The Survival and Treatment of Fusarium oxysporum f. sp. cubense in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Preparation of Foc Inoculum
2.3. Survival of Foc
2.4. Water Treatments
2.4.1. Foc Inoculum
2.4.2. Ozone
2.4.3. Ultraviolet Light Radiation
2.4.4. Chlorine
2.4.5. Peracetic Acid
2.5. Statistical Analysis
3. Results
3.1. Survival of Foc in Water
3.2. Water Treatments
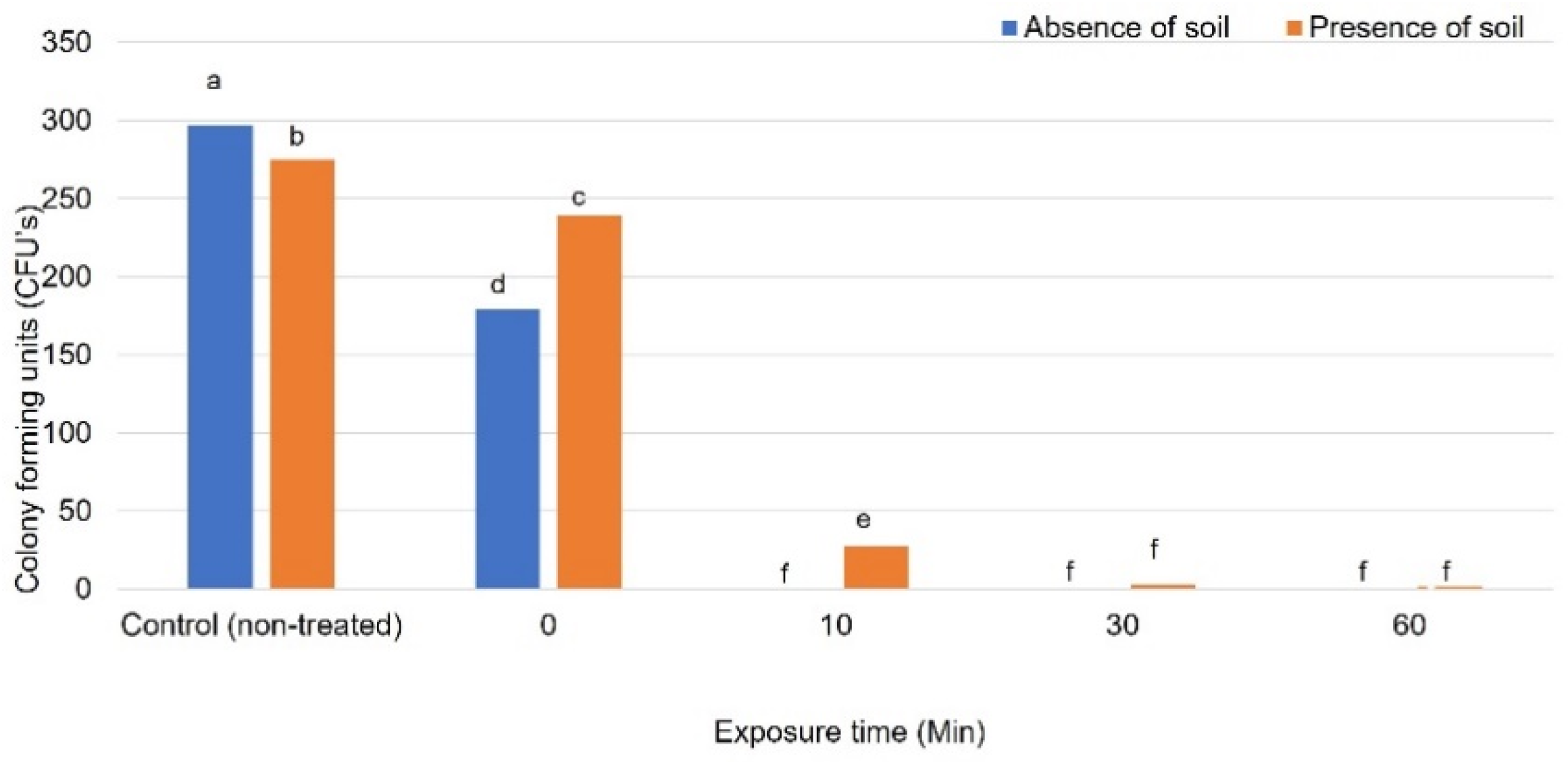
3.2.1. Ozone
3.2.2. Chlorine
3.2.3. Peracetic Acid
3.2.4. Ultraviolet Light Radiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stover, R.H. Fusarial Wilt (Panama Disease) of Bananas and Other Musa Species; The Commonwealth Mycological Institute: London, UK, 1962; p. 117. [Google Scholar]
- Stover, R.H. Studies on fusarium wilt of bananas: IX. Competitive saprophytic ability of Fusarium oxysporum f. sp. cubense. Can. J. Bot. 1962, 40, 1473–1481. [Google Scholar] [CrossRef]
- Ploetz, R.C. Panama Disease, an Old Nemesis Rears Its Ugly Head: Part 1, the Beginnings of the Banana Export Trades. Plant Health Prog. 2005, 6, 18. [Google Scholar] [CrossRef]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The epidemiology of Fusarium wilt of banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epp, M.D. Somaclonal variation in bananas: A case study with Fusarium wilt. Banana and plantain strategies, Cairns, Australia. ACIAR 1987, 21, 140–150. [Google Scholar]
- Viljoen, A.; Mostert, D.; Chiconela, T.; Beukes, I.; Fraser, C.; Dwyer, J.; Murray, H.; Amisse, J.; Matabuana, E.L.; Tazan, G.; et al. Occurrence and spread of the banana fungus Fusarium oxysporum f. sp. cubense TR4 in Mozambique. S. Afr. J. Sci. 2020, 116, 1–11. [Google Scholar]
- Xu, L.B.; Huang, B.Z.; Wei, Y.R. Production and banana R and D in China. In Advancing Banana and Plantain Research and Development in Asia and the Pacific; Molina, A.B., Eusebio, J.E., Roa, V.N., Van den Bergh, I., Maghuyop, M.A.G., Eds.; International Network for the Improvement of Banana and Plantain: Los Baños, Philippines, 2003; pp. 77–80. [Google Scholar]
- Viljoen, A.; Ma, L.-J.; Molina, A.B. Fusarium wilt (Panama disease) and monoculture banana production: Resurgence of a century-old disease. In Emerging Plant Diseases and Global Food Security; Ristaino, J.B., Records, A., Eds.; APS Press: St. Paul, MN, USA, 2020; pp. 159–184. [Google Scholar]
- Stover, R.H. Flooding of soil for disease control. In Developments in Agricultural and Managed Forest Ecology; Elsevier: Amsterdam, The Netherlands, 1979; pp. 19–28. [Google Scholar]
- Wardlaw, C.W. Banana Diseases Including Plantain and Abaca; John Wiley and Sons, Inc.: New York, NY, USA, 1961. [Google Scholar]
- Stover, R.H. Flood fallowing for eradication of Fusarium oxysporum f. sp. cubense: II. Some factors involved in fungus survival. Soil Sci. 1954, 77, 401–414. [Google Scholar]
- Newcombe, M. Some effects of water and anaerobic conditions on Fusarium oxysporum f. sp. cubense in soil. T. Brit. Mycol. Soc. 1960, 43, 51–59. [Google Scholar] [CrossRef]
- Stewart-Wade, S.M. Plant pathogens in recycled irrigation water in commercial plant nurseries and greenhouses: Their detection and management. Irrig. Sci. 2011, 29, 267–297. [Google Scholar] [CrossRef]
- Minuto, A.; Gaggero, L.; Gullino, M.L.; Garibaldi, A. Influence of pH, nutrient solution disinfestation and antagonists application in a closed soilless system on severity of Fusarium wilt of gerbera. Phytoparasitica 2008, 36, 294–303. [Google Scholar] [CrossRef]
- Runia, W.T.H.; Van Os, E.A.; Bollen, G.J. Disinfection of drain water from soilless cultures by heat treatment. Neth. J. Agr. Sci. 1988, 36, 231–238. [Google Scholar]
- Zheng, Y.; Dunets, S.; Cayanan, D. UV Light. Greenhouse and Nursery Water Treatment Information System; School of Environmental Sciences, University of Guelph: Guelph, ON, Canada, 2014; Available online: www.ces.uoguelph.ca (accessed on 30 August 2018).
- Beardsell, D.; Bankier, M. Monitoring and Treatment of Recycled Water for Nursery and Floriculture Production; Horticulture Australia Limited: Sydney, Australia, 1996. [Google Scholar]
- Igura, N.; Fujii, M.; Shimoda, M.; Hayakawa, I. Research note: Inactivation efficiency of ozonated water for Fusarium oxysporum conidia under hydroponic greenhouse conditions. Ozone-Sci. Eng. 2004, 26, 517–521. [Google Scholar] [CrossRef]
- Cayanan, D.F.; Zhang, P.; Weizhong, L.; Dixon, M.; Zheng, Y. Efficacy of chlorine in controlling five common plant pathogens. HortScience 2009, 44, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Scarlett, K.; Collins, D.; Tesoriero, L.; Jewell, L.; Van Ogtrop, F.; Daniel, R. Efficacy of chlorine, chlorine dioxide and ultraviolet radiation as disinfectants against plant pathogens in irrigation water. Eur. J. Plant Pathol. 2015, 145, 27–38. [Google Scholar] [CrossRef]
- Hanks, G.R.; Linfield, C.A. Evaluation of peroxyacetic acid disinfection in hot-water treatment for the control of basal rot (Fusarium oxysporum f. sp. narcissi) and stem nematode (Ditylenchus dipsaci) in narcissus. J. Phytopathol. 1999, 147, 271–279. [Google Scholar]
- Hopkins, D.L.; Thompson, C.M.; Hilgren, J.; Lovic, B. Wet seed treatment with peroxyacetic acid for the control of bacterial fruit blotch and other seedborne diseases of watermelon. Plant Dis. 2003, 87, 1495–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wyk, S.J.P.; Boutigny, A.L.; Coutinho, T.A.; Viljoen, A. Sanitation of a South African forestry nursery contaminated with Fusarium circinatum using hydrogen peroxide at specific oxidation reduction potentials. Plant Dis. 2012, 96, 875–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmer, W.H. Preventing the spread of Fusarium wilt of Hiemalis begonias in the greenhouse. Crop Prot. 2008, 27, 1078–1083. [Google Scholar] [CrossRef]
- White, G.C. The Handbook of Chlorination and Alternative Disinfectants, 3rd ed.; Van Nostrand Reinhold: New York, NY, USA, 1992. [Google Scholar]
- Graham, D.M. Use of ozone for food processing. Food Technol. 1997, 51, 72–75. [Google Scholar]
- Zheng, Y.; Dunets, S.; Cayanan, D. Ozonation; University of Guelph: Guelph, ON, Canada, 2018; Available online: www.ces.uoguelph.ca/water/PATHOGEN/Ozonation.pdf (accessed on 30 August 2018).
- Voigt, E.; Jaeger, H.; Knorr, D. Securing Safe Water Supplies: Comparison of Applicable Technologies; Oxford Academic Press: Oxford, UK, 2013; pp. 25–39. [Google Scholar]
- Hong, C.; Moorman, G.W.; Wohanka, W.; Buttner, C. Biology, Detection, and Management of Plant Pathogens in Irrigation Water; The American Phytopathological Society: Saint Paul, MN, USA, 2014. [Google Scholar]
- Ivey, M.L.I.; Miller, S.A. Assessing the efficacy of pre-harvest, chlorine-based sanitizers against human pathogen indicator microorganisms and Phytophyhora capsici in non-recycled irrigation water. Water Res. 2013, 47, 4639–4651. [Google Scholar] [CrossRef] [PubMed]
- Van Haute, S.; Sampers, I.; Holvoet, K.; Uyttendaele, M. Physiochemical quality and chemical safety of chlorine as a reconditioning agent and wash water disinfectant for fresh-cut lettuce washing. Appl. Environ. Microb. 2013, 79, 2850–2861. [Google Scholar] [CrossRef] [Green Version]
- Nel, N.; Steinberg, C.; Labuschagne, N.; Viljoen, A. Evaluation of fungicides and sterilants for potential application in the management of Fusarium wilt of banana. Crop Prot. 2007, 26, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Meldrum, R.A.; Daly, A.M.; Tran-Nguyen, L.T.T.; Aitken, E.A.B. The effect of surface sterilants on spore germination of Fusarium oxysporum f. sp. cubense tropical race 4. Crop Prot. 2013, 54, 194–198. [Google Scholar]
- Nguyen, T.V.; Tran-Nguyen, L.T.T.; Wright, C.L.; Trevorrow, P.; Grice, K. Evaluation of the efficacy of commercial disinfectants against Fusarium oxysporum f. sp. cubense race 1 and tropical race 4 propagules. Plant Dis. 2019, 103, 721–728. [Google Scholar]
- DWA (Department of Water Affairs). Revision of the General Authorizations in Terms of Section 39 of the National Water Act, 1998; Act no. 36 of 1998. Government Gazette no. 36820; DWA (Department of Water Affairs): Pretoria, South Africa, 2013.
- Alasri, A.; Roques, C.; Michel, G.; Cabassud, C.; Aptel, P. Bactericidal properties of peracetic acid and hydrogen peroxide, alone and in combination, and chlorine and formaldehyde against bacterial water strains. Can. J. Microbiol. 1992, 38, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- Fraser, J.A.L.; Godfree, A.F.; Jones, F. Use of peracetic acid in operational sludge disposal to pasture. Water Sci. Technol. 1984, 17, 456–466. [Google Scholar] [CrossRef]
- Tutumi, M.; Imamura, K.; Hatano, S.; Watanabe, T. Antimicrobial action of peracetic acid. J. Food Hyg. Soc. 1973, 15, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Freese, S.D.; Bailey, I.; Nozaic, D. The Evaluation of Alternative Disinfection Processes for the Removal of Protozaon Oocysts and Cysts and Other Micro-Organisms, in the Treatment of Final Wastewater; WRC Report No. 1030/1/03; WRC: Pretoria, South Africa, 2003. [Google Scholar]
- Akinbobola, A.B.; Sherry, L.; Mckay, W.G.; Ramage, G.; Williams, C. Tolerance of Pseudomonas aeruginosa in in-vitro biofilms to high-level peracetic acid disinfection. J. Hosp. Infect. 2017, 97, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runia, W.T.H. A review of possibilities for disinfection of recirculation water from soilless cultures. Acta Hortic. 1995, 382, 221–227. [Google Scholar] [CrossRef]
- Sequeira, L. Effect of urea applications on survival of Fusarium oxysporum f. cubense in soil. Phytopathology 1963, 53, 332–336. [Google Scholar]
- Deacon, J.W. Panama disease of bananas in South Africa. Hortic. Sci. 1984, 1, 29–31. [Google Scholar]
- Rattink, H. Epidemiology of Fusarium wilt in cyclamen in an ebb and flow system. Eur. J. Plant Pathol. 1990, 96, 171–177. [Google Scholar] [CrossRef]
- Horikawa, Y.; Terai, T.; Ogura, H. Mutual flocculation between selected clay minerals and some kinds of asexual spores from soilborne fungi. Soil Sci. Plant Nutr. 1979, 25, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Sichel, C.; De Cara, M.; Tello, J.; Blanco, J.; Fernández-Ibáñez, P. Solar photocatalytic disinfection of agricultural pathogenic fungi: Fusarium species. Appl. Catal. B-Environ. 2007, 74, 152–160. [Google Scholar] [CrossRef]
- Hong, C.X.; Moorman, G.W. Plant Pathogens in Irrigation Water: Challenges and Opportunities. Crit. Rev. Plant Sci. 2005, 24, 189–208. [Google Scholar] [CrossRef]
- Cateau, E.; Hechard, Y.; Fernandez, B.; Rodier, M.H. Free living amoebae could enhance Fusarium oxysporum growth. Fungal Ecol. 2014, 8, 12–17. [Google Scholar] [CrossRef]
- Zheng, C.; Zhao, L.; Zhou, X.; Fu, Z.; Li, A. Treatment Technologies for Organic Wastewater. In Water Treatment; Elshorbagy, W., Chowdhury, R.K., Eds.; IntechOpen: London, UK, 2013. [Google Scholar]
- Kim, J.; Huang, C.H. Reactivity of peracetic acid with organic compounds: A critical review. ACS ES&T Water 2020, 1, 15–33. [Google Scholar]
- Portjanskaja, E. Ozone Reactions with Inorganic and Organic Compounds in Water. Encyclopedia of Life Support Systems. Ozone Science and Technology. 2010. Available online: http://www.eolss.net/sample-chapters/c07/e6-192-06-00.pdf (accessed on 30 June 2018).
- Hunter, G. Bacterial Inactivation Using Radial Mode Ultrasonic Devices. Ph.D. Thesis, University of Glasgow, Glasgow, Scotland, 2008. [Google Scholar]
- Koivunen, J.; Heinonen-Tanski, H. Peracetic acid (PAA) disinfection of primary, secondary and tertiary treated municipal wastewaters. Water Res. 2005, 35, 4445–4453. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Mostert, D.; Serfontein, K.; Viljoen, A. The Survival and Treatment of Fusarium oxysporum f. sp. cubense in Water. J. Fungi 2021, 7, 796. https://doi.org/10.3390/jof7100796
Ullah S, Mostert D, Serfontein K, Viljoen A. The Survival and Treatment of Fusarium oxysporum f. sp. cubense in Water. Journal of Fungi. 2021; 7(10):796. https://doi.org/10.3390/jof7100796
Chicago/Turabian StyleUllah, Sahabne, Diane Mostert, Kobus Serfontein, and Altus Viljoen. 2021. "The Survival and Treatment of Fusarium oxysporum f. sp. cubense in Water" Journal of Fungi 7, no. 10: 796. https://doi.org/10.3390/jof7100796
APA StyleUllah, S., Mostert, D., Serfontein, K., & Viljoen, A. (2021). The Survival and Treatment of Fusarium oxysporum f. sp. cubense in Water. Journal of Fungi, 7(10), 796. https://doi.org/10.3390/jof7100796





