 16.08 | 16.08 | 16.08 | 16.08 | 16.08 |
| 5 | 125.00 | 124.93 | 62.01 | 61.93 | 41.08 | 20.08 | 20.08 | 20.08 | 20.08 |
| 6 | 150.00 | 149.93 | 87.01 | 86.93 | 66.08 | 24.08 | 24.08 | 24.08 | 24.08 |
| 7 | 175.00 | 174.93 | 112.01 | 111.93 | 91.08 | 49.08 | 28.08 | 28.08 | 28.08 |
| 8 | 200.00 | 199.93 | 137.01 | 136.93 | 116.08 | 74.08 | 32.08 | 32.08 | 32.08 |
| 9 | 225.00 | 224.93 | 162.01 | 161.93 | 141.08 | 99.08 | 36.08 | 36.08 | 36.08 |
| 10 | 250.00 | 249.93 | 187.01 | 186.93 | 166.08 | 124.08 | 40.08 | 40.08 | 40.08 |
| 11 | 275.00 | 274.93 | 212.01 | 211.93 | 191.08 | 149.08 | 65.08 | 44.08 | 44.08 |
| 12 | 300.00 | 299.93 | 237.01 | 236.93 | 216.08 | 174.08 | 90.08 | 69.08 | 48.08 |
| 13 | 325.00 | 324.93 | 262.01 | 261.93 | 241.08 | 199.08 | 115.08 | 94.08 | 52.08 |
| 14 | 350.00 | 349.93 | 287.01 | 286.93 | 266.08 | 224.08 | 140.08 | 119.08 | 77.08 |
| 15 | 375.00 | 374.93 | 312.01 | 311.93 | 291.08 | 249.08 | 165.08 | 144.08 | 102.08 |
| 16 | 400.00 | 399.93 | 337.01 | 336.93 | 316.08 | 274.08 | 190.08 | 169.08 | 127.08 |
| 17 | 425.00 | 424.93 | 362.01 | 361.93 | 341.08 | 299.08 | 215.08 | 194.08 | 152.08 |
| 18 | 450.00 | 449.93 | 387.01 | 386.93 | 366.08 | 324.08 | 240.08 | 219.08 | 177.08 |
| 19 | 475.00 | 474.93 | 412.01 | 411.93 | 391.08 | 349.08 | 265.08 | 244.08 | 202.08 |
| 20 | 500.00 | 499.93 | 437.01 | 436.93 | 416.08 | 374.08 | 290.08 | 269.08 | 227.08 |
| Number of Levels | df | F Values | p | ||
|---|---|---|---|---|---|
| Fixed Effects | Intercept | 1 | 1 | 73.649 | 0.000 |
| Treatment | 9 | 8 | 2.213 | 0.030 | |
| RH | 2 | 1 | 0.045 | 0.832 | |
| Treatment × RH | 18 | 8 | 1.477 | 0.171 | |
| Total | 53 | 144 | |||
| Treatments | Mean Latent Infection (%) * | R2 | RSS | A (ILmax) | μm (Rate Growth) | λ (Lag Phase) | Lag Phase (Days) observed vs. predicted |
|---|---|---|---|---|---|---|---|
| 1 | 8.57 a | 0.97 | 11.8532 | 11.94 ± 0.31 (p = 0.00000) # | 0.065 ± 0.008 (p = 0.00000) | 27.05 ± 10.97 (p = 0.02394) | 2-0 |
| 2 | 6.45 ab | 0.94 | 15.9857 | 9.40 ± 0.34 (p = 0.00000) | 0.068 ± 0.013 (p = 0.00005) | 72.64 ± 13.85 (p = 0.00005) | 2-2 |
| 3 | 4.18 bc | 0.97 | 6.7576 | 7.41 ± 0.23 (p = 0.00000) | 0.080 ± 0.015 (p = 0.00005) | 100.48 ± 9.25 (p = 0.00000) | 7-6 |
| 4 | 4.04 bc | 0.98 | 2.7736 | 6.42 ± 0.15 (p = 0.00000) | 0.053 ± 0.006 (p = 0.00000) | 51.53 ± 7.91 (p = 0.00000) | 4-4 |
| 5 | 4.04 bc | 0.95 | 9.8497 | 7.49 ± 0.36 (p = 0.00000) | 0.047 ± 0.009 (p = 0.00004) | 51.91 ± 14.88 (p = 0.00262) | 7-5 |
| 6 | 2.64 bc | 0.96 | 3.1223 | 4.50 ± 0.14 (p = 0.00000) | 0.056 ± 0.012 (p = 0.00016) | 32.40 ± 8.97 (p = 0.00199) | 7-6 |
| 7 | 2.06 c | 0.99 | 1.0660 | 6.86 ± 0.12 (p = 0.00000) | 0.137 ± 0.015 (p = 0.00000) | 115.20 ± 3.02 (p = 0.00000) | 14-13 |
| 8 | 3.70 bc | 0.99 | 3.3767 | 12.77 ± 0.20 (p = 0.00000) | 0.573 ± 0.091 (p = 0.00001) | 115.43 ± 1.18 (p = 0.00000) | 14-14 |
| 9 | 1.76 c | 0.81 | 47.4394 | 10,349.6 ± 333,500.5 (p = 0.97558) | 19.21 ± 533.57 (p = 0.97168) | 400.96 ± 1750.27 (p = 0.82139) | 13-16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Benitez, C.; Casals, C.; Usall, J.; Sánchez-Ramos, I.; Melgarejo, P.; De Cal, A. Impact of Postharvest Handling on Preharvest Latent Infections Caused by Monilinia spp. in Nectarines. J. Fungi 2020, 6, 266. https://doi.org/10.3390/jof6040266
Garcia-Benitez C, Casals C, Usall J, Sánchez-Ramos I, Melgarejo P, De Cal A. Impact of Postharvest Handling on Preharvest Latent Infections Caused by Monilinia spp. in Nectarines. Journal of Fungi. 2020; 6(4):266. https://doi.org/10.3390/jof6040266
Chicago/Turabian StyleGarcia-Benitez, Carlos, Carla Casals, Josep Usall, Ismael Sánchez-Ramos, Paloma Melgarejo, and Antonieta De Cal. 2020. "Impact of Postharvest Handling on Preharvest Latent Infections Caused by Monilinia spp. in Nectarines" Journal of Fungi 6, no. 4: 266. https://doi.org/10.3390/jof6040266
APA StyleGarcia-Benitez, C., Casals, C., Usall, J., Sánchez-Ramos, I., Melgarejo, P., & De Cal, A. (2020). Impact of Postharvest Handling on Preharvest Latent Infections Caused by Monilinia spp. in Nectarines. Journal of Fungi, 6(4), 266. https://doi.org/10.3390/jof6040266