Identification of Promising Antifungal Drugs against Scedosporium and Lomentospora Species after Screening of Pathogen Box Library
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Compounds
2.3. Screening of the Pathogen Box Library
2.4. Antifungal Susceptibility Testing
2.5. Biofilm Formation and the Preformed Biofilm Assay
2.6. Scanning Electron Microscopy
- fixation in 2.5% glutaraldehyde and 4% formaldehyde, in 0.1 M cacodylate buffer, for 30 min at room temperature;
- wash in 0.1 M cacodylate buffer;
- post-fixation in 1% osmium tetroxide in 0.1 M cacodylate buffer containing 1.25% potassium ferrocyanide for 30 min;
- wash in 0.1 M cacodylate buffer again;
- dehydration in a graded ethanol series (30–100%);
- critical point drying in CO2 (EM CPD300, Leica, German);
- adhesion to aluminum stubs with carbon tape; and
- coating with gold.
2.7. Antifungal Drug Synergy Assay
2.8. Statistical Analyses
3. Results
3.1. Screening of Pathogen Box Library
3.2. Minimum Inhibitory Concentration of Auranofin and Iodoquinol against Different Scedosporium and Lomentospora Species
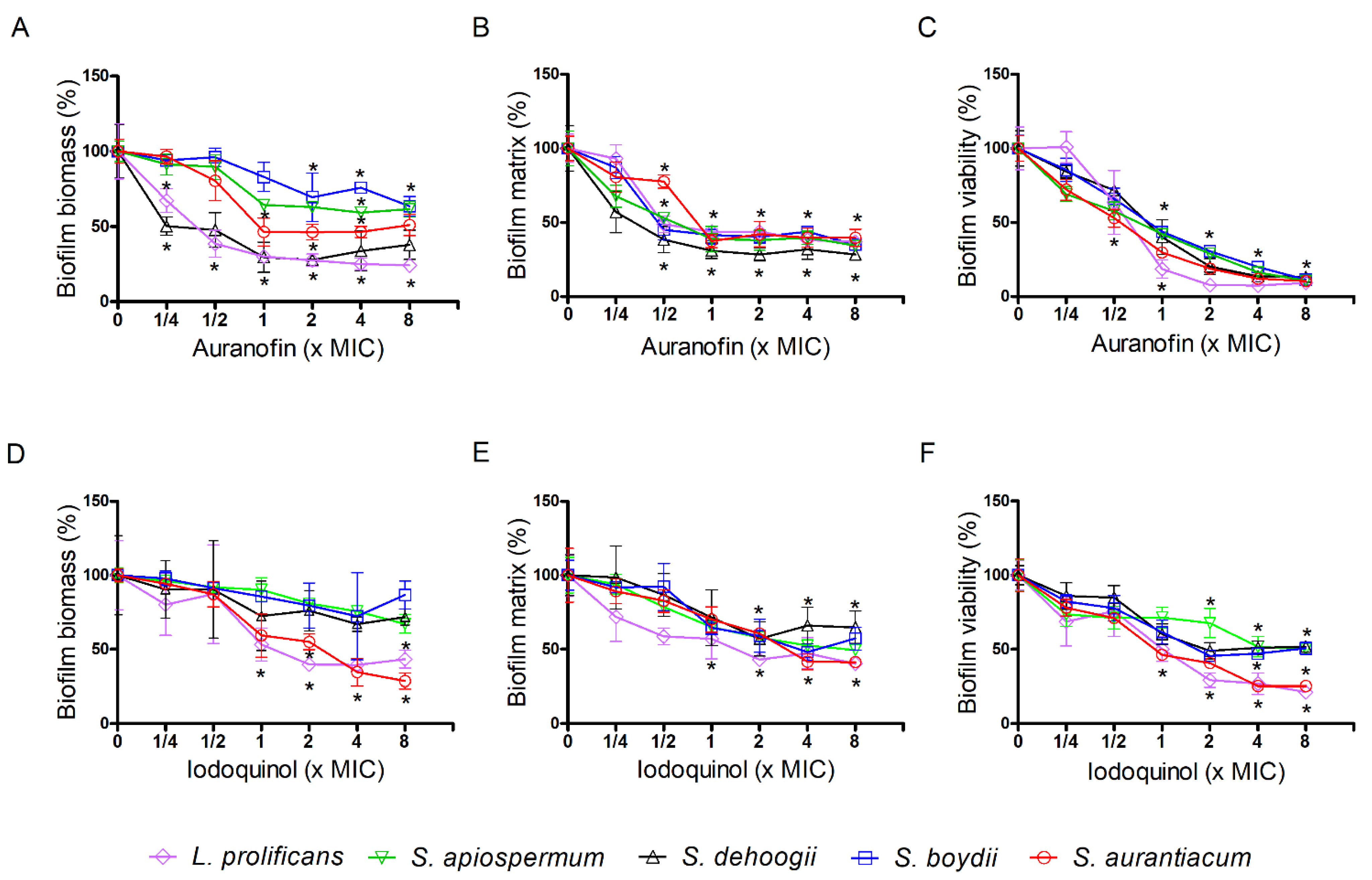
3.3. Effect of Auranofin and Iodoquinol on Fungal Biofilms
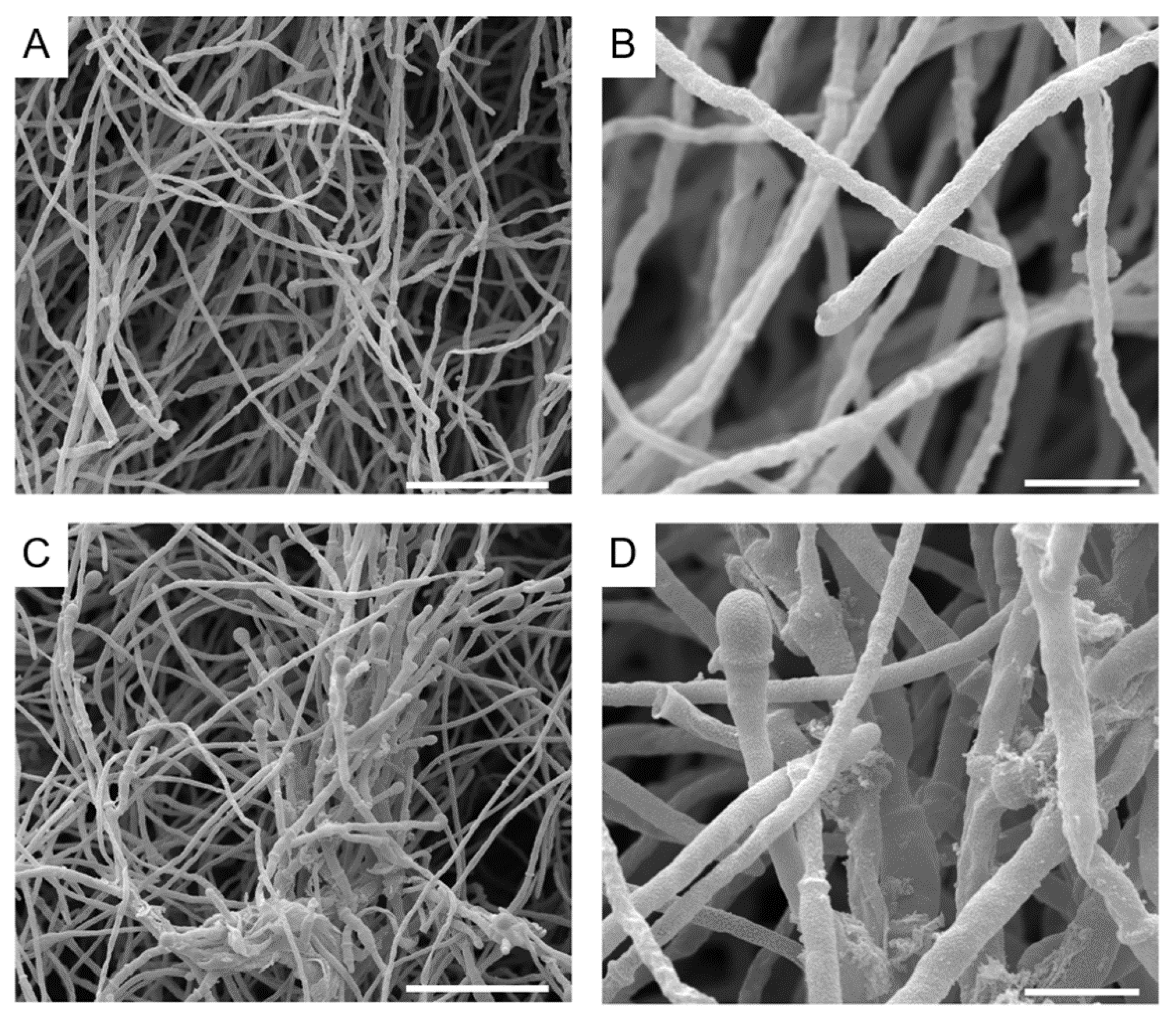
3.4. Alterations Caused by Auranofin and Iodoquinol on S. aurantiacum Morphology
3.5. Drug Interaction among Auranofin and Iodoquinol with Fluconazole, Voriconazole and Caspofungin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Cortez, K.J.; Roilides, E.; Quiroz-Telles, F.; Meletiadis, J.; Antachopoulos, C.; Knudsen, T.; Buchanan, W.; Milanovich, J.; Sutton, D.A.; Fothergill, A.; et al. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 2008, 21, 157–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilgado, F.; Cano, J.; Gene, J.; Serena, C.; Guarro, J. Different virulence of the species of the Pseudallescheria boydii complex. Med. Mycol. 2009, 47, 371–374. [Google Scholar] [CrossRef] [Green Version]
- Luplertlop, N. Pseudallescheria/Scedosporium complex species: From saprobic to pathogenic fungus. J. Mycol. Med. 2018, 28, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.G.P.; Slabbers, L.; de Jong, C.; Melchers, W.J.G.; Hagen, F.; Verweij, P.E.; Merkus, P.; Meis, J.F. Dutch Cystic Fibrosis Fungal Collection, C. Prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients—A Dutch, multicentre study. J. Cyst. Fibros. 2019, 18, 221–226. [Google Scholar] [CrossRef]
- Cowen, L.E. The evolution of fungal drug resistance: Modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 2008, 6, 187–198. [Google Scholar] [CrossRef]
- Rollin-Pinheiro, R.; Singh, A.; Barreto-Bergter, E.; Del Poeta, M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 2016, 8, 1469–1484. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Garcia, A.; Pellon, A.; Rementeria, A.; Buldain, I.; Barreto-Bergter, E.; Rollin-Pinheiro, R.; de Meirelles, J.V.; Xisto, M.; Ranque, S.; Havlicek, V.; et al. Scedosporium and Lomentospora: An updated overview of underrated opportunists. Med. Mycol. 2018, 56, 102–125. [Google Scholar] [CrossRef]
- Lackner, M.; de Hoog, G.S.; Verweij, P.E.; Najafzadeh, M.J.; Curfs-Breuker, I.; Klaassen, C.H.; Meis, J.F. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob. Agents Chemother. 2012, 56, 2635–2642. [Google Scholar] [CrossRef] [Green Version]
- Sedlacek, L.; Graf, B.; Schwarz, C.; Albert, F.; Peter, S.; Wurstl, B.; Wagner, S.; Klotz, M.; Becker, A.; Haase, G.; et al. Prevalence of Scedosporium species and Lomentospora prolificans in patients with cystic fibrosis in a multicenter trial by use of a selective medium. J. Cyst. Fibros. 2015, 14, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Tortorano, A.M.; Richardson, M.; Roilides, E.; van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.-D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. 2014, 20, 27–46. [Google Scholar] [CrossRef] [Green Version]
- Pellon, A.; Ramirez-Garcia, A.; Buldain, I.; Antoran, A.; Martin-Souto, L.; Rementeria, A.; Hernando, F.L. Pathobiology of Lomentospora prolificans: Could this species serve as a model of primary antifungal resistance? Int. J. Antimicrob. Agents 2018, 51, 10–15. [Google Scholar] [CrossRef]
- Hoenigl, M.; Salmanton-García, J.; Walsh, T.J.; Nucci, M.; Neoh, C.F.; Jenks, J.D.; Lackner, M.; Sprute, R.; Al-Hatmi, A.M.S.; Bassetti, M.; et al. Global guideline for the diagnosis and management of rare mould infections: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect. Dis. 2021, 21, e246–e257. [Google Scholar] [CrossRef]
- Seidel, D.; Meißner, A.; Lackner, M.; Piepenbrock, E.; Salmanton-García, J.; Stecher, M.; Mellinghoff, S.; Hamprecht, A.; Durán Graeff, L.; Köhler, P.; et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope®. Crit. Rev. Microbiol. 2019, 45, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Tudela, J.L.; Berenguer, J.; Guarro, J.; Kantarcioglu, A.S.; Horre, R.; de Hoog, G.S.; Cuenca-Estrella, M. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med. Mycol. 2009, 47, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquivel, B.D.; Rybak, J.M.; Barker, K.S.; Fortwendel, J.R.; Rogers, P.D.; White, T.C. Characterization of the Efflux Capability and Substrate Specificity of Aspergillus fumigatus PDR5-like ABC Transporters Expressed in Saccharomyces cerevisiae. mBio 2020, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Sobel, J.D.; White, T.C. A Combination Fluorescence Assay Demonstrates Increased Efflux Pump Activity as a Resistance Mechanism in Azole-Resistant Vaginal Candida albicans Isolates. Antimicrob. Agents Chemother. 2016, 60, 5858–5866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, S.; Holowka, T.; Orner, E.P.; Fries, B.C. Gene Duplication Associated with Increased Fluconazole Tolerance in Candida auris cells of Advanced Generational Age. Sci. Rep. 2019, 9, 5052. [Google Scholar] [CrossRef] [Green Version]
- Niimi, M.; Firth, N.A.; Cannon, R.D. Antifungal drug resistance of oral fungi. Odontology 2010, 98, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hampe, I.A.I.; Friedman, J.; Edgerton, M.; Morschhäuser, J. An acquired mechanism of antifungal drug resistance simultaneously enables Candida albicans to escape from intrinsic host defenses. PLoS Pathog. 2017, 13, e1006655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanden Bossche, H.; Marichal, P.; Gorrens, J.; Bellens, D.; Moereels, H.; Janssen, P.A. Mutation in cytochrome P-450-dependent 14 alpha-demethylase results in decreased affinity for azole antifungals. Biochem. Soc. Trans. 1990, 18, 56–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marichal, P.; Koymans, L.; Willemsens, S.; Bellens, D.; Verhasselt, P.; Luyten, W.; Borgers, M.; Ramaekers, F.C.S.; Odds, F.C.; Vanden Bossche, H. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 1999, 145, 2701–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, D.C.; Corran, A.; Baldwin, B.C.; Kwon-Chung, J.; Kelly, S.L. Resistant P45051A1 activity in azole antifungal tolerant Cryptococcus neoformans from AIDS patients. FEBS Lett. 1995, 368, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Sanglard, D.; Ischer, F.; Koymans, L.; Bille, J. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 1998, 42, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kelly, R.; Kahn, J.N.; Robles, J.; Hsu, M.J.; Register, E.; Li, W.; Vyas, V.; Fan, H.; Abruzzo, G.; et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 2005, 49, 3264–3273. [Google Scholar] [CrossRef] [Green Version]
- Balashov, S.V.; Park, S.; Perlin, D.S. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob. Agents Chemother. 2006, 50, 2058–2063. [Google Scholar] [CrossRef] [Green Version]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Chandra, J. Candida biofilm resistance. Drug Resist. Updates 2004, 7, 301–309. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Falkler, W.A.; Meiller, T.F. Fungal biofilms and drug resistance. Emerg. Infect. Dis. 2004, 10, 14–19. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 2006, 50, 1021–1033. [Google Scholar] [CrossRef] [Green Version]
- Hamal, P.; Ostransky, J.; Dendis, M.; Horváth, R.; Ruzicka, F.; Buchta, V.; Vejsova, M.; Sauer, P.; Hejnar, P.; Raclavsky, V. A case of endocarditis caused by the yeast Pichia fabianii with biofilm production and developed in vitro resistance to azoles in the course of antifungal treatment. Med. Mycol. 2008, 46, 601–605. [Google Scholar] [CrossRef] [Green Version]
- Di Bonaventura, G.; Pompilio, A.; Picciani, C.; Iezzi, M.; D’Antonio, D.; Piccolomini, R. Biofilm formation by the emerging fungal pathogen Trichosporon asahii: Development, architecture, and antifungal resistance. Antimicrob. Agents Chemother. 2006, 50, 3269–3276. [Google Scholar] [CrossRef] [Green Version]
- Seidler, M.J.; Salvenmoser, S.; Muller, F.M. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 2008, 52, 4130–4136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollin-Pinheiro, R.; de Meirelles, J.V.; Vila, T.V.M.; Fonseca, B.B.; Alves, V.; Frases, S.; Rozental, S.; Barreto-Bergter, E. Biofilm Formation by Pseudallescheria/Scedosporium Species: A Comparative Study. Front. Microbiol. 2017, 8, 1568. [Google Scholar] [CrossRef]
- Veale, C.G.L. Unpacking the Pathogen Box-An Open Source Tool for Fighting Neglected Tropical Disease. ChemMedChem 2019, 14, 386–453. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Lopez-Ribot, J.L. Screening the Pathogen Box for Identification of Candida albicans Biofilm Inhibitors. Antimicrob. Agents Chemother. 2017, 61, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, F.L.; Kronstad, J.W. Discovery of a Novel Antifungal Agent in the Pathogen Box. mSphere 2017, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, G.; Herrera, N.; Lopez-Ribot, J.L. Repositionable Compounds with Antifungal Activity against Multidrug Resistant Candida auris Identified in the Medicines for Malaria Venture’s Pathogen Box. J. Fungi 2019, 5, 92. [Google Scholar] [CrossRef] [Green Version]
- Coelho, R.A.; Joffe, L.S.; Alves, G.M.; Figueiredo-Carvalho, M.H.G.; Brito-Santos, F.; Amaral, A.C.F.; Rodrigues, M.L.; Almeida-Paes, R. A screening of the MMV Pathogen Box® reveals new potential antifungal drugs against the etiologic agents of chromoblastomycosis. PLoS ONE 2020, 15, e0229630. [Google Scholar] [CrossRef]
- Borba-Santos, L.P.; Vila, T.; Rozental, S. Identification of two potential inhibitors of Sporothrix brasiliensis and Sporothrix schenckii in the Pathogen Box collection. PLoS ONE 2020, 15, e0240658. [Google Scholar] [CrossRef]
- Taj-Aldeen, S.J.; Salah, H.; Al-Hatmi, A.M.; Hamed, M.; Theelen, B.; van Diepeningen, A.D.; Boekhout, T.; Lass-Florl, C. In vitro resistance of clinical Fusarium species to amphotericin B and voriconazole using the EUCAST antifungal susceptibility method. Diagn. Microbiol. Infect. Dis. 2016, 85, 438–443. [Google Scholar] [CrossRef]
- Rollin-Pinheiro, R.; Rochetti, V.P.; Xisto, M.; Liporagi-Lopes, L.C.; Bastos, B.; Rella, A.; Singh, A.; Rozental, S.; Del Poeta, M.; Barreto-Bergter, E. Sphingolipid biosynthetic pathway is crucial for growth, biofilm formation and membrane integrity of Scedosporium boydii. Future Med. Chem. 2019, 11, 2905–2917. [Google Scholar] [CrossRef]
- Mello, T.P.; Aor, A.C.; Goncalves, D.S.; Seabra, S.H.; Branquinha, M.H.; Santos, A.L. Assessment of biofilm formation by Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans. Biofouling 2016, 32, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.P.; Oliveira, S.S.C.; Frasés, S.; Branquinha, M.H.; Santos, A.L.S. Surface properties, adhesion and biofilm formation on different surfaces by Scedosporium spp. and Lomentospora prolificans. Biofouling 2018, 34, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 2008, 14, 982–984. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In vitro activity of olorofim against clinical isolates of Scedosporium species and Lomentospora prolificans using EUCAST and CLSI methodologies. J. Antimicrob. Chemother. 2020, 75, 3582–3585. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sachsenmeier, K.; Zhang, L.; Sult, E.; Hollingsworth, R.E.; Yang, H. A New Bliss Independence Model to Analyze Drug Combination Data. J. Biomol. Screen. 2014, 19, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Meletiadis, J.; Petraitis, V.; Petraitiene, R.; Lin, P.; Stergiopoulou, T.; Kelaher, A.M.; Sein, T.; Schaufele, R.L.; Bacher, J.; Walsh, T.J. Triazole-polyene antagonism in experimental invasive pulmonary aspergillosis: In vitro and in vivo correlation. J. Infect. Dis. 2006, 194, 1008–1018. [Google Scholar] [CrossRef]
- Harun, A.; Serena, C.; Gilgado, F.; Chen, S.C.; Meyer, W. Scedosporium aurantiacum is as virulent as S. prolificans, and shows strain-specific virulence differences, in a mouse model. Med. Mycol. 2010, 48 (Suppl. 1), S45–S51. [Google Scholar] [CrossRef] [Green Version]
- Panackal, A.A.; Marr, K.A. Scedosporium/Pseudallescheria infections. Semin. Respir. Crit. Care Med. 2004, 25, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Mursch, K.; Trnovec, S.; Ratz, H.; Hammer, D.; Horré, R.; Klinghammer, A.; de Hoog, S.; Behnke-Mursch, J. Successful treatment of multiple Pseudallescheria boydii brain abscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode. Childs Nerv. Syst. 2006, 22, 189–192. [Google Scholar] [CrossRef]
- Tammer, I.; Tintelnot, K.; Braun-Dullaeus, R.C.; Mawrin, C.; Scherlach, C.; Schluter, D.; Konig, W. Infections due to Pseudallescheria/Scedosporium species in patients with advanced HIV disease--a diagnostic and therapeutic challenge. Int. J. Infect. Dis. 2011, 15, e422–e429. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, C.; Brandt, C.; Antweiler, E.; Krannich, A.; Staab, D.; Schmitt-Grohe, S.; Fischer, R.; Hartl, D.; Thronicke, A.; Tintelnot, K. Prospective multicenter German study on pulmonary colonization with Scedosporium /Lomentospora species in cystic fibrosis: Epidemiology and new association factors. PLoS ONE 2017, 12, e0171485. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.H.; Slavin, M.A.; Sorrell, T.C.; Handke, R.; Harun, A.; Phillips, M.; Nguyen, Q.; Delhaes, L.; Ellis, D.; Meyer, W.; et al. Population-based surveillance for scedosporiosis in Australia: Epidemiology, disease manifestations and emergence of Scedosporium aurantiacum infection. Clin. Microbiol. Infect. 2009, 15, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, T.F.; Horta, M.A.C.; Colabardini, A.C.; Fernandes, C.M.; Silva, L.P.; Bastos, R.W.; Fonseca, M.V.L.; Wang, F.; Martins, C.; Rodrigues, M.L.; et al. Screening of Chemical Libraries for New Antifungal Drugs against Aspergillus fumigatus Reveals Sphingolipids Are Involved in the Mechanism of Action of Miltefosine. mBio 2021, 12, e01458-21. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.B.; RajaMuthiah, R.; Souza, A.C.; Eatemadpour, S.; Rossoni, R.D.; Santos, D.A.; Junqueira, J.C.; Rice, L.B.; Mylonakis, E. Inhibition of bacterial and fungal pathogens by the orphaned drug auranofin. Future Med. Chem. 2016, 8, 117–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiederhold, N.P.; Patterson, T.F.; Srinivasan, A.; Chaturvedi, A.K.; Fothergill, A.W.; Wormley, F.L.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. Repurposing auranofin as an antifungal: In vitro activity against a variety of medically important fungi. Virulence 2017, 8, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Yaakoub, H.; Staerck, C.; Mina, S.; Godon, C.; Fleury, M.; Bouchara, J.P.; Calenda, A. Repurposing of auranofin and honokiol as antifungals against Scedosporium species and the related fungus Lomentospora prolificans. Virulence 2021, 12, 1076–1090. [Google Scholar] [CrossRef]
- Siles, S.A.; Srinivasan, A.; Pierce, C.G.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. High-throughput screening of a collection of known pharmacologically active small compounds for identification of Candida albicans biofilm inhibitors. Antimicrob. Agents Chemother. 2013, 57, 3681–3687. [Google Scholar] [CrossRef] [Green Version]
- Thangamani, S.; Maland, M.; Mohammad, H.; Pascuzzi, P.E.; Avramova, L.; Koehler, C.M.; Hazbun, T.R.; Seleem, M.N. Repurposing Approach Identifies Auranofin with Broad Spectrum Antifungal Activity That Targets Mia40-Erv1 Pathway. Front. Cell. Infect. Microbiol. 2017, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- May, H.C.; Yu, J.J.; Guentzel, M.N.; Chambers, J.P.; Cap, A.P.; Arulanandam, B.P. Repurposing Auranofin, Ebselen, and PX-12 as Antimicrobial Agents Targeting the Thioredoxin System. Front. Microbiol. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Abutaleb, N.S.; Seleem, M.N. Repurposing the Antiamoebic Drug Diiodohydroxyquinoline for Treatment of Clostridioides difficile Infections. Antimicrob. Agents Chemother. 2020, 64, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yin, X.; Languino, L.R.; Altieri, D.C. Evaluation of drug combination effect using a Bliss independence dose-response surface model. Stat. Biopharm. Res. 2018, 10, 112–122. [Google Scholar] [CrossRef]
- Demidenko, E.; Miller, T.W. Statistical determination of synergy based on Bliss definition of drugs independence. PLoS ONE 2019, 14, e0224137. [Google Scholar] [CrossRef] [PubMed]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R., Jr.; Schultz, P.G.; et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Owings, J.P.; McNair, N.N.; Mui, Y.F.; Gustafsson, T.N.; Holmgren, A.; Contel, M.; Goldberg, J.B.; Mead, J.R. Auranofin and N-heterocyclic carbene gold-analogs are potent inhibitors of the bacteria Helicobacter pylori. FEMS Microbiol. Lett. 2016, 363, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Gandin, V.; Fernandes, A.P.; Rigobello, M.P.; Dani, B.; Sorrentino, F.; Tisato, F.; Björnstedt, M.; Bindoli, A.; Sturaro, A.; Rella, R.; et al. Cancer cell death induced by phosphine gold (I) compounds targeting thioredoxin reductase. Biochem. Pharmacol. 2010, 79, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Pomel, S.; Latre de Late, P.; Taravaud, A.; Loiseau, P.M.; Maes, L.; Cho-Ngwa, F.; Bulman, C.A.; Fischer, C.; Sakanari, J.A.; et al. Repurposing Auranofin and Evaluation of a New Gold (I) Compound for the Search of Treatment of Human and Cattle Parasitic Diseases: From Protozoa to Helminth Infections. Molecules 2020, 25, 5075. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.; Sobreira, T.J.; Seleem, M.N. Repurposing auranofin for the treatment of cutaneous staphylococcal infections. Int. J. Antimicrob. Agents 2016, 47, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kean, W.F.; Hart, L.; Buchanan, W.W. Auranofin. Br. J. Rheumatol. 1997, 36, 560–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an old drug for a golden new age. Drugs RD 2015, 15, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Lawung, R.; Cherdtrakulkiat, R.; Nabu, S.; Prachayasittikul, S.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. Repositioning of 8-hydroxyquinoline derivatives as a new promising candidate for combating multidrug resistant Neisseria gonorrhoeae. EXCLI J. 2018, 17, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, M.; Pacheco-Yepez, J.; Flores-Huerta, N.; Guzmán-Téllez, P.; Jarillo-Luna, R.A.; Cárdenas-Jaramillo, L.M.; Campos-Rodríguez, R.; Shibayama, M. Flavonoids as a Natural Treatment Against Entamoeba histolytica. Front. Cell. Infect. Microbiol. 2018, 8, 209. [Google Scholar] [CrossRef] [PubMed]





| Compound Code | % of Inhibition | Name | Antimicrobial Activity | Use | Mechanism of Action |
|---|---|---|---|---|---|
| MMV675968 | 80.21779 | 5-Chloro-6-[(2,5-dimethoxyanilino)methyl]quinazoline-2,4-diamine | Sporothrix spp. Candida albicans Cryptosporidium parvum | New compound (not commercially available) | Disruption of folate metabolism |
| MMV688978 | 87.72361 | Auranofin | Candida albicans Cryptococcus neoformans Blastomyces dermatitidis Aspergillus fumigatus Rhizopus oryzae Chromoblastomycosis agents Entamoeba hystolitica Staphylococcus aureus | Rheumatoid arthritis | Inhibition of thioredoxin reductase |
| MMV688943 | 89.10369 | Difenoconazole | Broad-range fungi Trypanosoma cruzi | Antifungal pesticide (agrochemical) | Inhibition of CYP51 (ergosterol synthesis) |
| MMV687807 | 89.10369 | N-[3,4-Bis(trifluoromethyl)phenyl]-5-chloro-2-hydroxybenzamide | Mycobacterium tuberculosis | New compound (not commercially available) | Salicylamide analogue |
| MMV002817 | 88.82634 | Iodoquinol | Sporothrix spp. Chromoblastomycosis agents Candida auris Entamoeba hystolitica | Amoebiasis | Ferrous ions chelate |
| MMV688774 | 87.88809 | Posaconazole | Broad-range fungi | Antifungal agent | Inhibition of CYP51 (ergosterol synthesis) |
| Reference drug | 81.84583 | Voriconazole | Broad-range fungi | Antifungal agent | Inhibition of CYP51 (ergosterol synthesis) |
| Auranofin (µm) | Iodoquinol (µm) | Voriconazole (µm) | ||||
|---|---|---|---|---|---|---|
| Fungal Species | Growth Inhibition | Viability Inhibition | Growth Inhibition | Viability Inhibition | Growth Inhibition | Viability Inhibition |
| S. aurantiacum | 5 | 5 | 5 | 5 | 3.75 | 3.75 |
| S. boydii | 5 | 5 | 0.625 | 0.625 | 0.94 | 1.88 |
| S. apiospermum | 5 | 10 | 1.25 | 5 | 3.75 | 7.5 |
| S. dehoogii | 5 | 5 | 1.25 | 1.25 | 1.88 | 1.88 |
| L. prolificans | 5 | 5 | 0.625 | 0.625 | 60 | 60 |
| MIC80/MEC80 Alone (µm) | MIC80/MEC80 Combined (µm) | FIC Index | |||
|---|---|---|---|---|---|
| Auranofin | 5 | Aur/Flc | 2.5/80 | Aur/Flc | 1.0 (no effect) |
| Iodoquinol | 5 | Aur/Vori | 5/3.75 | Aur/Vori | 2.0 (no effect) |
| Fluconazole | 160 | Aur/Casp | 2.5/5.0 | Aur/Casp | 0.75 (no effect) |
| Voriconazole | 3.75 | Iodo/Flc | 2.5/40 | Iodo/Flc | 0.75 (no effect) |
| Caspofungin | 20 | Iodo/Vori | 2.5/1.87 | Iodo/Vori | 1.0 (no effect) |
| Iodo/Casp | 0.62/1.25 | Iodo/Casp | 0.18 (synergic) | ||
| Efficacy of Combined Drugs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Efficacy of Drugs Alone (% of Inhibition) | Auranofin | Iodoquinol | ||||||
| MIC80 | 0.5 × MIC80 | Eobs | Eexp | ΔE, % (Interaction) | Eobs | Eexp | ΔE, % (Interaction) | |
| Auranofin | 87.72 | 20.28 | NP | NP | NP | NP | NP | NP |
| Iodoquinol | 88.82 | 44.15 | NP | NP | NP | NP | NP | NP |
| Fluconazole | 82.79 | 30.64 | 81.72 | 67.93 | 13.79 (S) | 21.14 | 51.77 | −30.69 (A) |
| Voriconazole | 81.84 | 49.75 | 57 | 55.71 | 1.29 (S) | 86.77 | 89.96 | −3.19 (A) |
| Caspofungin | 89.39 | 16.57 | 86.78 | 49.82 | 36.96 (S) | 87.30 | 73.34 | 13.96 (S) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollin-Pinheiro, R.; Borba-Santos, L.P.; da Silva Xisto, M.I.D.; de Castro-Almeida, Y.; Rochetti, V.P.; Rozental, S.; Barreto-Bergter, E. Identification of Promising Antifungal Drugs against Scedosporium and Lomentospora Species after Screening of Pathogen Box Library. J. Fungi 2021, 7, 803. https://doi.org/10.3390/jof7100803
Rollin-Pinheiro R, Borba-Santos LP, da Silva Xisto MID, de Castro-Almeida Y, Rochetti VP, Rozental S, Barreto-Bergter E. Identification of Promising Antifungal Drugs against Scedosporium and Lomentospora Species after Screening of Pathogen Box Library. Journal of Fungi. 2021; 7(10):803. https://doi.org/10.3390/jof7100803
Chicago/Turabian StyleRollin-Pinheiro, Rodrigo, Luana Pereira Borba-Santos, Mariana Ingrid Dutra da Silva Xisto, Yuri de Castro-Almeida, Victor Pereira Rochetti, Sonia Rozental, and Eliana Barreto-Bergter. 2021. "Identification of Promising Antifungal Drugs against Scedosporium and Lomentospora Species after Screening of Pathogen Box Library" Journal of Fungi 7, no. 10: 803. https://doi.org/10.3390/jof7100803







