Volatile Organic Compounds in the Azteca/Cecropia Ant-Plant Symbiosis and the Role of Black Fungi
Abstract
1. Introduction
2. Materials and Methods
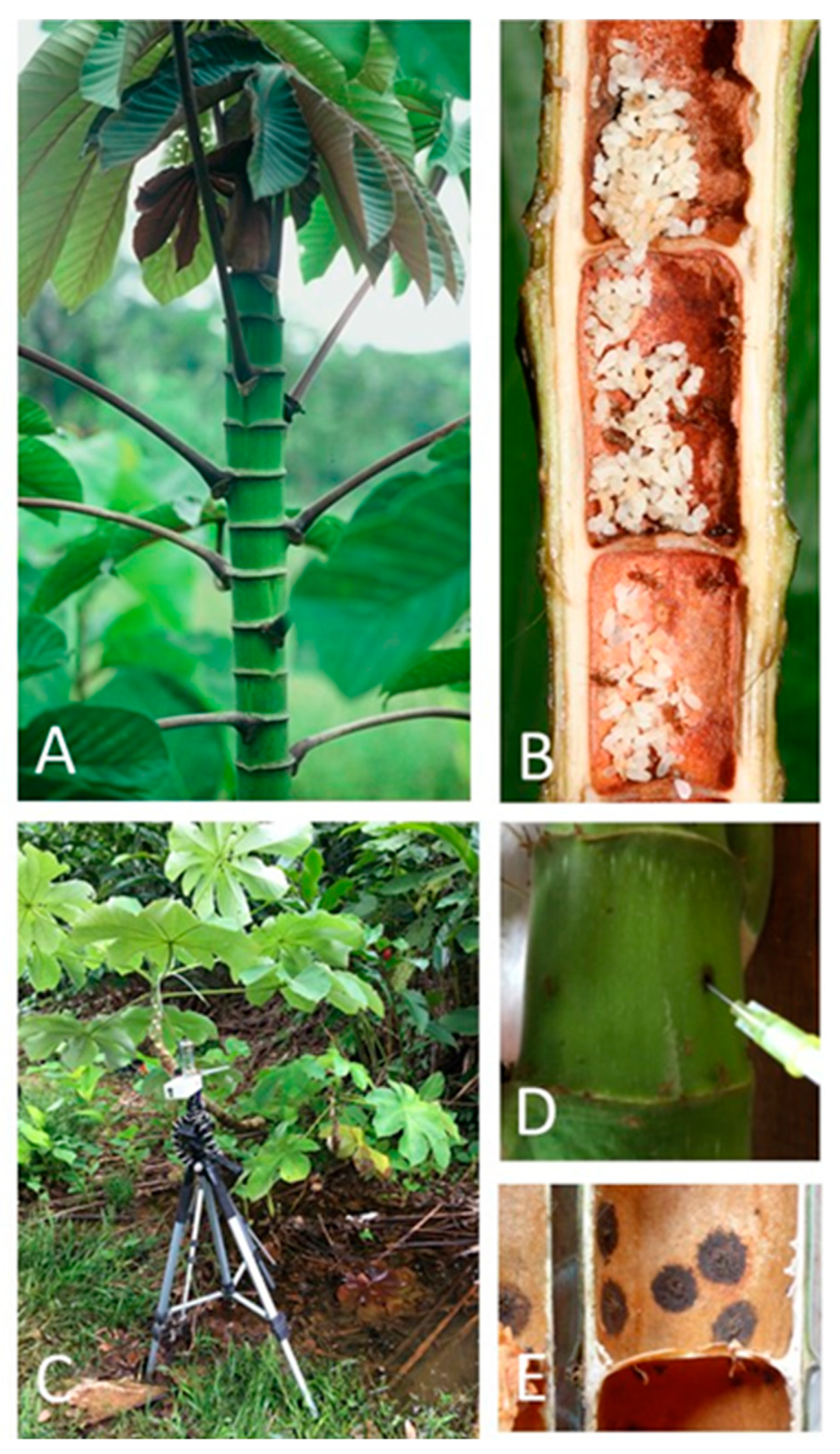
2.1. Sampling Campaign
2.2. Analytical Methods
2.3. Statistical Data Analysis
3. Results and Discussion
3.1. Predominant Volatile Compounds in the Cecropia/Azteca Symbiosis
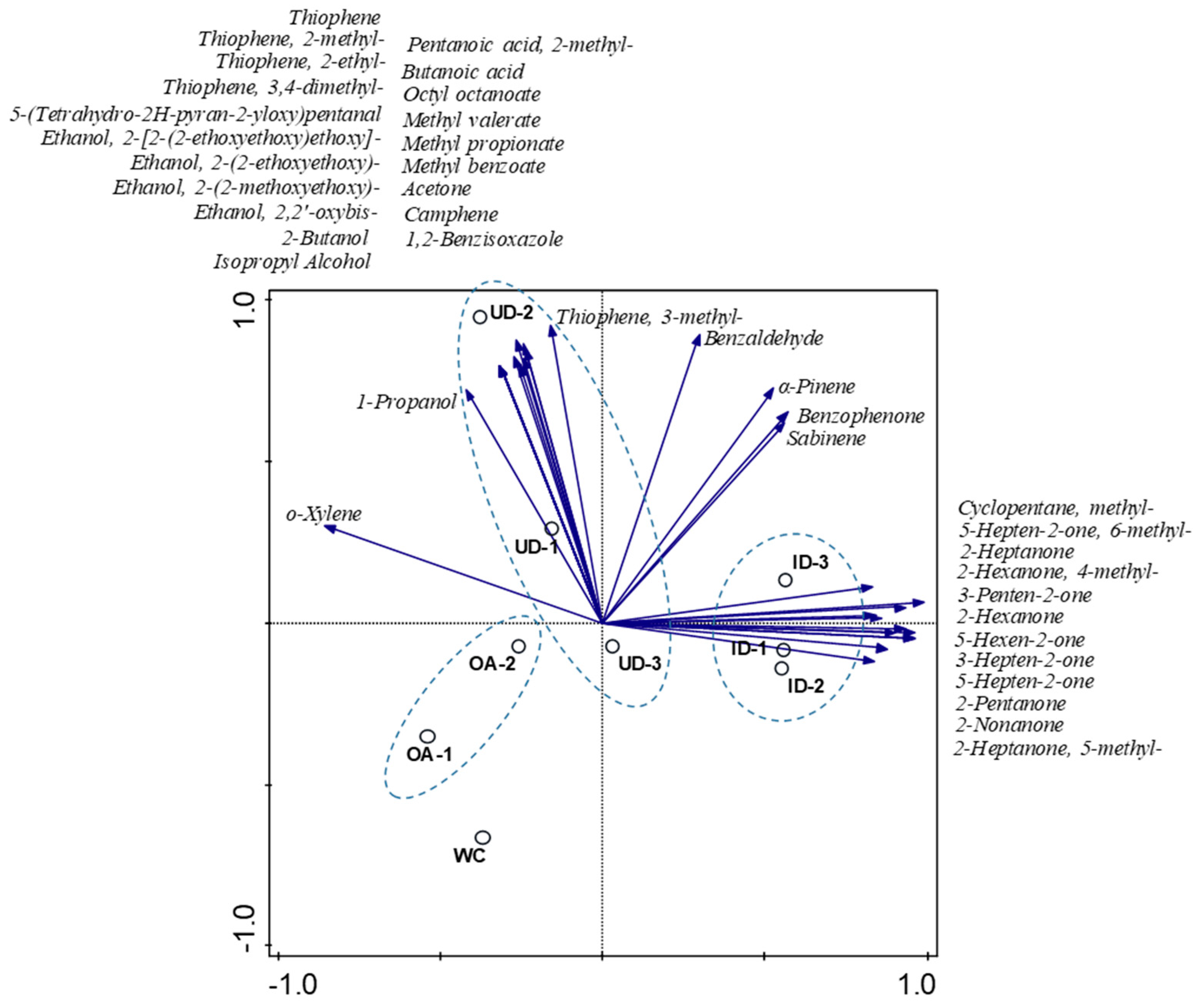
3.2. Multivariate Analysis of Chemical Profiles
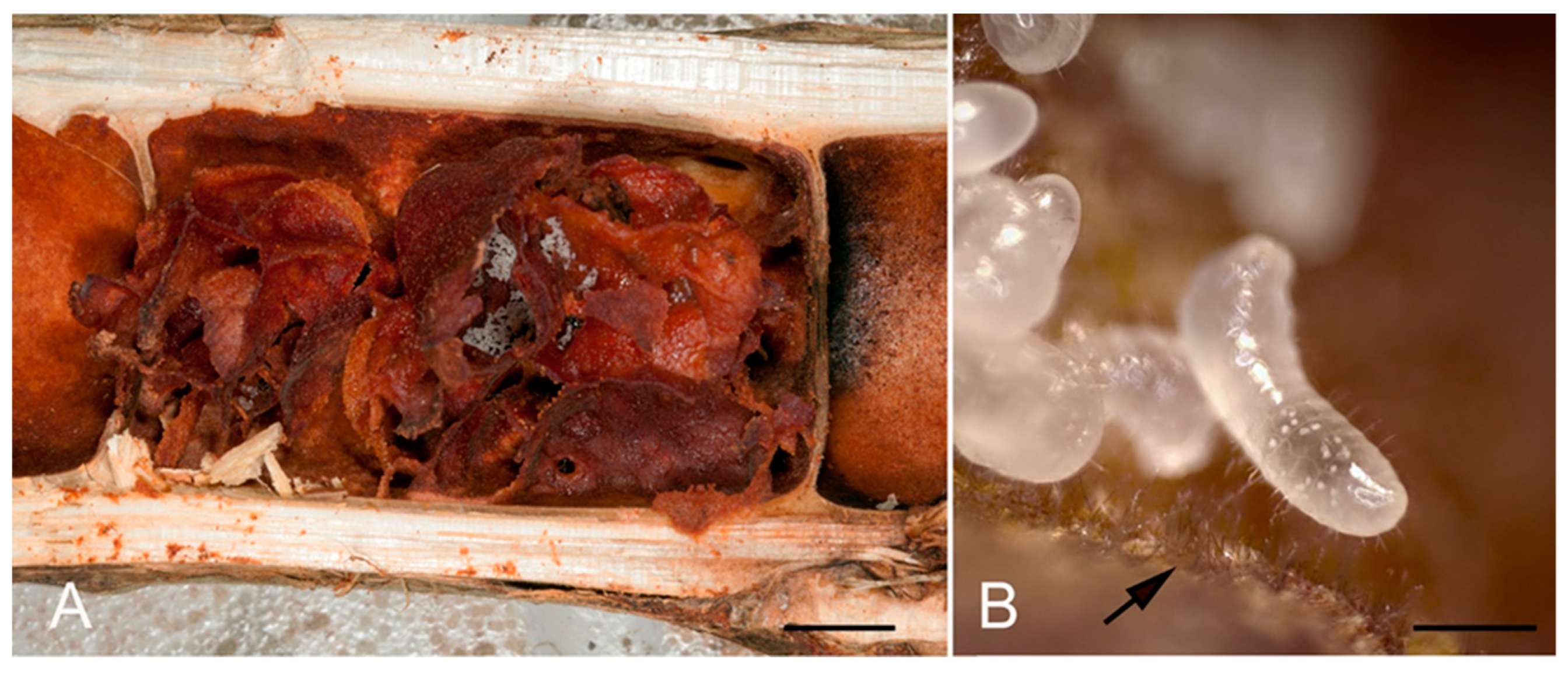
3.3. Role of Black Fungi in the Azteca/Cecropia Ant-Plant Symbiosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voglmayr, H.; Mayer, V.; Maschwitz, U.; Moog, J.; Djieto-Lordon, C.; Blatrix, R. The diversity of ant-associated black yeasts: Insights into a newly discovered world of symbiotic interactions. Fungal Biol. 2011, 115, 1077–1091. [Google Scholar] [CrossRef]
- Vasse, M.; Voglmayr, H.; Mayer, V.E.; Gueidan, C.; Nepel, M.; Moreno, L.; de Hoog, S.; Selosse, M.-A.; McKey, D.; Blatrix, R. A phylogenetic perspective on the association between ants (Hymenoptera: Formicidae) and black yeasts (Ascomycota: Chaetothyriales). Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- Nepel, M.; Voglmayr, H.; Blatrix, R.; Longino, J.T.; Fiedler, K.; Schönenberger, J.; Mayer, V.E. Ant-cultivated Chaetothyriales in hollow stems of myrmecophytic Cecropia sp. trees—diversity and patterns. Fungal Ecol. 2016, 23, 131–140. [Google Scholar] [CrossRef]
- Nepel, M.; Voglmayr, H.; Schönenberger, J.; Mayer, V.E. High diversity and low specificity of Chaetothyrialean fungi in carton galleries in a Neotropical ant-plant association. PLoS ONE 2014, 9, e112756. [Google Scholar] [CrossRef]
- Greenfield, M.J.; Lach, L.; Congdon, B.C.; Anslan, S.; Tedersoo, L.; Field, M.; Abell, S.E. Consistent patterns of fungal communities within ant-plants across a large geographic range strongly suggest a multipartite mutualism. Mycol. Prog. 2021, 20, 681–699. [Google Scholar] [CrossRef]
- Mayer, V.E.; Nepel, M.; Blatrix, R.; Oberhauser, F.B.; Fiedler, K.; Schönenberger, J.; Voglmayr, H. Transmission of fungal partners to incipient Cecropia-tree ant colonies. PLoS ONE 2018, 13, e0192207. [Google Scholar] [CrossRef]
- Defossez, E.; Selosse, M.A.; Dubois, M.P.; Mondolot, L.; Faccio, A.; Djieto-Lordon, C.; McKey, D.; Blatrix, R. Ant-plants and fungi: A new threeway symbiosis. New Phytol. 2009, 182, 942–949. [Google Scholar] [CrossRef]
- Defossez, E.; Djieto-Lordon, C.; McKey, D.; Selosse, M.A.; Blatrix, R. Plant-ants feed their host plant, but above all a fungal symbiont to recycle nitrogen. Proc. R. Soc. B-Biol. Sci. 2011, 278, 1419–1426. [Google Scholar] [CrossRef]
- Moreno, L.F.; Mayer, V.E.; Voglmayr, H.; Blatrix, R.; Stielow, B.; Teixeira, M.M.; Vicente, V.A.; de Hoog, S. Genomic analysis of ant domatia-associated melanized fungi (Chaetothyriales, Ascomycota). Mycol. Prog. 2019, 18, 541–552. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kiss, E.; Kuo, A.; Drula, E.; Kohler, A.; Sánchez-García, M.; Morin, E.; Andreopoulos, B.; Barry, K.W.; Bonito, G.; et al. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 2020, 11, 5125. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Kuo, A.; Nagy, L.G.; Morin, E.; Barry, K.W.; Buscot, F.; Canbäck, B.; Choi, C.; Cichocki, N.; Clum, A.; et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat. Genet. 2015, 47, 410–415. [Google Scholar] [CrossRef]
- Peter, M.; Kohler, A.; Ohm, R.A.; Kuo, A.; Krützmann, J.; Morin, E.; Arend, M.; Barry, K.W.; Binder, M.; Choi, C.; et al. Ectomycorrhizal ecology is imprinted in the genome of the dominant symbiotic fungus Cenococcum geophilum. Nat. Commun. 2016, 7, 12662. [Google Scholar] [CrossRef]
- Quan, Y.; Muggia, L.; Moreno, L.F.; Wang, M.; Al-Hatmi, A.M.S.; da Silva Menezes, N.; Shi, D.; Deng, S.; Ahmed, S.; Hyde, K.D.; et al. A re-evaluation of the Chaetothyriales using criteria of comparative biology. Fungal Divers. 2020, 103, 47–85. [Google Scholar] [CrossRef]
- Quan, Y.; van den Ende, B.G.; Shi, D.; Prenafeta-Boldú, F.X.; Liu, Z.; Al-Hatmi, A.M.S.; Ahmed, S.A.; Verweij, P.E.; Kang, Y.; de Hoog, S. A comparison of isolation methods for black fungi degrading aromatic toxins. Mycopathologia 2019, 184, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Blatrix, R.; Djiéto-Lordon, C.; Mondolot, L.; La Fisca, P.; Voglmayr, H.; McKey, D. Plant-ants use symbiotic fungi as a food source: New insight into the nutritional ecology of ant–plant interactions. Proc. R. Soc. London. Ser. B Biol. Sci. 2012, 279, 3940–3947. [Google Scholar] [CrossRef]
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 2019, 19, 58. [Google Scholar] [CrossRef]
- Wilson, E.O.; Regnier, F.E.J. The evolution of the alarm-defense system in the Formicine ants. Am. Nat. 1971, 105, 279–289. [Google Scholar] [CrossRef]
- Guerrieri, F.J.; Nehring, V.; Jørgensen, C.G.; Nielsen, J.; Galizia, C.G.; d’Ettorre, P. Ants recognize foes and not friends. Proc. R. Soc. London. Ser. B Biol. Sci. 2009, 276, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Dobler, S.; Petschenka, G.; Pankoke, H. Coping with toxic plant compounds—The insect’s perspective on iridoid glycosides and cardenolides. Phytochemistry 2011, 72, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A.; Petschenka, G.; Bingham, R.A.; Weber, M.G.; Rasmann, S. Toxic cardenolides: Chemical ecology and coevolution of specialized plant–herbivore interactions. New Phytol. 2012, 194, 28–45. [Google Scholar] [CrossRef]
- Prenafeta-Boldú, F.X.; de Hoog, G.S.; Summerbell, R.C. Fungal communities in hydrocarbon degradation. In Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology; McGenity, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–36. [Google Scholar]
- Prenafeta-Boldú, F.X.; Illa, J.; van Groenestijn, J.; Flotats, X. Influence of synthetic packing materials on the gas dispersion and biodegradation kinetics in fungal air biofilters. Appl. Microbiol. Biotechnol. 2008, 79, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.C.; Franco-Rosselli, P. Cecropia. In Flora Neotropica Monograph; New York Botanical Garden: New York, NY, USA, 2005; Volume 94. [Google Scholar]
- Longino, J.T. A taxonomic review of the genus Azteca (Hymenoptera: Formicidae) in Costa Rica and a global revision of the aurita group. Zootaxa 2007, 1491, 1–63. [Google Scholar] [CrossRef]
- Key to Costa Rica Azteca Queens. Available online: https://antwiki.org/wiki/Key_to_Costa_Rica_Azteca_queens (accessed on 14 August 2020).
- Prenafeta-Boldú, F.X.; Roca, N.; Villatoro, C.; Vera, L.; de Hoog, G.S. Prospective application of melanized fungi for the biofiltration of indoor air in closed bioregenerative systems. J. Hazard. Mater. 2019, 361, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Linstrom, P. NIST Standard Reference Database Number 69; National Institute of Standards and Technology Chemistry: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Haines, B.L. Identification and quantification of sulfur gases emitted from soils, leaf litter and live plant parts. Agric. Ecosyst. Environ. 1991, 34, 473–477. [Google Scholar] [CrossRef]
- Campion, R.; Martinez-Cruz, M.; Lecocq, T.; Caudron, C.; Pacheco, J.; Pinardi, G.; Hermans, C.; Carn, S.; Bernard, A. Space-and ground-based measurements of sulphur dioxide emissions from Turrialba Volcano (Costa Rica). Bull. Volcanol. 2012, 74, 1757–1770. [Google Scholar] [CrossRef]
- Andreae, M.O.; Talbot, R.W.; Andreae, T.W.; Harriss, R.C. Formic and acetic acid over the central Amazon region, Brazil: 1. Dry season. J. Geophys. Res. Atmos. 1988, 93, 1616–1624. [Google Scholar] [CrossRef]
- Carlton, A.G.; Barsanti, K.C.; Wiedinmyer, C.; Afreh, I. Detailed characterization of organic carbon from fire: Capitalizing on analytical advances to improve atmospheric models. In Multiphase Environmental Chemistry in the Atmosphere; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; Volume 1299, pp. 349–361. [Google Scholar]
- Kesselmeier, J.; Guenther, A.; Hoffmann, T.; Piedade, M.; Warnk, E.J. Natural volatile Organic compound emissions from plants and their roles in oxidant balance and particle formation. Amazon. Glob. Chang. 2009, 186, 183–206. [Google Scholar]
- Du, X.; Witzgall, P.; Wu, K.; Yan, F.; Ma, C.; Zheng, H.; Xu, F.; Ji, G.; Wu, X. Volatiles from Prunus persica flowers and their correlation with flower-visiting insect community in Wanbailin Ecological Garden, China. Adv. Entomol. 2018, 6, 116–133. [Google Scholar] [CrossRef][Green Version]
- Lenoir, A.; Boulay, R.; Dejean, A.; Touchard, A.; Cuvillier-Hot, V. Phthalate pollution in an Amazonian rainforest. Environ. Sci. Pollut. Res. Int. 2016, 23, 16865–16872. [Google Scholar] [CrossRef]
- Paralovo, S.L.; Borillo, G.C.; Barbosa, C.G.G.; Godoi, A.F.L.; Yamamoto, C.I.; de Souza, R.A.F.; Andreoli, R.V.; Costa, P.S.; Almeida, G.P.; Manzi, A.O.; et al. Observations of atmospheric monoaromatic hydrocarbons at urban, semi-urban and forest environments in the Amazon region. Atmos. Environ. 2016, 128, 175–184. [Google Scholar] [CrossRef]
- Adams, R.M.M.; Wells, R.L.; Yanoviak, S.P.; Frost, C.J.; Fox, E.G.P. Interspecific eavesdropping on ant chemical communication. Front. Ecol. Evol. 2020, 8, 24. [Google Scholar] [CrossRef]
- Papachristoforou, A.; Kagiava, A.; Papaefthimiou, C.; Termentzi, A.; Fokialakis, N.; Skaltsounis, A.-L.; Watkins, M.; Arnold, G.; Theophilidis, G. The bite of the honeybee: 2-heptanone secreted from honeybee mandibles during a bite acts as a local anaesthetic in insects and mammals. PLoS ONE 2012, 7, e47432. [Google Scholar] [CrossRef]
- Jardine, K.J.; Luani, R.D.; Rodrigues, T.B.; Spanner, G.C.; Rodrigues, J.R.; Menezes, V.S.; Sampaio, I.; Oliveira, D.C.; Gimenez, B.O.; Higuchi, N.; et al. Volatiles defenses of Amazon Azteca ants (repellent ants). bioRxiv 2020. [Google Scholar] [CrossRef]
- Francelino, M.R.; Mendonca, A.L.; Do Nascimento, R.R.; Sant’ana, A.E.G. The mandibular gland secretions of the leaf-cutting ants Atta sexdens sexdens and Atta opaciceps exhibit intercaste and intercolony variations. J. Chem. Ecol. 2006, 32, 643–656. [Google Scholar] [CrossRef]
- López-Riquelme, G.O.; Manolo, E.A.; Cruz-López, L.; Fanjul-Moles, M.L. Antennal olfactory sensitivity in response to task-related odours of three castes of the ant Atta mexicana (hymenoptera: Formicidae). Physiol. Entomol. 2006, 31, 353–360. [Google Scholar] [CrossRef]
- Hoenigsberger, M.; Kopchinskiy, A.G.; Bueschl, C.; Parich, A.; Laciny, A.; Zettel, H.; Salim, K.A.; Lim, L.B.; Druzhinina, I.S.; Schuhmacher, R. Volatiles from the mandibular gland reservoir content of Colobopsis explodens Laciny and Zettel, 2018, worker ants (Hymenoptera: Formicidae). Molecules 2019, 24, 3468. [Google Scholar] [CrossRef]
- Metcalf, R.A.; Metcalf, R.L. Effects of isosteres of 2-Heptanone on the alarm behavior of the ant Conomyrma pyramica. Ann. Entomol. Soc. Am. 1970, 63, 34–35. [Google Scholar] [CrossRef]
- Cometto-Muñiz, J.E.; Abraham, M.H. Olfactory psychometric functions for homologous 2-ketones. Behav. Brain Res. 2009, 201, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, A.; Feldhaar, H.; Feldmeyer, B.; Fiala, B. Chemical composition of leaf volatiles in Macaranga species (Euphorbiaceae) and their potential role as olfactory cues in host-localization of foundress queens of specific ant partners. Biochem. Syst. Ecol. 2006, 34, 97–113. [Google Scholar] [CrossRef]
- Mayer, V.; Schaber, D.; Hadacek, F. Volatiles of myrmecophytic Piper plants signal stem tissue damage to inhabiting Pheidole ant-partners. J. Ecol. 2008, 96, 962–970. [Google Scholar] [CrossRef]
- Karr, L.L.; Coats, J.R. Insecticidal Properties of d-Limonene. J. Pestic. Sci. 1988, 13, 287–290. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene—a review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1934578X0800300728. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural products for controlling insects of importance to human health—a structure-activity relationship study. Psyche 2016, 2016, 4595823. [Google Scholar] [CrossRef]
- Hollingsworth, R.G. Limonene, a citrus extract, for control of mealybugs and scale Insects. J. Econ. Entomol. 2005, 98, 772–779. [Google Scholar] [CrossRef]
- Gershenzon, J.; Croteau, R. Terpenoides. In Herbivores. Their Interaction with Secondary Plant Metabolites; Rosenthal, G.A., Berenbaum, M.R., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 165–219. [Google Scholar]
- Showler, A.T.; Harlien, J.L.; Perez de Léon, A.A. Effects of laboratory grade limonene and a commercial limonene-based insecticide on Haematobia irritans irritans (Muscidae: Diptera): Deterrence, mortality, and reproduction. J. Med. Entomol. 2019, 56, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Skopec, M.M.; Adams, R.P.; Muir, J.P. Terpenes may serve as feeding deterrents and foraging cues for mammalian herbivores. J. Chem. Ecol. 2019, 45, 993–1003. [Google Scholar] [CrossRef]
- Liao, C.; Kim, U.-J.; Kannan, K. A Review of Environmental Occurrence, Fate, Exposure, and Toxicity of Benzothiazoles. Environ. Sci. Technol. 2018, 52, 5007–5026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cui, K.; Xu, C.; Wang, Q.; Wang, Y.; Zhang, Z.; Liu, F.; Mu, W. Proteomic profile of the Bradysia odoriphaga in response to the microbial secondary metabolite benzothiazole. Sci. Rep. 2016, 6, 37730. [Google Scholar] [CrossRef]
- Davidson, D.W.; Seidel, J.L.; Epstein, W.W. Neotropical ant gardens. 2. Bioassays of seed compounds. J. Chem. Ecol. 1990, 16, 2993–3013. [Google Scholar] [CrossRef] [PubMed]
- Orivel, J.; Dejean, A. Selection of epiphyte seeds by ant-garden ants. Écoscience 1999, 6, 51–55. [Google Scholar] [CrossRef]
- Truong, D.-H.; Delory, B.; Vanderplanck, M.; Brostaux, Y.; Vandereycken, A.; Heuskin, S.; Delaplace, P.; Francis, F.; Lognay, G. Temperature regimes and aphid density interactions differentially influence VOC emissions in Arabidopsis. Arthropod-Plant Interact. 2014, 8, 317–327. [Google Scholar] [CrossRef][Green Version]
- Swale, D.; Bloomquist, J. Is DEET a dangerous neurotoxicant? Pest Manag. Sci. 2019, 75, 2068–2070. [Google Scholar] [CrossRef]
- Mekonnen, B.; Cheseto, X.; Pirk, C.; Yusuf, A.; Ekesi, S.; Deletre, E.; Torto, B. Re-analysis of abdominal gland volatilome secretions of the African Weaver ant, Oecophylla longinoda (Hymenoptera: Formicidae). Molecules 2021, 26, 871. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.R.F.; Vasconcellos, P.C.; Mantovani, W.; Pool, C.S.; Pisani, S.O. Measurements of biogenic hydrocarbons and carbonyl compounds emitted by trees from temperate warm Atlantic rainforest, Brazil. J. Environ. Monit. 2005, 7, 493–499. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, B.M.S.; Melo, C.R.; Santos, A.C.C.; Nascimento, L.F.A.; Nízio, D.A.C.; Cristaldo, P.F.; Blank, A.F.; Bacci, L. Essential oils from Varronia curassavica (Cordiaceae) accessions and their compounds (E)-caryophyllene and α-humulene as an alternative to control Dorymyrmex thoracius (Formicidae: Dolichoderinae). Environ. Sci. Pollut. Res. 2019, 26, 6602–6612. [Google Scholar] [CrossRef]
- Wheeler, J.W.; Evans, S.L.; Blum, M.S.; Torgerson, R.L. Cyclopentyl ketones: Identification and function in Azteca ants. Science 1975, 187, 254–255. [Google Scholar] [CrossRef]
- Saeed Al Kaabi, S.K.S. Evaluation of phytochemical composition and anti-cancer potential in root extracts of Moringa peregrina (Forssk.)Fiori. Master’s Thesis, United Arab Emirates University, Abu Dhabi, United Arab Emirates, 2019. [Google Scholar]
- Prakash, B.; Dubey, N.K. Evaluation of chemically characterised essential oils of Coleus aromaticus, Hyptis suaveolens and Ageratum conyzoides against storage fungi and aflatoxin contamination of food commodities. Int. J. Food Sci. Technol. 2011, 46, 754–760. [Google Scholar] [CrossRef]
- Nair, J.V.; Shanmugam, P.V.; Karpe, S.D.; Ramakrishnan, U.; Olsson, S. An optimized protocol for large-scale in situ sampling and analysis of volatile organic compounds. Ecol. Evol. 2018, 8, 5924–5936. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Mohamed, G.A. Naturally occurring thiophenes: Isolation, purification, structural elucidation, and evaluation of bioactivities. Phytochem. Rev. 2016, 15, 197–220. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Li, H.; Sivasithamparam, K.; Jones, M.G.K.; Du, X.; Ren, Y.; Wylie, S.J. Metabolic responses of endophytic Nicotiana benthamiana plants experiencing water stress. Environ. Exp. Bot. 2017, 143, 59–71. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Halim, N.H.A.; Abidin, N.A.Z.; Me, R. A study of chemical compounds in Rhizophora apiculata. Open Conf. Proc. J. 2014, 4, 108–110. [Google Scholar] [CrossRef]
- Römer, D.; Bollazzi, M.; Roces, F. Carbon dioxide sensing in the social context: Leaf-cutting ants prefer elevated CO2 levels to tend their brood. J. Insect Physiol. 2018, 108, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.G.; Rangel, S.; Kubo, I. Volatile aldehydes are promising broad-spectrum postharvest insecticides. J. Agric. Food Chem. 2000, 48, 4410–4417. [Google Scholar] [CrossRef]
- Hubert, J.; Münzbergová, Z.; Santino, A. Plant volatile aldehydes as natural insecticides against stored-product beetles. Pest Manag. Sci. 2008, 64, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.; Bikoba, V.; Mitcham, E.J. Effects of acetaldehyde on fruit quality and target pest mortality for harvested strawberries. Postharvest Biol. Technol. 2003, 28, 405–416. [Google Scholar] [CrossRef]
- Stewart, J.K.; Aharoni, Y.; Hartsell, P.L.; Young, D.K. Acetaldehyde fumigation at reduced pressures to control the green peach aphid on wrapped and packed head lettuce. J. Econ. Entomol. 1980, 73, 149–152. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, A.; Ali, L.; Khan, A.; Waqas, M.; Hussain, J.; Lee, I.-J.; Shin, J.-H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2014, 53, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Bond, E.J. Manual of Fumigation for Insect Control; Food and Agriculture Organization of the United Nations: Rome, Italy, 1984. [Google Scholar]
- Govindan, M.; Cutkomp, L.K. Carbon disulfide effectiveness on flour beetles in relation to temperature and exposure time. J. Econ. Entomol. 1961, 54, 1121–1127. [Google Scholar] [CrossRef]
- Cui, K.; He, L.; Zhang, Z.; Zhang, L.; Mu, W.; Liu, F. Effects of benzothiazole on survival for reduced reproduction and development in Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Pest Manag. Sci. 2020, 76, 3088–3095. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Jiang, H.Y.; Zhang, W.J.; Guo, S.S.; Yang, K.; Lei, N.; Ma, P.; Geng, Z.F.; Du, S.S. Contact toxicity and repellency of the main components from the essential oil of Clausena anisum-olens against two stored product insects. J. Insect Sci. 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Zhang, X.; Wang, Y.; Chen, Z.-Y.; Lu, X.-X.; Du, Y.-S.; Du, S.-S. The potential contribution of cymene isomers to insecticidal and repellent activities of the essential oil from Alpinia zerumbet. Int. Biodeterior. Biodegrad. 2021, 157, 105138. [Google Scholar] [CrossRef]
- Zhang, N.; Liao, Y.; Xie, L.; Zhang, Z.; Hu, W. Using essential oils from Citrus paradisi as a fumigant for Solenopsis invicta workers and evaluating the oils’ effect on worker behavior. Environ. Sci. Pollut. Res. Int. 2021, 1–8. [Google Scholar] [CrossRef]
- Vogt, J.; Shelt, V.; Merchant, M.; Russell, S.; Tanle, M.; Appe, A. Efficacy of three citrus oil formulations against Solenopsis invicta Buren (Hymenoptera: Formicidae), the red imported fire ant. J. Agric. Urban Entomol. 2002, 19, 159–171. [Google Scholar]
- Verza, S.S.; Nagamoto, N.S.; Forti, L.C.; Noronha Jr, N.C. Preliminary studies on the effects of d-limonene to workers of the leaf-cutting ant Atta sexdens rubropilosa and its implications for control. Bull. Insectology 2011, 64, 27–32. [Google Scholar]
- Gostinčar, C.; Zajc, J.; Lenassi, M.; Plemenitaš, A.; de Hoog, S.; Al-Hatmi, A.M.S.; Gunde-Cimerman, N. Fungi between extremotolerance and opportunistic pathogenicity on humans. Fungal Divers. 2018, 93, 195–213. [Google Scholar] [CrossRef]
- Marting, P.R.; Wcislo, W.T.; Pratt, S.C. Colony personality and plant health in the Azteca-Cecropia mutualism. Behav. Ecol. 2018, 29, 264–271. [Google Scholar] [CrossRef]
| Chemical Families and Compounds | Origin a | Outdoor Air b | Empty Domatia c | Colonized Domatia c | Weak Colony d | Reduction e (%) | ||
|---|---|---|---|---|---|---|---|---|
| Alcohols | 23.4 | 17.5 | ±6.8 | 13.4 | ±5.9 | 28.4 | 24 | |
| Ethanol | P | 8.4 | 3.5 | ±3.4 | 3.8 | ±1.6 | 0.8 | 43 |
| 2-Ethyl-1-hexanol | P | 10.3 | 5.1 | ±2.5 | 9.0 | ±3.9 | 22.7 | −76 |
| Aldehydes | 30.5 | 51.7 | ±19.2 | 31.5 | ±10.9 | 19.9 | 39 | |
| Acetaldehyde | P | 8.3 | 11.7 | ±3.7 | 4.0 | ±4.9 | 4.5 | 66 * |
| Benzaldehyde | P | 3.5 | 12.0 | ±9.9 | 8.8 | ±2.4 | 2.1 | 27 |
| Nonanal | P | 3.2 | 9.2 | ±10.3 | 4.5 | ±0.7 | 2.5 | 51 |
| Aliphatic Hydrocarbons | 38.8 | 25.8 | ±15.8 | 39.3 | ±1.2 | 106.2 | −52 | |
| n-Hexane | P | 1.8 | 0.8 | ±0.5 | 1.1 | ±0.3 | 7.3 | −26 |
| Octane | I | 0.4 | 0.4 | ±0.1 | 0.4 | ±0.4 | 71.4 | 2 |
| Amines | 0.1 | 0.1 | ±0.2 | 0.0 | ±0.0 | 0.0 | 100 | |
| Aromatic Alcohols | 2.0 | 1.8 | ±1.8 | 1.0 | ±0.7 | 0.7 | 46 | |
| Aromatic Hydrocarbons | 11.5 | 17.2 | ±13.8 | 7.6 | ±1.3 | 8.3 | 56 | |
| Toluene | A | 5.2 | 3.7 | ±2.4 | 3.1 | ±1.0 | 2.7 | 17 |
| Cyclic Hydrocarbons | 7.9 | 7.3 | ±4.7 | 39.6 | ±29.0 | 3.6 | −439 | |
| Methyl-cyclopentane | I | 0.0 | 1.0 | ±0.4 | 30.5 | ±28.7 | 0.0 | −2808 |
| Ethyl-cyclohexane | P | 6.4 | 3.6 | ±3.8 | 6.3 | ±4.8 | 0.0 | −75 |
| Esters | 23.5 | 32.1 | ±23.3 | 83.0 | ±42.6 | 10.1 | −158 | |
| Methyl-acetate | P | 5.6 | 1.7 | ±1.5 | 0.6 | ±0.6 | 0.4 | 64 |
| Methyl−2-ethylpentanoate | P | 0.0 | 0.1 | ±0.2 | 6.0 | ±3.9 | 2.2 | −4798 |
| Dibutyl-phthalate | A | 7.1 | 16.5 | ±14.3 | 39.7 | ±20.9 | 0.0 | −141 |
| Diisobutyl phthalate | A | 7.1 | 11.9 | ±10.4 | 31.8 | ±15.4 | 3.4 | −167 |
| Ethers | 1.4 | 1.8 | ±1.6 | 3.2 | ±1.8 | 2.9 | −72 | |
| Furans | 0.9 | 1.9 | ±2.8 | 2.7 | ±0.8 | 0.8 | −38 | |
| Halogen-containing compounds | 0.3 | 0.2 | ±0.0 | 0.1 | ±0.1 | 0.0 | 14 | |
| Heterogroups | 0.6 | 15.7 | ±19.3 | 27.9 | ±28.1 | 2.6 | −77 | |
| Diethyltoluamide | A | 0.6 | 14.8 | ±19.2 | 27.9 | ±28.1 | 0.0 | 89 |
| Ketones | 73.8 | 406.0 | ±414.8 | 5853.5 | ±2022.6 | 172.9 | −1342 ** | |
| Acetone | P | 3.3 | 14.0 | ±19.1 | 1.8 | ±3.2 | 0.0 | 87 |
| 2-Pentanone | I | 1.3 | 10.5 | ±15.2 | 240.2 | ±196.9 | 2.7 | −2179 |
| 2-Hexanone | I | 0.2 | 1.3 | ±1.9 | 38.4 | ±22.5 | 0.5 | −2791 ** |
| 2-Methyl-cyclopentanone | I | 0.0 | 0.0 | 0.0 | 7.2 | - | ||
| 5-Hepten−2-one | I | 0.0 | 1.1 | ±1.9 | 138.6 | ±65.5 | 0.0 | −12,760 ** |
| 2-Heptanone | I | 63.5 | 358.0 | ±412.2 | 5186.2 | ±1647.7 | 136.3 | −1349 ** |
| 6-Methyl-5-hepten-2-one | I | 2.0 | 6.3 | ±5.2 | 223.8 | ±79.0 | 0.4 | −3429 ** |
| 1-Cyclohexyl-ethanone | I | 0.0 | 0.0 | ±0.0 | 0.0 | ±0.0 | 20.0 | - |
| Acetophenone | P | 1.7 | 8.7 | ±12.4 | 2.0 | ±0.4 | 1.2 | 77 |
| Lactones | 1.2 | 1.4 | ±0.3 | 0.8 | ±0.5 | 1.7 | 42 | |
| Nitrogen-containing compounds | 5.6 | 7.4 | ±3.4 | 3.9 | ±1.3 | 8.1 | 47 | |
| Organic Acids | 33.2 | 16.9 | ±13.2 | 11.9 | ±3.6 | 18.0 | 29 | |
| Acetic acid | P | 31.0 | 14.9 | ±12.1 | 9.8 | ±4.4 | 13.1 | 34 |
| Oxygen-containing compounds | 0.3 | 2.1 | ±3.2 | 0.4 | ±0.1 | 0.7 | 79 | |
| Sulfur-containing compounds | 408.2 | 672.1 | ±250.7 | 205.3 | ±300.5 | 59.3 | 69 | |
| Sulfur dioxide | G | 366.3 | 524.7 | ±266.1 | 171.7 | ±248.8 | 56.0 | 67 |
| Carbon disulphide | G | 38.4 | 113.6 | ±108.9 | 31.0 | ±49.4 | 1.8 | 73 |
| Benzothiazole | P | 1.5 | 27.9 | ±45.4 | 1.3 | ±0.3 | 1.6 | 95 |
| Terpenes | 2.8 | 177.7 | ±163.5 | 41.1 | ±16.6 | 12.6 | 77 | |
| α-Phellandrene | P | 0.0 | 9.6 | ±16.2 | 1.6 | ±2.3 | 0.0 | 84 |
| D-Limonene | P | 1.7 | 86.5 | ±125.9 | 5.3 | ±2.7 | 1.0 | 94 |
| p-Cymene | P | 0.4 | 50.4 | ±45.9 | 9.8 | ±8.4 | 0.5 | 81 |
| β-Phellandrene | P | 0.0 | 9.0 | ±14.8 | 2.6 | ±2.6 | 0.0 | 72 |
| Caryophyllene | P | 0.0 | 0.0 | ±0.0 | 0.0 | ±0.0 | 9.4 | - |
| Total concentration: | 1151.1 | 1456.9 | ±435.3 | 6366.3 | ±2234.5 | 457.1 | −337 ** | |
| Partial concentration f: | 1021.2 | 1078.0 | ±114.0 | 501.1 | ±264.9 | 225.7 | 54 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, V.E.; de Hoog, S.; Cristescu, S.M.; Vera, L.; Prenafeta-Boldú, F.X. Volatile Organic Compounds in the Azteca/Cecropia Ant-Plant Symbiosis and the Role of Black Fungi. J. Fungi 2021, 7, 836. https://doi.org/10.3390/jof7100836
Mayer VE, de Hoog S, Cristescu SM, Vera L, Prenafeta-Boldú FX. Volatile Organic Compounds in the Azteca/Cecropia Ant-Plant Symbiosis and the Role of Black Fungi. Journal of Fungi. 2021; 7(10):836. https://doi.org/10.3390/jof7100836
Chicago/Turabian StyleMayer, Veronika E., Sybren de Hoog, Simona M. Cristescu, Luciano Vera, and Francesc X. Prenafeta-Boldú. 2021. "Volatile Organic Compounds in the Azteca/Cecropia Ant-Plant Symbiosis and the Role of Black Fungi" Journal of Fungi 7, no. 10: 836. https://doi.org/10.3390/jof7100836
APA StyleMayer, V. E., de Hoog, S., Cristescu, S. M., Vera, L., & Prenafeta-Boldú, F. X. (2021). Volatile Organic Compounds in the Azteca/Cecropia Ant-Plant Symbiosis and the Role of Black Fungi. Journal of Fungi, 7(10), 836. https://doi.org/10.3390/jof7100836








