Bias of the Immune Response to Pneumocystis murina Does Not Alter the Ability of Neonatal Mice to Clear the Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Generation of LyzM-Cre x Argfl/fl Mice and Genotyping
2.3. P. murina Isolation and Infection
2.4. Isolation of Cells from Alveolar Spaces, Lungs, and Lymph Nodes
2.5. Enumeration of P. murina
2.6. Flow Cytometric Analysis
2.7. Cell Sorting
2.8. Cytokine Analysis
2.9. Analysis of Arginase Production
2.10. Statistical Analysis
3. Results
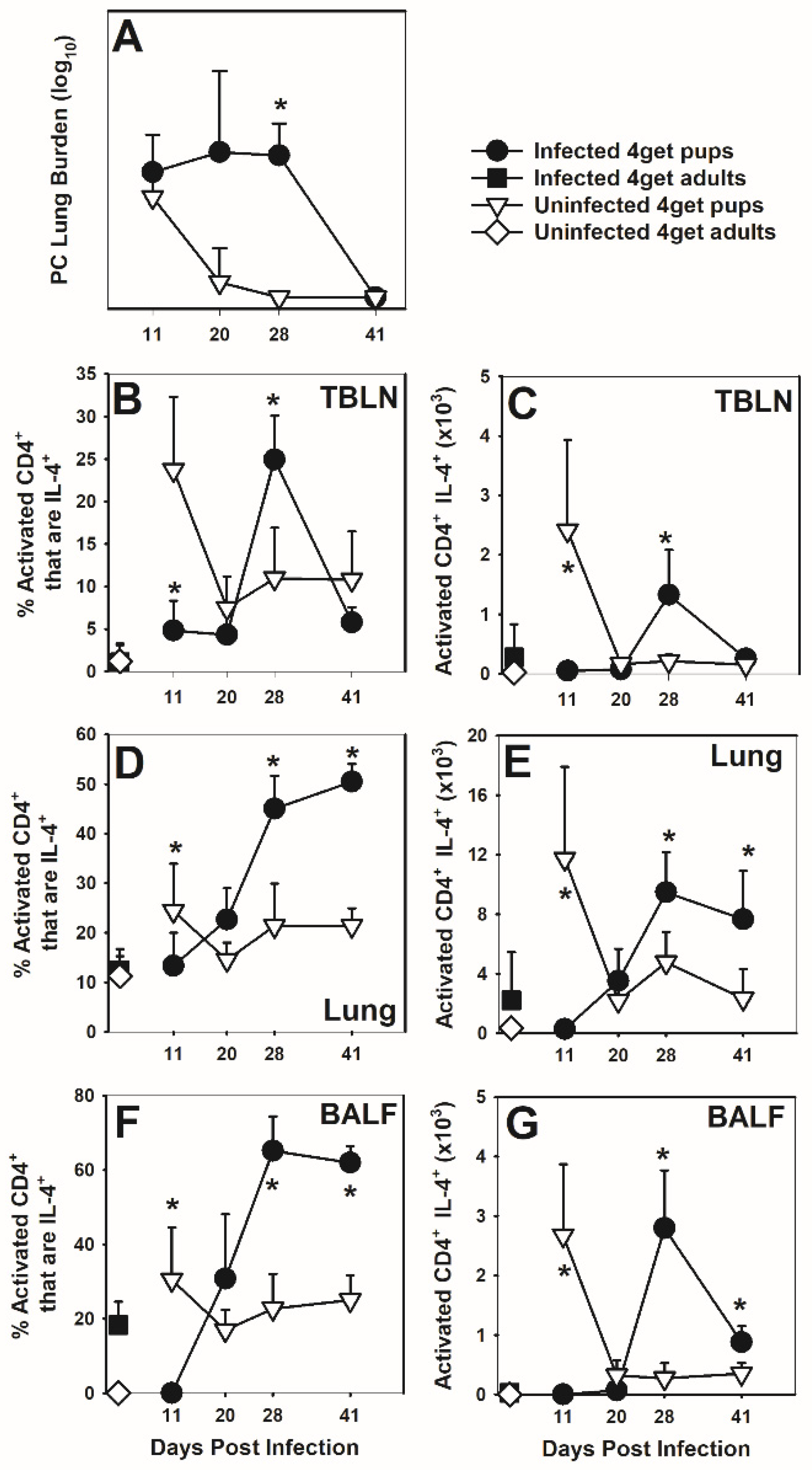
3.1. CD4 Lymphocytes Express IL-4 in Response P. murina
3.2. Cells of the Myeloid Lineage Express IL-4 in Response to P. murina
3.3. Arginase Is Not Necessary in the Neonatal Immune Response to P. murina
3.4. IL-4 Has a Modest Impact on Clearance of P. murina
3.5. IL-4Rα Deficiency Alters T Cell Activation in the Neonatal Immune Response to P. murina
3.6. Deficiency in IL-4Rα Results in a Reduction in Alternatively Activated Macrophages
3.7. IL-4Rα Deficiency Affects the Cytokine Environment and Antibody Response to P. murina
3.8. IFNγ or IL-23 p19 Deficiency Alters the Immune Response but Not Ability to Clear P. murina Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Gajdusek, D.C. Pneumocystis carinii- etiological agent of interstitial plasma cell pneumonia of premature and young infants. Pediatrics 1957, 19, 543–565. [Google Scholar]
- Vanek, J. Atypicka (interstitialni) pneumonie deti, vyvolana Pneumocysti carinii (Atypical intersitial pneumonia of infants produced by Pneumocystis carinii). Casop Lek Cesk 1951, 90, 1121. [Google Scholar]
- Pifer, L.L.; Hughes, W.T.; Stagno, S.; Woods, D. Pneumocystis carinii infection: Evidence for high prevalence in normal and immunosuppressed children. Pediatrics 1978, 61, 35–41. [Google Scholar]
- Meuwissen, J.H.; Tauber, I.; Leeuwenberg, A.D.; Beckers, P.J.; Sieben, M. Parasitologic and serologic observations of infection with Pneumocystis in humans. J. Infect. Dis. 1977, 136, 43–49. [Google Scholar] [CrossRef]
- Morgan, D.J.; Vargas, S.L.; Reyes-Mugica, M.; Walterspiel, J.N.; Carver, W.; Gigliotti, F. Identification of Pneumocystis carinii in the lungs of infants dying of sudden infant death syndrome. Pediatr. Infect. Dis. J. 2001, 20, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.L.; Hughes, W.T.; Santolaya, M.E.; Ulloa, A.V.; Ponce, C.A.; Cabrera, C.E.; Cumsille, F.; Gigliotti, F. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 2001, 32, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.L.; Ponce, C.A.; Hughes, W.T.; Wakefield, A.E.; Weitz, J.C.; Donoso, S.; Ulloa, A.V.; Madrid, P.; Gould, S.; Latorre, J.J.; et al. Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin. Infect. Dis. 1999, 29, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Totet, A.; Pautard, J.C.; Raccurt, C.; Roux, P.; Nevez, G. Genotypes at the internal transcribed spacers of the nuclear rRNA operon of Pneumocystis jiroveci in nonimmunosuppressed infants without severe pneumonia. J. Clin. Microbiol. 2003, 41, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Garvy, B.A.; Harmsen, A.G. Susceptibility to Pneumocystis carinii infection: Host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect. Immun. 1996, 64, 3987–3992. [Google Scholar] [CrossRef] [PubMed]
- Garvy, B.A.; Qureshi, M.H. Delayed inflammatory response to Pneumocystis carinii infection in neonatal mice is due to an inadequate lung environment. J. Immunol. 2000, 165, 6480–6486. [Google Scholar] [CrossRef] [PubMed]
- Adkins, B.; Leclerc, C.; Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004, 4, 553–564. [Google Scholar] [CrossRef]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef]
- Harmsen, A.G.; Stankiewicz, M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J. Exp. Med. 1990, 172, 937–945. [Google Scholar] [CrossRef]
- Limper, A.H.; Hoyte, J.S.; Standing, J.E. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 1997, 99, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E.; Schuer, K.; Hollifield, M.; Randall, T.D.; Garvy, B.A. Clearance of Pneumocystis carinii in Mice Is Dependent on B Cells But Not on P. carinii-Specific Antibody. J. Immunol. 2003, 171, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, H.; Levesque, D.; Delanay, K.; Bourgeault, A.; de la Durantaye, R.; Brochu, S.; Lavoie, M.C. Pneumocystis carinii infection in transgenic B cell-deficient mice. J. Infect. Dis. 1996, 173, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Roths, J.B.; Sidman, C.L. Single and combined humoral and cell-mediated immunotherapy of Pneumocystis carinii pneumonia in immunodeficient scid mice. Infect. Immun. 1993, 61, 1641–1649. [Google Scholar] [CrossRef]
- Qureshi, M.H.; Garvy, B.A. Neonatal T cells in an adult lung environment are competent to resolve Pneumocystis carinii pneumonia. J. Immunol. 2001, 166, 5704–5711. [Google Scholar] [CrossRef]
- Qureshi, M.H.; Cook-Mills, J.; Doherty, D.E.; Garvy, B.A. TNF-{alpha}-Dependent ICAM-1- and VCAM-1-Mediated Inflammatory Responses Are Delayed in Neonatal Mice Infected with Pneumocystis carinii. J. Immunol. 2003, 171, 4700–4707. [Google Scholar] [CrossRef]
- Empey, K.M.; Hollifield, M.; Schuer, K.; Gigliotti, F.; Garvy, B.A. Passive immunization of neonatal mice against Pneumocystis carinii f.sp. muris enhances control of infection without stimulating inflammation. Infect. Immun. 2004, 72, 6211–6220. [Google Scholar] [CrossRef]
- Qureshi, M.; Empey, K.M.; Garvy, B.A. Modulation of proinflammatory responses to Pneumocystis carinii f. sp. muris in neonatal mice by granulocyte-macrophage colony-stimulating factor and IL-4: Role of APCs. J. Immunol. 2005, 174, 441–448. [Google Scholar] [CrossRef]
- Empey, K.M.; Hollifield, M.; Garvy, B.A. Exogenous heat-killed Escherichia coli improves alveolar macrophage activity and reduces Pneumocystis carinii lung burden in infant mice. Infect. Immun. 2007, 75, 3382–3393. [Google Scholar] [CrossRef]
- Kurkjian, C.; Hollifield, M.; Lines, J.L.; Rogosky, A.; Empey, K.M.; Qureshi, M.; Brown, S.A.; Garvy, B.A. Alveolar Macrophages in Neonatal Mice Are Inherently Unresponsive to Pneumocystis murina Infection. Infect. Immun. 2012, 80, 2835–2846. [Google Scholar] [CrossRef]
- Adkins, B. T-cell function in newborn mice and humans. Immunol. Today 1999, 20, 330–335. [Google Scholar] [CrossRef]
- Adkins, B.; Ghanei, A.; Hamilton, K. Up-regulation of murine neonatal T helper cell responses by accessory cell factors. J. Immunol. 1994, 153, 3378–3385. [Google Scholar]
- Adkins, B.; Hamilton, K. Freshly isolated, murine neonatal T cells produce IL-4 in response to anti-CD3 stimulation. J. Immunol. 1992, 149, 3448–3455. [Google Scholar]
- Rose, S.; Lichtenheld, M.; Foote, M.R.; Adkins, B. Murine Neonatal CD4+ Cells Are Poised for Rapid Th2 Effector-Like Function. J. Immunol. 2007, 178, 2667–2678. [Google Scholar] [CrossRef]
- Nelson, M.P.; Christmann, B.S.; Werner, J.L.; Metz, A.E.; Trevor, J.L.; Lowell, C.A.; Steele, C. IL-33 and M2a Alveolar Macrophages Promote Lung Defense against the Atypical Fungal Pathogen Pneumocystis murina. J. Immunol. 2011, 186, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.M.; Kottom, T.J.; Hebrink, D.M.; Moua, T.; Singh, R.D.; Pagano, R.E.; Limper, A.H. Glycosphingolipids mediate pneumocystis cell wall beta-glucan activation of the IL-23/IL-17 axis in human dendritic cells. Am. J. Respir. Cell Mol. Biol. 2012, 47, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Shellito, J.; Tate, C.; Ruan, S.; Kolls, J. Murine CD4+ T lymphocyte subsets and host defense against Pneumocystis carinii. J. Infect. Dis. 2000, 181, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- McKinley, L.; Logar, A.J.; McAllister, F.; Zheng, M.; Steele, C.; Kolls, J.K. Regulatory T Cells Dampen Pulmonary Inflammation and Lung Injury in an Animal Model of Pneumocystis Pneumonia. J. Immunol. 2006, 177, 6215–6226. [Google Scholar] [CrossRef] [PubMed]
- Rudner, X.L.; Happel, K.I.; Young, E.A.; Shellito, J.E. Interleukin-23 (IL-23)-IL-17 Cytokine Axis in Murine Pneumocystis carinii Infection. Infect. Immun. 2007, 75, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.C.; Dunaway, C.W.; Nelson, M.P.; Trevor, J.L.; Morris, A.; Steele, C. STAT4-Dependent and -Independent Th2 Responses Correlate with Protective Immunity against Lung Infection with Pneumocystis murina. J. Immunol. 2013, 190, 6287–6294. [Google Scholar] [CrossRef] [PubMed]
- Linke, M.J.; Ashbaugh, A.; Collins, M.S.; Lynch, K.; Cushion, M.T. Characterization of a Distinct Host Response Profile to Pneumocystis murina Asci during Clearance of Pneumocystis Pneumonia. Infect. Immun. 2013, 81, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Garvy, B.A.; Ezekowitz, R.A.B.; Harmsen, A.G. Role of interferon-g in the host immune and inflammatory response to P. carinii infection. Infect. Immun 1997, 65, 373. [Google Scholar] [CrossRef] [PubMed]
- Garvy, B.A.; Wiley, J.A.; Gigliotti, F.; Harmsen, A.H. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect. Immun. 1997, 65, 5052–5056. [Google Scholar] [CrossRef] [PubMed]
- Perez-Nazario, N.; Rangel-Moreno, J.; O’Reilly, M.A.; Pasparakis, M.; Gigliotti, F.; Wright, T.W. Selective Ablation of Lung Epithelial IKK2 Impairs Pulmonary Th17 Responses and Delays the Clearance of Pneumocystis. J. Immunol. 2013, 191, 4720–4730. [Google Scholar] [CrossRef]
- Raes, G.; Brys, L.; Dahal, B.K.; Brandt, J.; Grooten, J.; Brombacher, F.; Vanham, G.; Noel, W.; Bogaert, P.; Boonefaes, T.; et al. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J. Leukoc. Biol. 2005, 77, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.P.; Metz, A.E.; Li, S.; Lowell, C.A.; Steele, C. The Absence of Hck, Fgr, and Lyn Tyrosine Kinases Augments Lung Innate Immune Responses to Pneumocystis murina. Infect. Immun. 2009, 77, 1790–1797. [Google Scholar] [CrossRef]
- Pitts, M.G.; Myers-Morales, T.; D’Orazio, S.E.F. Type I IFN Does Not Promote Susceptibility to Foodborne Listeria monocytogenes. J. Immunol. 2016, 196, 3109–3116. [Google Scholar] [CrossRef]
- Thompson, J.S.; Chu, Y.; Glass, J.F.; Brown, S.A. Absence of IL-23p19 in donor allogeneic cells reduces mortality from acute GVHD. Bone Marrow Transplant. 2010, 45, 712–722. [Google Scholar] [CrossRef]
- Corraliza, I.M.; Campo, M.L.; Soler, G.; Modolell, M. Determination of arginase activity in macrophages: A micromethod. J. Immunol. Methods 1994, 174, 231–235. [Google Scholar] [CrossRef]
- Shirey, K.A.; Pletneva, L.M.; Puche, A.C.; Keegan, A.D.; Prince, G.A.; Blanco, J.C.; Vogel, S.N. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 2010, 3, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lei, G.S.; Shao, S.; Jung, H.W.; Durant, P.J.; Lee, C.H. Accumulation of myeloid-derived suppressor cells in the lungs during Pneumocystis pneumonia. Infect. Immun. 2012, 80, 3634–3641. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of T Cell Receptor CD3ζ Chain Expression by l-Arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- McKenzie, G.J.; Fallon, P.G.; Emson, C.L.; Grencis, R.K.; McKenzie, A.N.J. Simultaneous Disruption of Interleukin (IL)-4 and IL-13 Defines Individual Roles in T Helper Cell Type 2–mediated Responses. J. Exp. Med. 1999, 189, 1565–1572. [Google Scholar] [CrossRef]
- Rudmann, D.G.; Preston, A.M.; Moore, M.W.; Beck, J.M. Susceptibility to Pneumocystis carinii in mice is dependent on simultaneous deletion of IFN-gamma and type 1 and 2 TNF receptor genes. J. Immunol. 1998, 161, 360–366. [Google Scholar] [PubMed]
- Adkins, B.; Bu, Y.; Cepero, E.; Perez, R. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J. Immunol. 2000, 164, 2347–2353. [Google Scholar] [CrossRef]
- Adkins, B. Development of neonatal Th1/Th2 function. Int. Rev. Immunol. 2000, 19, 157–171. [Google Scholar] [CrossRef]
- Zaghouani, H.; Hoeman, C.M.; Adkins, B. Neonatal immunity: Faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009, 30, 585–591. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Ezekowitz, R.A.; Williams, D.J.; Koziel, H.; Armstrong, M.Y.; Warner, A.; Richards, F.F.; Rose, R.M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature 1991, 351, 155–158. [Google Scholar] [CrossRef]
- Steele, C.; Marrero, L.; Swain, S.; Harmsen, A.G.; Zheng, M.; Brown, G.D.; Gordon, S.; Shellito, J.E.; Kolls, J.K. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor. J. Exp. Med. 2003, 198, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Milde, R.; Varin, A.; Melgert, B.N.; Draijer, C.; Thomas, B.; Fabbri, M.; Crawshaw, A.; Ho, L.P.; et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: Similarities and differences. Blood 2013, 121, e57–e69. [Google Scholar] [CrossRef] [PubMed]
- Willment, J.A.; Lin, H.H.; Reid, D.M.; Taylor, P.R.; Williams, D.L.; Wong, S.Y.; Gordon, S.; Brown, G.D. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J. Immunol. 2003, 171, 4569–4573. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Havell, E.A.; Harmsen, A.G. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect. Immun. 1992, 60, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Kolls, J.K.; Beck, J.M.; Nelson, S.; Summer, W.R.; Shellito, J. Alveolar macrophage release of tumor necrosis factor during murine Pneumocystis carinii pneumonia. Am. J. Respir. Cell Mol. Biol. 1993, 8, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Kolls, J.K.; Lei, K.; Vazquez, C.; Odom, G.; Summer, W.R.; Nelson, S.; Shellito, J. Exacerbation of murine Pneumocystis carinii infection by adenoviral-mediated gene transfer of TNF inhibitor. Am. J. Respir. Cell Mol. Biol. 1997, 16, 112–118. [Google Scholar] [CrossRef]
- Deckman, J.M.; Kurkjian, C.J.; McGillis, J.P.; Cory, T.J.; Birket, S.E.; Schutzman, L.M.; Murphy, B.S.; Garvy, B.A.; Feola, D.J. Pneumoycstis infection alters the activation state of pulmonary macrophages. Immunobiology 2017, 222, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Wang, J.; Hoy, Z.; Keegan, A.; Bhagwat, S.; Gigliotti, F.; Wright, T.W.; Deepe, G.S. Neither Classical nor Alternative Macrophage Activation Is Required for Pneumocystis Clearance during Immune Reconstitution Inflammatory Syndrome. Infect. Immun. 2015, 83, 4594–4603. [Google Scholar] [CrossRef] [PubMed][Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurkjian, C.; Hollifield, M.; Feola, D.J.; Garvy, B.A. Bias of the Immune Response to Pneumocystis murina Does Not Alter the Ability of Neonatal Mice to Clear the Infection. J. Fungi 2021, 7, 827. https://doi.org/10.3390/jof7100827
Kurkjian C, Hollifield M, Feola DJ, Garvy BA. Bias of the Immune Response to Pneumocystis murina Does Not Alter the Ability of Neonatal Mice to Clear the Infection. Journal of Fungi. 2021; 7(10):827. https://doi.org/10.3390/jof7100827
Chicago/Turabian StyleKurkjian, Cathryn, Melissa Hollifield, David J. Feola, and Beth A. Garvy. 2021. "Bias of the Immune Response to Pneumocystis murina Does Not Alter the Ability of Neonatal Mice to Clear the Infection" Journal of Fungi 7, no. 10: 827. https://doi.org/10.3390/jof7100827
APA StyleKurkjian, C., Hollifield, M., Feola, D. J., & Garvy, B. A. (2021). Bias of the Immune Response to Pneumocystis murina Does Not Alter the Ability of Neonatal Mice to Clear the Infection. Journal of Fungi, 7(10), 827. https://doi.org/10.3390/jof7100827





