Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance
Abstract
:1. History of Polyenes as Antifungal Drugs
2. Strengths and Drawbacks of Polyene Use in the Clinic
3. Mode of Action of Polyene Antifungal Drugs
3.1. Polyene—Sterol Interactions
3.2. Pore Forming Models
3.3. Surface Adsorption and Sterol Sponge Models
3.4. Other Proposed Modes of Action
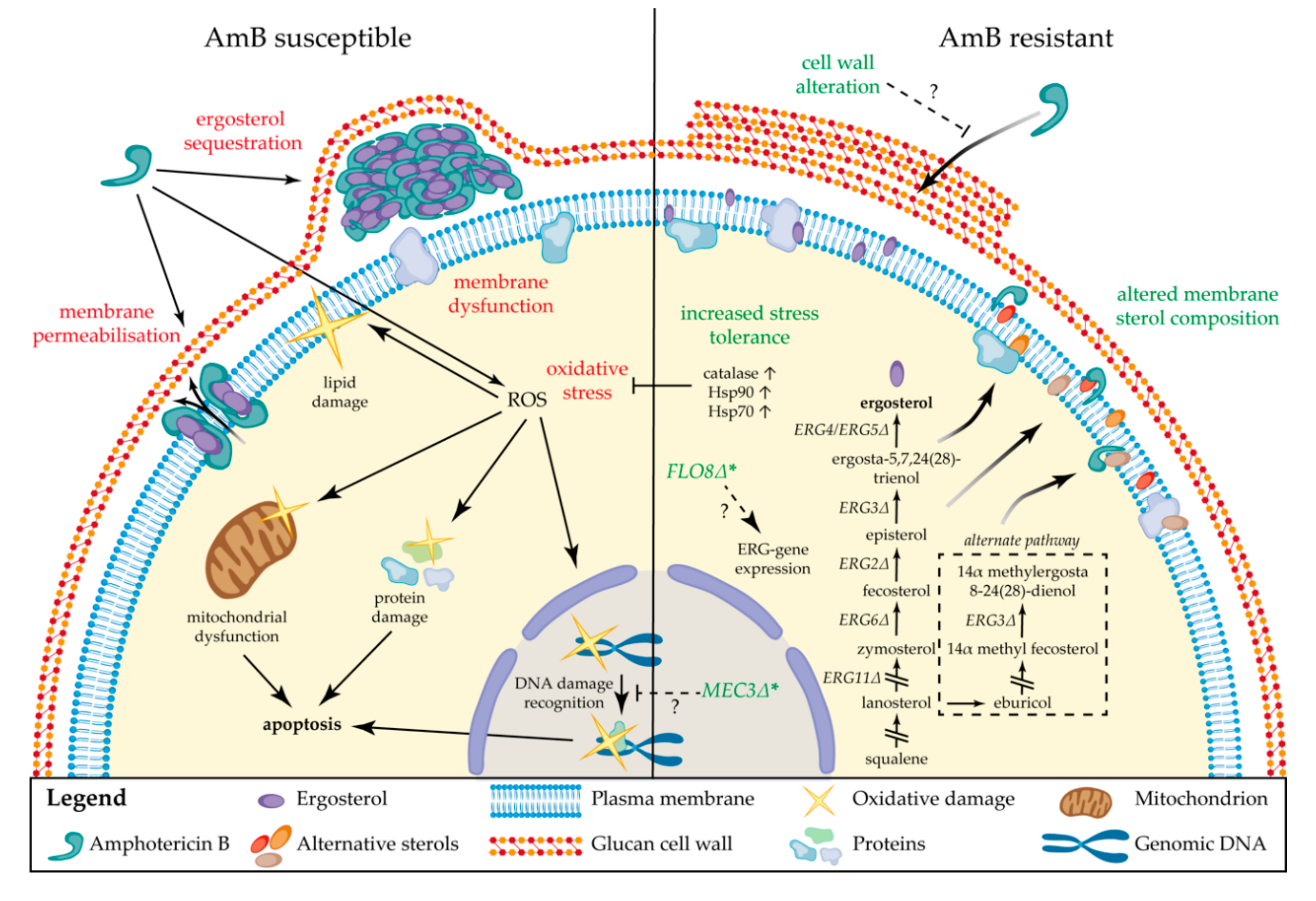
4. Drug Resistance to Polyenes
4.1. Molecular Mechanisms of Resistance
4.2. Epidemiology of Polyene Resistance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar]
- Zotchev, S.B. Polyene macrolide antibiotics and their applications in human therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [PubMed]
- Dixon, D.M.; Walsh, T.J. Antifungal Agents. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Hazen, E.L.; Brown, R. Fungicidin, an antibiotic produced by a soil actinomycete. Proc. Soc. Exp. Biol. Med. 1951, 76, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Lattif, A.A.; Swindell, K. History of Antifungals. In Antifungal Therapy; Ghannoum, A.M., Perfect, R.J., Eds.; CRC Press: Boca Raton, FL, USA, 2009; Volume 1. [Google Scholar]
- Espinell-Ingroff, A. Medical Mycology and Training in the United States: A Historical Analysis (1894–1996); Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Dutcher, J.D. The discovery and development of amphotericin B. Dis. Chest 1968, 54, 296–298. [Google Scholar]
- Borowski, E.; Zieliński, J.; Ziminski, T.; Falkowski, L.; Kołodziejczyk, P.; Golik, J.; Jereczek, E.; Adlercreutz, H. Chemical studies with amphotericin B III. The complete structure of the antibiotic. Tetrahedron Lett. 1970, 11, 3909–3914. [Google Scholar]
- Hamilton-Miller, J. Chemistry and biology of the polyene macrolide antibiotics. Bacteriol. Rev. 1973, 37, 166. [Google Scholar]
- Kinsky, S.C. Polyene Antibiotics. In Antibiotics; Springer: Berlin/Heidelberg, Germany, 1967; pp. 122–141. [Google Scholar]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar]
- Saravolatz, L.D.; Bern, C.; Adler-Moore, J.; Berenguer, J.; Boelaert, M.; den Boer, M.; Davidson, R.N.; Figueras, C.; Gradoni, L.; Kafetzis, D.A. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin. Infect. Dis. 2006, 43, 917–924. [Google Scholar]
- Chandrasekar, P. Management of invasive fungal infections: A role for polyenes. J. Antimicrob. Chemother. 2011, 66, 457–465. [Google Scholar]
- Cornely, O.A.; Vehreschild, J.J.; Ullmann, A.J. Is there a role for polyenes in treating invasive mycoses? Curr. Opin. Infect. Dis. 2006, 19, 565–570. [Google Scholar]
- Stone, N.R.; Bicanic, T.; Salim, R.; Hope, W. Liposomal amphotericin B (AmBisome®): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016, 76, 485–500. [Google Scholar] [PubMed] [Green Version]
- Steinbach, W.J.; Benjamin, D.K., Jr.; Kontoyiannis, D.P.; Perfect, J.R.; Lutsar, I.; Marr, K.A.; Lionakis, M.S.; Torres, H.A.; Jafri, H.; Walsh, T.J. Infections due to Aspergillus terreus: A multicenter retrospective analysis of 83 cases. Clin. Infect. Dis. 2004, 39, 192–198. [Google Scholar] [PubMed] [Green Version]
- Steinbach, W.J.; Schell, W.A.; Miller, J.L.; Perfect, J.R. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J. Clin. Microbiol. 2003, 41, 3981–3985. [Google Scholar] [PubMed] [Green Version]
- Escandon, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin. Infect. Dis. 2019, 68, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristanc, L.; Božič, B.; Jokhadar, Š.Z.; Dolenc, M.S.; Gomišček, G. The pore-forming action of polyenes: From model membranes to living organisms. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 418–430. [Google Scholar]
- Vincent, B.M.; Lancaster, A.K.; Scherz-Shouval, R.; Whitesell, L.; Lindquist, S. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol. 2013, 11, e1001692. [Google Scholar]
- Rambach, G.; Oberhauser, H.; Speth, C.; Lass-Flörl, C. Susceptibility of Candida species and various moulds to antimycotic drugs: Use of epidemiological cutoff values according to EUCAST and CLSI in an 8-year survey. Med. Mycol. 2011, 49, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A. Treatment of aspergillosis: Clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef]
- Readio, J.D.; Bittman, R. Equilibrium binding of amphotericin B and its methyl ester and borate complex to sterols. Biochim. Biophys. Acta (BBA)-Biomembr. 1982, 685, 219–224. [Google Scholar] [CrossRef]
- Tevyashova, A.N.; Bychkova, E.N.; Solovieva, S.E.; Zatonsky, G.V.; Grammatikova, N.E.; Isakova, E.B.; Mirchink, E.P.; Treshchalin, I.D.; Pereverzeva, E.R.; Bykov, E.E. Discovery of Amphamide, a Drug Candidate for the Second Generation of Polyene Antibiotics. ACS Infect. Dis. 2020, 6, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, M.; Yang, Z. Design of amphotericin B oral formulation for antifungal therapy. Drug Deliv. 2017, 24, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuddihy, G.; Wasan, E.K.; Di, Y.; Wasan, K.M. The development of oral amphotericin b to treat systemic fungal and parasitic infections: Has the myth been finally realized? Pharmaceutics 2019, 11, 99. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, D.; Carter, H.E.; Sloneker, J.H.; Ammann, A. Protection of fungi against polyene antibiotics by sterols. Science 1958, 128, 361. [Google Scholar] [CrossRef]
- Cotero, B.V.; Rebolledo-Antúnez, S.; Ortega-Blake, I. On the role of sterol in the formation of the amphotericin B channel. Biochim. Biophys. Acta (BBA)-Biomembr. 1998, 1375, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400. [Google Scholar] [CrossRef]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef] [Green Version]
- Palacios, D.S.; Anderson, T.M.; Burke, M.D. A Post-PKS Oxidation of the Amphotericin B Skeleton Predicted to be Critical for Channel Formation Is Not Required for Potent Antifungal Activity. J. Am. Chem. Soc. 2007, 129, 13804–13805. [Google Scholar] [CrossRef] [Green Version]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Gianinni, M.J.S.M.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Welscher, Y.M.; Jones, L.; Van Leeuwen, M.R.; Dijksterhuis, J.; De Kruijff, B.; Eitzen, G.; Breukink, E. Natamycin inhibits vacuole fusion at the priming phase via a specific interaction with ergosterol. Antimicrob. Agents Chemother. 2010, 54, 2618–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Welscher, Y.M.; ten Napel, H.H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; De Kruijff, B.; Breukink, E. Natamycin blocks fungal growth by binding specifically to ergosterol without permeabilizing the membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Welscher, Y.M.; van Leeuwen, M.R.; de Kruijff, B.; Dijksterhuis, J.; Breukink, E. Polyene antibiotic that inhibits membrane transport proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 11156–11159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiński, D.M. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 2014, 43, 453–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, M.L. The multifunctional fungal ergosterol. mBio 2018, 9, e01755-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heese-Peck, A.; Pichler, H.; Zanolari, B.; Watanabe, R.; Daum, G.; Riezman, H. Multiple functions of sterols in yeast endocytosis. Mol. Biol. Cell 2002, 13, 2664–2680. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; McCaffery, J.M.; Grote, E. Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J. Cell Biol. 2008, 180, 813–826. [Google Scholar] [CrossRef] [Green Version]
- Baran, M.; Borowski, E.; Mazerski, J. Molecular modeling of amphotericin B–ergosterol primary complex in water II. Biophys. Chem. 2009, 141, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Foglia, F.; Lawrence, M.J.; Demeė, B.; Fragneto, G.; Barlow, D. Neutron diffraction studies of the interaction between amphotericin B and lipid-sterol model membranes. Sci. Rep. 2012, 2, 778. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, S.; Ikeuchi, H.; Matsumori, N.; Murata, M. Dominant formation of a single-length channel by amphotericin B in dimyristoylphosphatidylcholine membrane evidenced by 13C−31P rotational echo double resonance. Biochemistry 2005, 44, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E. Amphotericin B membrane action: Role for two types of ion channels in eliciting cell survival and lethal effects. J. Membr. Biol. 2010, 238, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ostroumova, O.S.; Efimova, S.S.; Schagina, L.V. Probing amphotericin B single channel activity by membrane dipole modifiers. PLoS ONE 2012, 7, e30261. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-S.; Ou, K.-L.; Peng, P.-W.; Liou, B.-C.; Wang, W.-T.; Huang, Y.-C.; Tsai, C.-M.; Su, C.-H. Quantifying membrane permeability of amphotericin B ion channels in single living cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1794–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouri, R.; Konoki, K.; Matsumori, N.; Oishi, T.; Murata, M. Complex formation of amphotericin B in sterol-containing membranes as evidenced by surface plasmon resonance. Biochemistry 2008, 47, 7807–7815. [Google Scholar] [CrossRef] [PubMed]
- Szpilman, A.M.; Cereghetti, D.M.; Manthorpe, J.M.; Wurtz, N.R.; Carreira, E.M. Synthesis and Biophysical Studies on 35-Deoxy Amphotericin B Methyl Ester. Chem. Eur. J. 2009, 15, 7117–7128. [Google Scholar] [CrossRef] [PubMed]
- Sokol-Anderson, M.; Sligh, J.E., Jr.; Elberg, S.; Brajtburg, J.; Kobayashi, G.S.; Medoff, G. Role of cell defense against oxidative damage in the resistance of Candida albicans to the killing effect of amphotericin B. Antimicrob. Agents Chemother. 1988, 32, 702–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol-Anderson, M.L.; Brajtburg, J.; Medoff, G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 1986, 154, 76–83. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Zhu, Z.; Chen, X.; Yao, X.; Zhao, L.; Wang, H.; Yan, L.; Wu, H.; Chai, Y.; Jiang, Y. Effect of amphotericin B on the metabolic profiles of Candida albicans. J. Proteome Res. 2013, 12, 2921–2932. [Google Scholar] [CrossRef]
- Liu, T.T.; Lee, R.E.; Barker, K.S.; Lee, R.E.; Wei, L.; Homayouni, R.; Rogers, P.D. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother. 2005, 49, 2226–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamp-Freund, M.T.; Ferreira, V.F.; Schreier, S. Mechanism of inactivation of the polyene antibiotic amphotericin B. J. Antibiot. 1985, 38, 753–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geber, A.; Hitchcock, C.A.; Swartz, J.E.; Pullen, F.S.; Marsden, K.E.; Kwon-Chung, K.J.; Bennett, J.E. Deletion of the Candida glabrata ERG3 and ERG11 genes: Effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob. Agents Chemother. 1995, 39, 2708–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanglard, D.; Ischer, F.; Parkinson, T.; Falconer, D.; Bille, J. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob. Agents Chemother. 2003, 47, 2404–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. A clinical isolate of Candida albicans with mutations in ERG11 (encoding sterol 14α-demethylase) and ERG5 (encoding C22 desaturase) is cross resistant to azoles and amphotericin B. Antimicrob. Agents Chemother. 2010, 54, 3578–3583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, L.Y.; Hull, C.M.; Heitman, J. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob. Agents Chemother. 2003, 47, 2717–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Joseph, L.; Parker, J.E.; Asadzadeh, M.; Kelly, S.L.; Meis, J.F.; Khan, Z. ERG6 and ERG2 are major targets conferring reduced susceptibility to amphotericin B in clinical Candida glabrata isolates in Kuwait. Antimicrob. Agents Chemother. 2019, 63, e01900-18. [Google Scholar] [CrossRef] [Green Version]
- Kelly, S.L.; Lamb, D.C.; Taylor, M.; Corran, A.J.; Baldwin, B.C.; Powderly, W.G. Resistance to amphotericin B associated with defective sterol Δ 8→7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiol. Lett. 1994, 122, 39–42. [Google Scholar] [CrossRef]
- Joseph-Horne, T.; Loeffler, R.; Hollomon, D.; Kelly, S. Amphotericin B resistant isolates of Cryptococcus neoformans without alteration in sterol biosynthesis. J. Med. Vet. Mycol. 1996, 34, 223–225. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.N.; Oliveira, S.S.; Magalhães, L.B.; Andrade Neto, V.V.; Torres-Santos, E.C.; Carvalho, M.D.; Pereira, M.D.; Branquinha, M.H.; Santos, A.L. Unmasking the amphotericin B resistance mechanisms in Candida haemulonii species complex. ACS Infect. Dis. 2020, 6, 1273–1282. [Google Scholar] [CrossRef]
- Joseph-Horne, T.; Hollomon, D.; Loeffler, R.; Kelly, S.L. Cross-resistance to polyene and azole drugs in Cryptococcus neoformans. Antimicrob. Agents Chemother. 1995, 39, 1526–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, S.; Lamb, D.; Kelly, D.; Manning, N.; Loeffler, J.; Hebart, H.; Schumacher, U.; Einsele, H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5, 6-desaturation. FEBS Lett. 1997, 400, 80–82. [Google Scholar] [CrossRef] [Green Version]
- Posch, W.; Blatzer, M.; Wilflingseder, D.; Lass-Flörl, C. Aspergillus terreus: Novel lessons learned on amphotericin B resistance. Med. Mycol. 2018, 56, S73–S82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, G.; Perkhofer, S.; Haas, H.; Schrettl, M.; Würzner, R.; Dierich, M.P.; Lass-Flörl, C. Potential basis for amphotericin B resistance in Aspergillus terreus. Antimicrob. Agents Chemother. 2008, 52, 1553–1555. [Google Scholar] [CrossRef] [Green Version]
- Andrews, F.A.; Sarosi, G.A.; Beggs, W.H. Enhancement of amphotericin B activity by a series of compounds related to phenolic antioxidants. J. Antimicrob. Chemother. 1979, 5, 173–177. [Google Scholar] [CrossRef]
- Blatzer, M.; Jukic, E.; Posch, W.; Schöpf, B.; Binder, U.; Steger, M.; Blum, G.; Hackl, H.; Gnaiger, E.; Lass-Flörl, C. Amphotericin B resistance in Aspergillus terreus is overpowered by coapplication of pro-oxidants. Antioxid. Redox Signal. 2015, 23, 1424–1438. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef] [Green Version]
- Cowen, L.E.; Lindquist, S. Hsp90 potentiates the rapid evolution of new traits: Drug resistance in diverse fungi. Science 2005, 309, 2185–2189. [Google Scholar] [CrossRef]
- De Aguiar Cordeiro, R.; de Jesus Evangelista, A.J.; Serpa, R.; de Farias Marques, F.J.; de Melo, C.V.S.; de Oliveira, J.S.; da Silva Franco, J.; de Alencar, L.P.; Bandeira, T.d.J.P.G.; Brilhante, R.S.N. Inhibition of heat-shock protein 90 enhances the susceptibility to antifungals and reduces the virulence of Cryptococcus neoformans/Cryptococcus gattii species complex. Microbiology 2016, 162, 309–317. [Google Scholar] [CrossRef]
- LaFayette, S.L.; Collins, C.; Zaas, A.K.; Schell, W.A.; Betancourt-Quiroz, M.; Gunatilaka, A.L.; Perfect, J.R.; Cowen, L.E. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 2010, 6, e1001069. [Google Scholar] [CrossRef] [Green Version]
- Blatzer, M.; Blum, G.; Jukic, E.; Posch, W.; Gruber, P.; Nagl, M.; Binder, U.; Maurer, E.; Sarg, B.; Lindner, H.; et al. Blocking Hsp70 Enhances the Efficiency of Amphotericin B Treatment against Resistant Aspergillus terreus Strains. Antimicrob. Agents Chemother. 2015, 59, 3778–3788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, K.; Akiyoshi, H.; Ohnishi, Y. Alteration of cell wall composition leads to amphotericin B resistance in Aspergillus flavus. Microbiol. Immunol. 1999, 43, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Rueda, C.; Román, E.; Quintin, J.; Terrón, M.C.; Luque, D.; Netea, M.G.; Pla, J.; Zaragoza, O. Cell wall changes in amphotericin B-resistant strains from Candida tropicalis and relationship with the immune responses elicited by the host. Antimicrob. Agents Chemother. 2016, 60, 2326–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casadevall, A.; Pirofski, L.-A. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 2003, 1, 17–24. [Google Scholar] [CrossRef]
- O’Keeffe, J.; Kavanagh, K. Adriamycin alters the expression of drug efflux pumps and confers amphotericin B tolerance in Candida albicans. Anticancer Res. 2004, 24, 405–408. [Google Scholar]
- Niimi, M.; Niimi, K.; Takano, Y.; Holmes, A.R.; Fischer, F.J.; Uehara, Y.; Cannon, R.D. Regulated overexpression of CDR1 in Candida albicans confers multidrug resistance. J. Antimicrob. Chemother. 2004, 54, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 2020. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Kean, R.; Ramage, G. Combined antifungal resistance and biofilm tolerance: The global threat of Candida auris. mSphere 2019, 4, e00458-19. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, J.F.; Gade, L.; Chow, N.A.; Loparev, V.N.; Juieng, P.; Berkow, E.L.; Farrer, R.A.; Litvintseva, A.P.; Cuomo, C.A. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat. Commun. 2018, 9, 5346. [Google Scholar] [CrossRef] [Green Version]
- Woods, K.; Hofken, T. The zinc cluster proteins Upc2 and Ecm22 promote filamentation in Saccharomyces cerevisiae by sterol biosynthesis-dependent and -independent pathways. Mol. Microbiol. 2016, 99, 512–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carolus, H.; Pierson, S.; Muñoz, J.F.; Subotić, A.; Cruz, R.B.; Cuomo, C.A.; Van Dijck, P. Genome-wide analysis of experimentally evolved Candida auris reveals multiple novel mechanisms of multidrug-resistance. bioRxiv 2020. [Google Scholar] [CrossRef]
- Majka, J.; Burgers, P.M. Yeast Rad17/Mec3/Ddc1: A sliding clamp for the DNA damage checkpoint. Proc. Natl. Acad. Sci. USA 2003, 100, 2249–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.; Liang, J.; Chaturvedi, S.; Jacobs, J.; Chaturvedi, V. Pan-resistant Candida auris: New York Sub-cluster Susceptible to Antifungal Combinations. bioRxiv 2020. [Google Scholar] [CrossRef]
- Arikan, S.; Lozano-Chiu, M.; Paetznick, V.; Nangia, S.; Rex, J.H. Microdilution Susceptibility Testing of Amphotericin B, Itraconazole, and Voriconazole against clinical Isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 1999, 37, 3946–3951. [Google Scholar] [CrossRef] [Green Version]
- Anaissie, E.J.; Hachem, R.; Legrand, C.; Legenne, P.; Nelson, P.; Bodey, G.P. Lack of activity of amphotericin B in systemic murine fusarial infection. J. Infect. Dis. 1992, 165, 1155–1157. [Google Scholar] [CrossRef]
- Al-Hatmi, A.; Curfs-Breuker, I.; De Hoog, G.S.; Meis, J.F.; Verweij, P.E. Antifungal susceptibility testing of Fusarium: A practical approach. J. Fungi 2017, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Goldman, C.; Akiyama, M.J.; Torres, J.; Louie, E.; Meehan, S.A. Scedosporium apiospermum infections and the role of combination antifungal therapy and GM-CSF: A case report and review of the literature. Med. Mycol. Case Rep. 2016, 11, 40–43. [Google Scholar] [CrossRef]
- Lamaris, G.A.; Chamilos, G.; Lewis, R.E.; Safdar, A.; Raad, I.I.; Kontoyiannis, D.P. Scedosporium infection in a tertiary care cancer center: A review of 25 cases from 1989–2006. Clin. Infect. Dis. 2006, 43, 1580–1584. [Google Scholar] [CrossRef]
- Howden, B.; Slavin, M.; Schwarer, A.; Mijch, A. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M.; Ruiz-Díez, B.; Martínez-Suárez, J.V.; Monzón, A.; Rodríguez-Tudela, J.L. Comparative In Vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 1999, 43, 149–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, C.; Chowdhary, A. Molecular bases of antifungal resistance in filamentous fungi. Int. J. Antimicrob. Agents 2017, 50, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on antifungal resistance mechanisms in the emerging pathogen Candida auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, M.-N.; Jang, S.J.; Ju, M.Y.; Kim, S.H.; Shin, M.G.; Suh, S.P.; Ryang, D.W. Detection of amphotericin B resistance in Candida haemulonii and closely related species by use of the Etest, Vitek-2 yeast susceptibility system, and CLSI and EUCAST broth microdilution methods. J. Clin. Microbiol. 2012, 50, 1852–1855. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-L.; Li, S.-Y.; Cheng, H.-H.; Lo, H.-J. The trend of susceptibilities to amphotericin B and fluconazole of Candida species from 1999 to 2002 in Taiwan. BMC Infect. Dis. 2005, 5, 99. [Google Scholar] [CrossRef] [Green Version]
- Ostrosky-Zeichner, L.; Rex, J.H.; Pappas, P.G.; Hamill, R.J.; Larsen, R.A.; Horowitz, H.W.; Powderly, W.G.; Hyslop, N.; Kauffman, C.A.; Cleary, J. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 2003, 47, 3149–3154. [Google Scholar] [CrossRef] [Green Version]
- Ellis, D. Amphotericin B: Spectrum and resistance. J. Antimicrob. Chemother. 2002, 49, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Lopez, A.; Cuenca-Estrella, M.; Monzon, A.; Rodriguez-Tudela, J. In Vitro susceptibility of clinical isolates of Zygomycota to amphotericin B, flucytosine, itraconazole and voriconazole. J. Antimicrob. Chemother. 2001, 48, 919–921. [Google Scholar] [CrossRef] [Green Version]
- Almyroudis, N.G.; Sutton, D.A.; Fothergill, A.W.; Rinaldi, M.G.; Kusne, S. In Vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob. Agents Chemother. 2007, 51, 2587–2590. [Google Scholar] [CrossRef] [Green Version]
- Maraki, S.; Mavromanolaki, V.E.; Stafylaki, D.; Nioti, E.; Hamilos, G.; Kasimati, A. Epidemiology and antifungal susceptibility patterns of Candida isolates from Greek women with vulvovaginal candidiasis. Mycoses 2019, 62, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Alborzi, A. Susceptibility of clinical Candida species isolates to antifungal agents by E-test, Southern Iran: A five year study. Iran. J. Microbiol. 2011, 3, 183–188. [Google Scholar] [PubMed]
- Bourgeois, N.; Dehandschoewercker, L.; Bertout, S.; Bousquet, P.-J.; Rispail, P.; Lachaud, L. Antifungal susceptibility of 205 Candida spp. isolated primarily during invasive candidiasis and comparison of the Vitek 2 system with the CLSI broth microdilution and Etest methods. J. Clin. Microbiol. 2010, 48, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iatta, R.; Caggiano, G.; Cuna, T.; Montagna, T. Antifungal susceptibility testing of a 10-year collection of Candida spp. isolated from patients with candidemia. J. Chemother. 2011, 23, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-I.; Shin, J.H.; Choi, H.-J.; Ju, M.-Y.; Kim, S.-H.; Lee, W.G.; Park, Y.-J.; Lee, K. Antifungal susceptibility to amphotericin B, fluconazole, voriconazole, and flucytosine in Candida bloodstream isolates from 15 tertiary hospitals in Korea. Ann. Lab. Med. 2012, 32, 426–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockhart, S.R.; Iqbal, N.; Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Bolden, C.B.; Baughman, W.; Stein, B.; Hollick, R.; Park, B.J. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two US cities from 2008 to 2011. J. Clin. Microbiol. 2012, 50, 3435–3442. [Google Scholar] [CrossRef] [Green Version]
- Tsega, A.; Mekonnen, F. Prevalence, risk factors and antifungal susceptibility pattern of Candida species among pregnant women at Debre Markos Referral Hospital, Northwest Ethiopia. BMC Pregnancy Childbirth 2019, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-L.; Wang, A.-H.; Wang, C.-W.; Cheng, W.-T.; Li, S.-Y.; Lo, H.-J. Susceptibilities to amphotericin B and fluconazole of Candida species in Taiwan Surveillance of Antimicrobial Resistance of Yeasts 2006. Diagn. Microbiol. Infect. Dis. 2008, 61, 175–180. [Google Scholar] [CrossRef]
- Christenson, J.C.; Guruswamy, A.; Mukwaya, G.; Retting, P.J. Candida lusitaniae: An emerging human pathogen. Pediatr. Infect. Dis. J. 1987, 6, 755–757. [Google Scholar] [CrossRef]
- Hawkins, J.L.; Baddour, L.M. Candida lusitaniae infections in the era of fluconazole availability. Clin. Infect. Dis. 2003, 36, e14–e18. [Google Scholar] [CrossRef] [Green Version]
- Krcmery, V.; Barnes, A. Non-albicans Candida spp. causing fungaemia: Pathogenicity and antifungal resistance. J. Hosp. Infect. 2002, 50, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Khan, S.; Joseph, L. Candida lusitaniae in Kuwait: Prevalence, antifungal susceptibility and role in neonatal fungemia. PLoS ONE 2019, 14, e0213532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minari, A.; Hachem, R.; Raad, I. Candida lusitaniae: A cause of breakthrough fungemia in cancer patients. Clin. Infect. Dis. 2001, 32, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.; Diekema, D.; Messer, S.; Boyken, L.; Hollis, R.; Jones, R. In Vitro activities of voriconazole, posaconazole, and four licensed systemic antifungal agents against Candida species infrequently isolated from blood. J. Clin. Microbiol. 2003, 41, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, S.L.; Francisco, E.C.; de Almeida, J.N., Jr.; Santos, D.W.d.C.L.; Carlesse, F.; Queiroz-Telles, F.; Melo, A.S.d.A.; Colombo, A.L. Increasing Prevalence of Multidrug-Resistant Candida haemulonii Species Complex among All Yeast Cultures Collected by a Reference Laboratory over the Past 11 Years. J. Fungi 2020, 6, 110. [Google Scholar] [CrossRef]
- Cendejas-Bueno, E.; Kolecka, A.; Alastruey-Izquierdo, A.; Theelen, B.; Groenewald, M.; Kostrzewa, M.; Cuenca-Estrella, M.; Gómez-López, A.; Boekhout, T. Reclassification of the Candida haemulonii complex as Candida haemulonii (C. haemulonii group I.), C. duobushaemulonii sp. nov.(C. haemulonii group II), and C. haemulonii var. vulnera var. nov.: Three multiresistant human pathogenic yeasts. J. Clin. Microbiol. 2012, 50, 3641–3651. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, J.N., Jr.; Assy, J.G.P.L.; Levin, A.S.; Del Negro, G.M.; Giudice, M.C.; Tringoni, M.P.; Thomaz, D.Y.; Motta, A.L.; Abdala, E.; Pierroti, L.C. Candida haemulonii complex species, Brazil, January 2010–March 2015. Emerg. Infect. Dis. 2016, 22, 561. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Xiao, M.; Chen, S.C.-A.; Wang, H.; Cheng, J.-W.; Chen, X.-X.; Xu, Z.-P.; Fan, X.; Kong, F.; Xu, Y.-C. Identification and antifungal susceptibility profiles of Candida haemulonii species complex clinical isolates from a multicenter study in China. J. Clin. Microbiol. 2016, 54, 2676–2680. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, S.R. Candida auris and multidrug resistance: Defining the new normal. Fungal Genet. Biol. 2019, 131, 103243. [Google Scholar] [CrossRef]
- Pfaller, M.; Diekema, D.; Gibbs, D.; Newell, V.; Meis, J.; Gould, I.; Fu, W.; Colombo, A.; Rodriguez-Noriega, E. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: An 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 2007, 45, 1735–1745. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xiao, M.; Chen, S.C.; Kong, F.; Sun, Z.-Y.; Liao, K.; Lu, J.; Shao, H.-F.; Yan, Y.; Fan, H. Results from the National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) Study, 2010: Yeast species and in vitro susceptibilities to fluconazole and voriconazole. J. Clin. Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arechavala, A.I.; Ochiuzzi, M.E.; Borgnia, M.D.; Santiso, G.M. Fluconazole and amphotericin B susceptibility testing of Cryptococcus neoformans: Results of minimal inhibitory concentrations against 265 isolates from HIV-positive patients before and after two or more months of antifungal therapy. Rev. Iberoam. Micol. 2009, 26, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.E.; Pfaller, M.A.; Hajjeh, R.A.; Hamill, R.J.; Pappas, P.G.; Reingold, A.L.; Rimland, D.; Warnock, D.W. Trends in antifungal drug susceptibility of Cryptococcus neoformans isolates in the United States: 1992 to 1994 and 1996 to 1998. Antimicrob. Agents Chemother. 2001, 45, 3065–3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandenier, J.; Adou-Bryn, K.; Douchet, C.; Sar, B.; Kombila, M.; Swinne, D.; Therizol-Ferly, M.; Buisson, Y.; Richard-Lenoble, D. In Vitro activity of amphotericin B, fluconazole and voriconazole against 162 Cryptococcus neoformans isolates from Africa and Cambodia. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 506–508. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Aller, A.; Canton, E.; Castañón-Olivares, L.; Chowdhary, A.; Cordoba, S.; Cuenca-Estrella, M.; Fothergill, A.; Fuller, J.; Govender, N. Cryptococcus neoformans-Cryptococcus gattii species complex: An international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob. Agents Chemother. 2012, 56, 5898–5906. [Google Scholar] [CrossRef] [Green Version]
- Hagen, F.; Hare Jensen, R.; Meis, J.F.; Arendrup, M.C. Molecular epidemiology and In Vitro antifungal susceptibility testing of 108 clinical Cryptococcus neoformans sensu lato and Cryptococcus gattii sensu lato isolates from Denmark. Mycoses 2016, 59, 576–584. [Google Scholar] [CrossRef]
- Lei, Y.; Xiao, Y.; He, C.; Zhang, C.; Xie, Y.; Kang, M. Genotypes and in vitro antifungal susceptibility of Cryptococcus isolates in Sichuan Province. Sichuan Da Xue Xue Bao Yi Xue Ban J. Sichuan Univ. Med. Sci. Ed. 2015, 46, 82–86. [Google Scholar]
- Perkins, A.; Gomez-Lopez, A.; Mellado, E.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Rates of antifungal resistance among Spanish clinical isolates of Cryptococcus neoformans var. neoformans. J. Antimicrob. Chemother. 2005, 56, 1144–1147. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Zaragoza, O.; Martins, M.D.A.; Melhem, M.; Rodriguez-Tudela, J.; Cuenca-Estrella, M. In Vitro susceptibility of Cryptococcus gattii clinical isolates. Clin. Microbiol. Infect. 2008, 14, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Francisco, E.; de Almeida, J.N., Jr.; de Queiroz Telles, F.; Aquino, V.; Mendes, A.; de Andrade Barberino, M.; Castro, P.d.T.O.; Guimarães, T.; Hahn, R.; Padovan, A. Species distribution and antifungal susceptibility of 358 Trichosporon clinical isolates collected in 24 medical centres. Clin. Microbiol. Infect. 2019, 25, 909.e1–909.e5. [Google Scholar] [CrossRef]
- Guo, L.-N.; Yu, S.-Y.; Hsueh, P.-R.; Al-Hatmi, A.M.; Meis, J.F.; Hagen, F.; Xiao, M.; Wang, H.; Barresi, C.; Zhou, M.-L. Invasive infections due to Trichosporon: Species distribution, genotyping, and antifungal susceptibilities from a multicenter study in China. J. Clin. Microbiol. 2019, 57, e01505-18. [Google Scholar]
- Taj-Aldeen, S.J.; Al-Ansari, N.; El Shafei, S.; Meis, J.F.; Curfs-Breuker, I.; Theelen, B.; Boekhout, T. Molecular identification and susceptibility of Trichosporon species isolated from clinical specimens in Qatar: Isolation of Trichosporon dohaense Taj-Aldeen, Meis & Boekhout sp. nov. J. Clin. Microbiol. 2009, 47, 1791–1799. [Google Scholar] [PubMed] [Green Version]
- Rodriguez-Tudela, J.L.; Diaz-Guerra, T.M.; Mellado, E.; Cano, V.; Tapia, C.; Perkins, A.; Gomez-Lopez, A.; Rodero, L.; Cuenca-Estrella, M. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob. Agents Chemother. 2005, 49, 4026–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabatzis, M.; Abel, P.; Kanellopoulou, M.; Adamou, D.; Alexandrou-Athanasoulis, H.; Stathi, A.; Platsouka, E.; Milioni, A.; Pangalis, A.; Velegraki, A. Sequence-based identification, genotyping and EUCAST antifungal susceptibilities of Trichosporon clinical isolates from Greece. Clin. Microbiol. Infect. 2014, 20, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, A.M.; Sánchez González, A.; Palma-Nicolás, J.P.; Gómez-Treviño, A.; González, J.G.; González, G.M. Genotyping, extracellular compounds, and antifungal susceptibility testing of Trichosporon asahii isolated from Mexican patients. Med. Mycol. 2015, 53, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Chagas-Neto, T.C.; Chaves, G.M.; Melo, A.S.; Colombo, A.L. Bloodstream infections due to Trichosporon spp.: Species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing, and antifungal susceptibility testing. J. Clin. Microbiol. 2009, 47, 1074–1081. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.A.; Alastruey-Izquierdo, A.; Gomez-Lopez, A.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Molecular identification and susceptibility testing of Trichosporon isolates from a Brazilian hospital. Rev. Iberoam Micol. 2008, 25, 221–225. [Google Scholar]
- Borman, A.M.; Muller, J.; Walsh-Quantick, J.; Szekely, A.; Patterson, Z.; Palmer, M.D.; Fraser, M.; Johnson, E.M. MIC distributions for amphotericin B, fluconazole, itraconazole, voriconazole, flucytosine and anidulafungin and 35 uncommon pathogenic yeast species from the UK determined using the CLSI broth microdilution method. J. Antimicrob. Chemother. 2020, 75, 1194–1205. [Google Scholar] [CrossRef]
- Messer, S.A.; Jones, R.N.; Fritsche, T.R. International surveillance of Candida spp. and Aspergillus spp.: Report from the SENTRY Antimicrobial Surveillance Program (2003). J. Clin. Microbiol. 2006, 44, 1782–1787. [Google Scholar] [CrossRef] [Green Version]
- Reichert-Lima, F.; Lyra, L.; Pontes, L.; Moretti, M.L.; Pham, C.D.; Lockhart, S.R.; Schreiber, A.Z. Surveillance for azoles resistance in Aspergillus spp. highlights a high number of amphotericin B-resistant isolates. Mycoses 2018, 61, 360–365. [Google Scholar] [CrossRef]
- Van Der Linden, J.W.; Warris, A.; Verweij, P.E. Aspergillus species intrinsically resistant to antifungal agents. Med. Mycol. 2011, 49, S82–S89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashu, E.E.; Korfanty, G.A.; Samarasinghe, H.; Pum, N.; You, M.; Yamamura, D.; Xu, J. Widespread amphotericin B-resistant strains of Aspergillus fumigatus in Hamilton, Canada. Infect. Drug Resist. 2018, 11, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadeganipour, M.; Mohammadi, R. A 9-Year Experience of Aspergillus Infections from Isfahan, Iran. Infect. Drug Resist. 2020, 13, 2301. [Google Scholar] [CrossRef] [PubMed]
- Koss, T.; Bagheri, B.; Zeana, C.; Romagnoli, M.F.; Grossman, M.E. Amphotericin B-resistant Aspergillus flavus infection successfully treated with caspofungin, a novel antifungal agent. J. Am. Acad. Dermatol. 2002, 46, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fuoli, L.; Mellado, E.; Alastruey-Izquierdo, A.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Aspergillus section Fumigati: Antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 2008, 52, 1244–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manikandan, P.; Abdel-Hadi, A.; Randhir Babu Singh, Y.; Revathi, R.; Anita, R.; Banawas, S.; Bin Dukhyil, A.A.; Alshehri, B.; Shobana, C.S.; Panneer Selvam, K. Fungal keratitis: Epidemiology, rapid detection, and antifungal susceptibilities of Fusarium and Aspergillus isolates from corneal scrapings. BioMed Res. Int. 2019, 2019, 6395840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baddley, J.W.; Marr, K.A.; Andes, D.R.; Walsh, T.J.; Kauffman, C.A.; Kontoyiannis, D.P.; Ito, J.I.; Balajee, S.A.; Pappas, P.G.; Moser, S.A. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J. Clin. Microbiol. 2009, 47, 3271–3275. [Google Scholar] [CrossRef] [Green Version]
- Husain, S.; Muñoz, P.; Forrest, G.; Alexander, B.D.; Somani, J.; Brennan, K.; Wagener, M.M.; Singh, N. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: Clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 2005, 40, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, W.; Perfect, J. Scedosporium species infections and treatments. J. Chemother. 2003, 15, 16–27. [Google Scholar] [CrossRef]
- Dalle Rosa, P.; Sheid, K.; Locatelli, C.; Marinho, D.; Goldani, L. Fusarium solani keratitis: Role of antifungal susceptibility testing and identification to the species level for proper management. Braz. J. Infect. Dis. 2019, 23, 197–199. [Google Scholar] [CrossRef]
- Dallé da Rosa, P.; Ramirez-Castrillon, M.; Borges, R.; Aquino, V.; Fuentefria, A.M.; Goldani, L.Z. Epidemiological aspects and characterization of the resistance profile of Fusarium spp. in patients with invasive fusariosis. J. Med. Microbiol. 2019, 68, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Lee, S.A. Emerging moulds: Epidemiological trends and antifungal resistance. Mycoses 2011, 54, e666–e678. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, M.; Nordoff, N.; Li, R.-K.; Pasarell, L.; Warnock, D. Sporothrix schenckii sensitivity to voriconazole, itraconazole and amphotericin B. Med. Mycol. 2001, 39, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.M.; Soares, B.M.; de Assis Santos, D.; Da Silva Barros, M.E.; Hamdan, J.S. In Vitro susceptibility of isolates of Sporothrix schenckii to amphotericin B, itraconazole, and terbinafine: Comparison of yeast and mycelial forms. Can. J. Microbiol. 2006, 52, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Trilles, L.; Fernández-Torres, B.; dos Santos Lazéra, M.; Wanke, B.; de Oliveira Schubach, A.; de Almeida Paes, R.; Inza, I.; Guarro, J. In Vitro antifungal susceptibilities of Sporothrix schenckii in two growth phases. Antimicrob. Agents Chemother. 2005, 49, 3952–3954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, S.; Zaini, F.; Kordbacheh, P.; Safara, M.; Heidari, M. Sporothrix schenckii complex in Iran: Molecular identification and antifungal susceptibility. Sabouraudia 2016, 54, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreu, C.; León, A.; Medina, Y.; Machín, G.; Lancha, M.; Zaragozi, M. In Vitro sensitivity of Histoplasma capsulatum var. capsulatum to amphotericin B, ketoconazole, itroconazole and fluconazole. Rev. Cuba. Med. Trop. 2003, 55, 76. [Google Scholar]
- Li, R.K.; Ciblak, M.A.; Nordoff, N.; Pasarell, L.; Warnock, D.W.; McGinnis, M.R. In Vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob. Agents Chemother. 2000, 44, 1734–1736. [Google Scholar] [CrossRef] [Green Version]
- Wagner, L.; de Hoog, S.; Alastruey-Izquierdo, A.; Voigt, K.; Kurzai, O.; Walther, G. A revised species concept for opportunistic Mucor species reveals species-specific antifungal susceptibility profiles. Antimicrob. Agents Chemother. 2019, 63, e00653-19. [Google Scholar] [CrossRef] [Green Version]
- Riley, T.T.; Muzny, C.A.; Swiatlo, E.; Legendre, D.P. Breaking the mold: A review of mucormycosis and current pharmacological treatment options. Ann. Pharmacother. 2016, 50, 747–757. [Google Scholar] [CrossRef]
- Drogari-Apiranthitou, M.; Mantopoulou, F.-D.; Skiada, A.; Kanioura, L.; Grammatikou, M.; Vrioni, G.; Mitroussia-Ziouva, A.; Tsakris, A.; Petrikkos, G. In Vitro antifungal susceptibility of filamentous fungi causing rare infections: Synergy testing of amphotericin B, posaconazole and anidulafungin in pairs. J. Antimicrob. Chemother. 2012, 67, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Alastruey-Izquierdo, A.; Castelli, M.; Cuesta, I.; Zaragoza, O.; Monzón, A.; Mellado, E.; Rodríguez-Tudela, J. In vitro activity of antifungals against Zygomycetes. Clin. Microbiol. Infect. 2009, 15, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, R.G.; de Hoog, G.S.; Schwarz, P.; Dannaoui, E.; Deng, S.; Machouart, M.; Voigt, K.; van de Sande, W.W.; Dolatabadi, S.; Meis, J.F. Antifungal susceptibility and phylogeny of opportunistic members of the order mucorales. J. Clin. Microbiol. 2012, 50, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.J.; Arthington-Skaggs, B.A.; Hajjeh, R.A.; Iqbal, N.; Ciblak, M.A.; Lee-Yang, W.; Hairston, M.D.; Phelan, M.; Plikaytis, B.D.; Sofair, A.N.; et al. Evaluation of Amphotericin B Interpretive Breakpoints for Candida Bloodstream Isolates by Correlation with Therapeutic Outcome. Antimicrob. Agents Chemother. 2006, 50, 1287–1292. [Google Scholar] [CrossRef] [Green Version]
- Rex, J.H.; Pfaller, M.A.; Barry, A.L.; Nelson, P.W.; Webb, C.D. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Antimicrob. Agents Chemother. 1995, 39, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.H.; Clancy, C.J.; Yu, V.L.; Yu, Y.C.; Morris, A.J.; Snydman, D.R.; Sutton, D.A.; Rinaldi, M.G. Do In Vitro Susceptibility Data Predict the Microbiologic Response to Amphotericin B? Results of a Prospective Study of Patients with Candida Fungemia. J. Infect. Dis. 1998, 177, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Clancy, C.J.; Nguyen, M.H. Correlation between In Vitro Susceptibility Determined by E Test and Response to Therapy with Amphotericin B: Results from a Multicenter Prospective Study of Candidemia. Antimicrob. Agents Chemother. 1999, 43, 1289–1290. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. https://doi.org/10.3390/jof6040321
Carolus H, Pierson S, Lagrou K, Van Dijck P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. Journal of Fungi. 2020; 6(4):321. https://doi.org/10.3390/jof6040321
Chicago/Turabian StyleCarolus, Hans, Siebe Pierson, Katrien Lagrou, and Patrick Van Dijck. 2020. "Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance" Journal of Fungi 6, no. 4: 321. https://doi.org/10.3390/jof6040321
APA StyleCarolus, H., Pierson, S., Lagrou, K., & Van Dijck, P. (2020). Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. Journal of Fungi, 6(4), 321. https://doi.org/10.3390/jof6040321







