Fungal Enzymes as Catalytic Tools for Polyethylene Terephthalate (PET) Degradation
Abstract
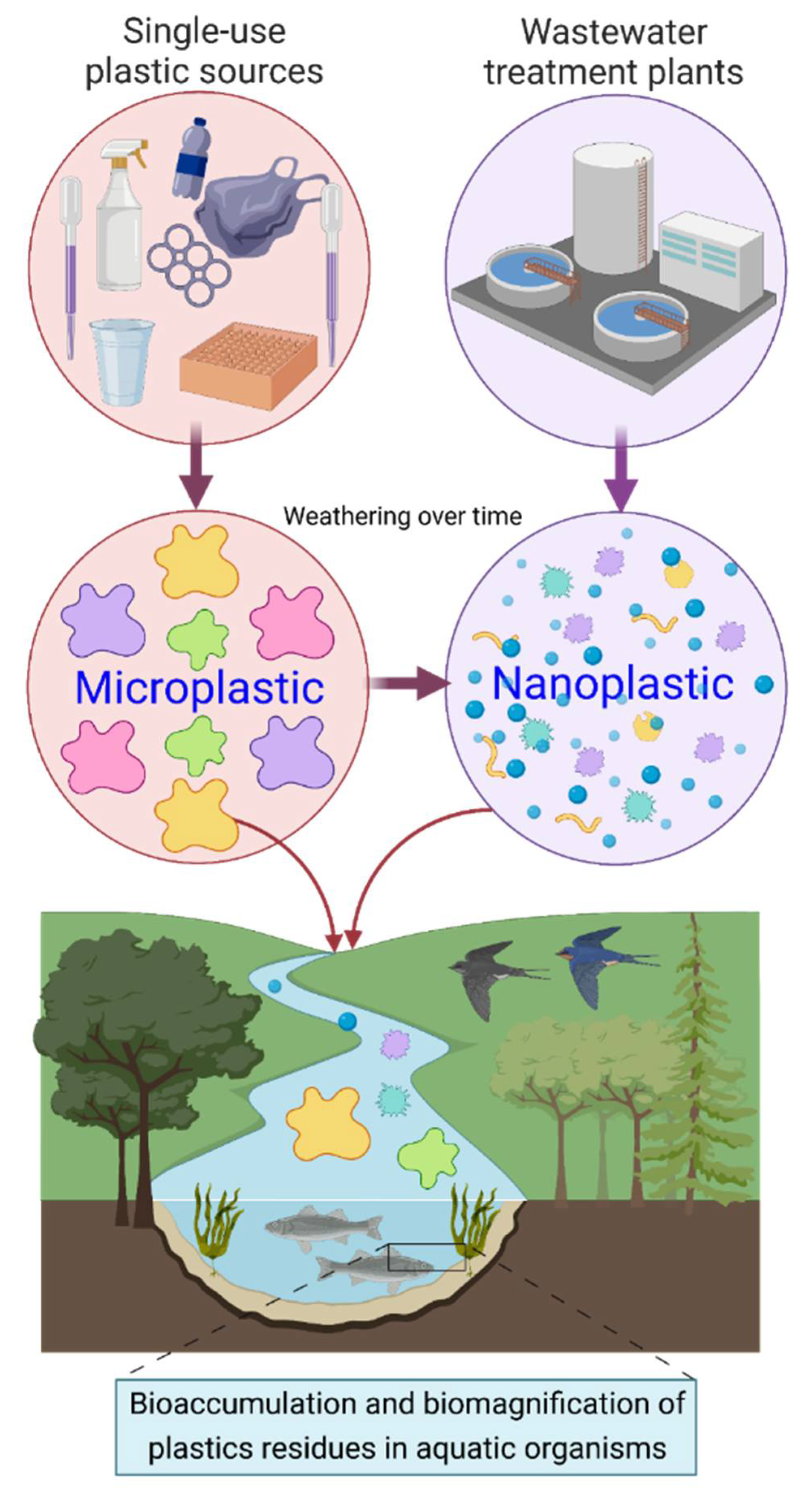
:1. Introduction
2. Synthetic Plastics—Categories and PET
3. Fungal Enzyme-Mediated PET Degradation
4. Catalytic Mechanism of Cutinases for PET Hydrolysis
5. Limitations Hindering Enzymatic PET Biodegradation
6. Strategies to Enhance Enzyme-Based PET Biodegradation
6.1. Thermostable Enzymes
6.2. Use of Surfactants and Additives
6.3. Enzyme Tailoring and Genetic Modification
7. Conclusions and Future Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.; Sun, J. Degradation of conventional plastic wastes in the environment. A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 2020, 759, 143536. [Google Scholar] [CrossRef]
- Feil, A.; Pretz, T. Mechanical recycling of packaging waste. In Plastic Waste and Recycling; Academic Press: Cambridge, MA, USA, 2020; pp. 283–319. [Google Scholar]
- Nielsen, T.D.; Hasselbalch, J.; Holmberg, K.; Stripple, J. Politics and the plastic crisis: A review throughout the plastic life cycle. Wiley Interdiscip. Rev. Energy Environ. 2020, 9, 360. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Song, Y.; Zhang, X.; Xie, B.; He, D. Microplastics in Urban Environments: Sources, Pathways, and Distribution. In The Handbook of Environmental Chemistry; He, D., Luo, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lahive, E.; Walton, A.; Horton, A.A.; Spurgeon, D.J.; Svendsen, C. Microplastic particles reduce reproduction in the terrestrial worm Enchytraeus crypticus in a soil exposure. Environ. Pollut. 2019, 255, 113174. [Google Scholar] [CrossRef] [PubMed]
- Selonen, S.; Dolar, A.; Kokalj, A.J.; Skalar, T.; Dolcet, L.P.; Hurley, R.; Van Gestel, C.A. Exploring the impacts of plastics in soil–The effects of polyester textile fibers on soil invertebrates. Sci. Total Environ. 2020, 700, 134451. [Google Scholar] [CrossRef] [PubMed]
- Vadera, S.; Khan, S. A Critical Analysis of the Rising Global Demand of Plastics and its Adverse Impact on Environmental Sustainability. J. Environ. Pollut. Manag. 2021, 3, 105. [Google Scholar] [CrossRef]
- Whitmee, S.; Haines, A.; Beyrer, C.; Boltz, F.; Capon, A.G.; de Souza Dias, B.F.; Yach, D. Safeguarding human health in the Anthropocene epoch: Report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet 2015, 386, 1973–2028. [Google Scholar] [CrossRef]
- Barceló, D.; Pico, Y. Microplastics in the global aquatic environment: Analysis, effects, remediation and policy solutions. J. Environ. Chem. Eng. 2019, 7, 103421. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Vega, J.M.; Quej, V.K.; de los Angeles Chi, J.; Del Cid, L.S.; Chi, C.; Geissen, V. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef]
- Ryberg, M.W.; Hauschild, M.Z.; Wang, F.; Averous-Monnery, S.; Laurent, A. Global environmental losses of plastics across their value chains. Resour. Conserv. Recycl. 2019, 151, 104459. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besseling, E.; Quik, J.T.; Sun, M.; Koelmans, A.A. Fate of nano-and microplastic in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2019, 14, 16–22. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.; Hardesty, B.D. Plastic pollution challenges in marine and coastal environments: From local to global governance. Restor. Ecol. 2017, 25, 123–128. [Google Scholar] [CrossRef]
- Crippa, M.; De Wilde, B.; Koopmans, R.; Leyssens, J.; Muncke, J.; Ritschkoff, A.C.; Van Doorsselaer, K.; Velis, C.; Wagner, M. A Circular Economy for Plastics: Insights from Research and Innovation to Inform Policy and Funding Decisions; European Commission: Brussels, Belgium, 2019; p. 239. ISBN 978-92-79-98429-7. Available online: https://hdl.handle.net/10.2777/269031 (accessed on 4 August 2021).
- Samak, N.A.; Jia, Y.; Sharshar, M.M.; Mu, T.; Yang, M.; Peh, S.; Xing, J. Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling. Environ. Int. 2020, 145, 106144. [Google Scholar] [CrossRef] [PubMed]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and biotechnological perspectives on microbial degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef] [Green Version]
- Inderthal, H.; Tai, S.L.; Harrison, S.T. Non-hydrolyzable plastics–an interdisciplinary look at plastic bio-oxidation. Trends Biotechnol. 2021, 39, 12–23. [Google Scholar] [CrossRef]
- Grand Review Research. Plastic Market Size, Share & Trends Analysis Report By Product (PE, PP, PU, PVC, PET, Polystyrene, ABS, PBT, PPO, Epoxy Polymers, LCP, PC, Polyamide), By Application, By End-Use, By Region, And Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/global-plastics-market (accessed on 20 August 2021).
- Leonard, S.; Barra, R. Plastics and the Circular Economy. Scientific and Technical Advisory Panel to the Global Environment Facility; The Scientific and Technical Advisory Panel (STAP) to the Global Environment Facility: Washington, DC, USA, 2018. [Google Scholar]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. Polyethylene terephthalate (PET) in the packaging industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- PlasticsEurope. 2020. Available online: https://www.plasticseurope.org/application/files/5716/0752/4286/AF_Plastics_the_facts-WEB-2020-ING_FINAL (accessed on 13 September 2021).
- Statista. Distribution of Polyethylene Terephthalate (PET) Packaging Consumption Worldwide in 2019, by End-Use Sector. 2020. Available online: https://www.statista.com/statistics/858624/global-polyethylene-terephthalate-consumption-distribution-by-end-use (accessed on 13 September 2021).
- Statista. Annual Production of Plastics Worldwide from 1950 to 2020. 2021. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950 (accessed on 13 September 2021).
- Statista. Demand for Polyethylene Terephthalate Worldwide from 2010 to 2020 with a Forecast for 2021 to 2030. 2021. Available online: https://www.statista.com/statistics/1128658/polyethylene-terephthalate-demand-worldwide (accessed on 13 September 2021).
- Intelligence, M. Gaming Market-Growth, Trends, Forecasts (2020–2025); Mordor Intelligence: Hyderabad, India, 2020; Available online: https://www.mordorintelligence.com/industry-reports/pet-bottles-market (accessed on 3 September 2021).
- WHO. Antimony in Drinking-Water. 2013. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/antimony.pdf (accessed on 4 September 2021).
- Wittkowski, P.; Marx-Stoelting, P.; Violet, N.; Fetz, V.; Schwarz, F.; Oelgeschläger, M.; Vogl, S. Caenorhabditis elegans as a promising alternative model for environmental chemical mixture effect assessment—A comparative study. Environ. Sci. Technol. 2019, 53, 12725–12733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerhoff, P.; Prapaipong, P.; Shock, E.; Hillaireau, A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008, 42, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shi, H.; Adams, C.D.; Ma, Y. Assessment of metal contaminations leaching out from recycling plastic bottles upon treatments. Environ. Sci. Pollut. Res. 2010, 17, 1323–1330. [Google Scholar] [CrossRef]
- Filella, M. Antimony and PET bottles: Checking facts. Chemosphere 2020, 261, 127732. [Google Scholar] [CrossRef]
- Konieczna, A.; Rutkowska, A.; Rachon, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Państwowego Zakładu 2015, 66, 5–11. [Google Scholar]
- Ito, Y.; Kamijima, M.; Nakajima, T. Di (2-ethylhexyl) phthalate-induced toxicity and peroxisome proliferator-activated receptor alpha: A review. Environ. Health Prev. Med. 2019, 24, 47. [Google Scholar] [CrossRef] [Green Version]
- Minnesota Department of Health. Toxicological Summary for: Butyl Benzyl Phthalate. 2015. Available online: https://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/butylbenzylsumm (accessed on 13 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 13 September 2021).
- Zellmer, S.; Heiserich, L.; Kappenstein, O.; Merkel, S.; Schulte, A.; Luch, A. MCCP: Are medium-chain chlorinated paraffins of concern for humans? Arch. Toxicol. 2020, 94, 955–957. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.D.; Lee, J.Y.; Kwack, S.J.; Shin, C.Y.; Jang, H.J.; Kim, H.Y.; Kim, K.B. Risk assessment of triclosan, a cosmetic preservative. Toxicol. Res. 2019, 35, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Yost, E.E.; Euling, S.Y.; Weaver, J.A.; Beverly, B.E.; Keshava, N.; Mudipalli, A.; Makris, S.L. Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies. Environ. Int. 2019, 125, 579–594. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Hu, G.; Li, L.; Su, H.; Wang, Y.; Ge, R.S. Effects of in utero exposure to dicyclohexyl phthalate on rat fetal leydig cells. Int. J. Environ. Res. Public Health 2016, 13, 246–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddela, N.R.; Venkateswarlu, K.; Megharaj, M. Tris (2-chloroethyl) phosphate, a pervasive flame retardant: Critical perspective on its emissions into the environment and human toxicity. Environ. Sci. Process. Impacts 2020, 22, 1809–1827. [Google Scholar] [CrossRef]
- Thoene, M.; Dzika, E.; Slawomir, S.; Wojtkiewicz, J. Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, J.C.; Downs, C.A. Dermatological and environmental toxicological impact of the sunscreen ingredient oxybenzone/benzophenone-3. J. Cosmet. Dermatol. 2018, 17, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Antimony Trioxide. Available online: https://www.nj.gov/health/eoh/rtkweb/documents/fs/0149 (accessed on 18 September 2021).
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, S.; Nayak, S.G.; Labde, J.V.; Gharal, P.R.; Rao, K.; Kelkar, A.K. Degradation and recyclability of poly (ethylene terephthalate). In Polyester; Saleh, H.E.D.M., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 4; pp. 75–98. [Google Scholar]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of various plastics in the environment. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 71–92. [Google Scholar]
- Farzi, A.; Dehnad, A.; Fotouhi, A.F. Biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatal. Agric. Biotechnol. 2019, 17, 25–31. [Google Scholar] [CrossRef]
- Narciso-Ortiz, L.; Coreño-Alonso, A.; Mendoza-Olivares, D.; Lucho-Constantino, C.A.; Lizardi-Jiménez, M.A. Baseline for plastic and hydrocarbon pollution of rivers, reefs, and sediment on beaches in Veracruz State, México, and a proposal for bioremediation. Environ. Sci. Pollut. Res. 2020, 27, 23035–23047. [Google Scholar] [CrossRef]
- Torena, P.; Alvarez-Cuenca, M.; Reza, M. Biodegradation of polyethylene terephthalate microplastics by bacterial communities from activated sludge. Canad. J. Chem. Eng. 2021, 99, 69–82. [Google Scholar] [CrossRef]
- Djapovic, M.; Milivojevic, D.; Ilic-Tomic, T.; Lješević, M.; Nikolaivits, E.; Topakas, E.; Nikodinovic-Runic, J. Synthesis and characterization of polyethylene terephthalate (PET) precursors and potential degradation products: Toxicity study and application in discovery of novel PETases. Chemosphere 2021, 275, 130005. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Sun, C. A marine bacterial community capable of degrading poly (ethylene terephthalate) and polyethylene. J. Hazard. Mater. 2021, 416, 125928. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, S.; Venkatakrishnan, H.R.R.; Ravindran, J.; Sathyamoorthy, J.; Rangabashyam, K.A.; Ragini, Y.P.; Sureshbabu, K. Hydrolytic Degradation of Polyethylene Terephthalate by Cutinase Enzyme Derived from Fungal Biomass–Molecular Characterization. BioInterface Res. Appl. Chem. 2021, 12, 653–667. [Google Scholar]
- Moyses, D.N.; Teixeira, D.A.; Waldow, V.A.; Freire, D.M.; Castro, A.M. Fungal and enzymatic bio-depolymerization of waste post-consumer poly (ethylene terephthalate)(PET) bottles using Penicillium species. 3 Biotech 2021, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Marty, A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Sarkhel, R.; Sengupta, S.; Das, P.; Bhowal, A. Comparative biodegradation study of polymer from plastic bottle waste using novel isolated bacteria and fungi from marine source. J. Polym. Res. 2020, 27, 16. [Google Scholar] [CrossRef]
- Groß, C.; Hamacher, K.; Schmitz, K.; Jager, S. Cleavage product accumulation decreases the activity of cutinase during PET hydrolysis. J. Chem. Inf. Model. 2017, 57, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Dimarogona, M.; Nikolaivits, E.; Kanelli, M.; Christakopoulos, P.; Sandgren, M.; Topakas, E. Structural and functional studies of a Fusarium oxysporum cutinase with polyethylene terephthalate modification potential. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2308–2317. [Google Scholar] [CrossRef] [PubMed]
- Kanelli, M.; Vasilakos, S.; Nikolaivits, E.; Ladas, S.; Christakopoulos, P.; Topakas, E. Surface modification of poly (ethylene terephthalate)(PET) fibers by a cutinase from Fusarium oxysporum. Proc. Biochem. 2015, 50, 1885–1892. [Google Scholar] [CrossRef]
- Carniel, A.; Valoni, É.; Junior, J.N.; da Conceição Gomes, A.; de Castro, A.M. Lipase from Candida antarctica (CALB) and cutinase from Humicola insolens act synergistically for PET hydrolysis to terephthalic acid. Proc. Biochem. 2017, 59, 84–90. [Google Scholar] [CrossRef]
- Sulaiman, S.; Yamato, S.; Kanaya, E.; Kim, J.J.; Koga, Y.; Takano, K.; Kanaya, S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl. Environ. Microbiol. 2012, 78, 1556–1562. [Google Scholar] [CrossRef] [Green Version]
- Nowak, B.; Pająk, J.; Łabużek, S.; Rymarz, G.; Talik, E. Biodegradation of poly (ethylene terephthalate) modified with polyester” Bionolle®” by Penicillium funiculosum. Polimery 2011, 56, 35–44. [Google Scholar] [CrossRef]
- Eberl, A.; Heumann, S.; Brückner, T.; Araujo, R.; Cavaco-Paulo, A.; Kaufmann, F.; Guebitz, G.M. Enzymatic surface hydrolysis of poly (ethylene terephthalate) and bis (benzoyloxyethyl) terephthalate by lipase and cutinase in the presence of surface active molecules. J. Biotechnol. 2009, 143, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ronkvist, Å.M.; Xie, W.; Lu, W.; Gross, R.A. Cutinase-catalyzed hydrolysis of poly (ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar] [CrossRef]
- Araújo, R.; Silva, C.; O’Neill, A.; Micaelo, N.; Guebitz, G.; Soares, C.M.; Cavaco-Paulo, A. Tailoring cutinase activity towards polyethylene terephthalate and polyamide 6, 6 fibers. J. Biotechnol. 2007, 128, 849–857. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, A.; Araújo, R.; Casal, M.; Guebitz, G.; Cavaco-Paulo, A. Effect of the agitation on the adsorption and hydrolytic efficiency of cutinases on polyethylene terephthalate fibres. Enzyme Microb. Technol. 2007, 40, 1801–1805. [Google Scholar] [CrossRef] [Green Version]
- Liebminger, S.; Eberl, A.; Sousa, F.; Heumann, S.; Fischer-Colbrie, G.; Cavaco-Paulo, A.; Guebitz, G.M. Hydrolysis of PET and bis-(benzoyloxyethyl) terephthalate with a new polyesterase from Penicillium citrinum. Biocatal. Biotransform. 2007, 25, 171–177. [Google Scholar] [CrossRef]
- Nimchua, T.; Punnapayak, H.; Zimmermann, W. Comparison of the hydrolysis of polyethylene terephthalate fibers by a hydrolase from Fusarium oxysporum LCH I and Fusarium solani f. sp. pisi. Biotechnol. J. Healthc. Nutr. Technol. 2007, 2, 361–364. [Google Scholar]
- Alisch-Mark, M.; Herrmann, A.; Zimmermann, W. Increase of the hydrophilicity of polyethylene terephthalate fibres by hydrolases from Thermomonospora fusca and Fusarium solani f. sp. pisi. Biotechnol. Lett. 2006, 28, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Vertommen, M.A.M.E.; Nierstrasz, V.A.; Van Der Veer, M.; Warmoeskerken, M.M.C.G. Enzymatic surface modification of poly (ethylene terephthalate). J. Biotechnol. 2005, 120, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Oeser, T.; Schmidt, J.; Meier, R.; Barth, M.; Then, J.; Zimmermann, W. Engineered bacterial polyester hydrolases efficiently degrade polyethylene terephthalate due to relieved product inhibition. Biotechnol. Bioeng. 2016, 113, 1658–1665. [Google Scholar] [CrossRef]
- Bermúdez-García, E.; Peña-Montes, C.; Castro-Rodríguez, J.A.; González-Canto, A.; Navarro-Ocaña, A.; Farrés, A. ANCUT2, a thermo-alkaline cutinase from Aspergillus nidulans and its potential applications. Appl. Biochem. Biotechnol. 2017, 182, 1014–1036. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Wei, R.; Zimmermann, W. Biocatalysis as a green route for recycling the recalcitrant plastic polyethylene terephthalate. Microb. Biotechnol. 2017, 10, 1302–1307. [Google Scholar] [CrossRef]
- Sepperumal, U.; Markandan, M.; Palraja, I. Micromorphological and chemical changes during biodegradation of polyethylene terephthalate (PET) by Penicillium sp. J. Microbiol. Biotechnol. Res. 2013, 3, 47–53. [Google Scholar]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Zimmermann, W. Biocatalytic degradation efficiency of postconsumer polyethylene terephthalate packaging determined by their polymer microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef] [Green Version]
- Oda, M.; Yamagami, Y.; Inaba, S.; Oida, T.; Yamamoto, M.; Kitajima, S.; Kawai, F. Enzymatic hydrolysis of PET: Functional roles of three Ca2+ ions bound to a cutinase-like enzyme, Cut190*, and its engineering for improved activity. Appl. Microbiol. Biotechnol. 2018, 102, 10067–10077. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Huo, Y.; Yang, Y. Microbial degradation and valorization of plastic wastes. Front. Microbiol. 2020, 11, 442. [Google Scholar] [CrossRef] [Green Version]
- Barth, M.; Oeser, T.; Wei, R.; Then, J.; Schmidt, J.; Zimmermann, W. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 2015, 93, 222–228. [Google Scholar] [CrossRef]
- Sánchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro-and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; De Geus, P.; Lauwereys, M.; Matthyssens, G.; Cambillau, C. Fusarium solani cutinase is a lipolytic enzyme with a catalytic serine accessible to solvent. Nature 1992, 356, 615–618. [Google Scholar] [CrossRef]
- Shirke, A.N.; White, C.; Englaender, J.A.; Zwarycz, A.; Butterfoss, G.L.; Linhardt, R.J.; Gross, R.A. Stabilizing leaf and branch compost cutinase (LCC) with glycosylation: Mechanism and effect on PET hydrolysis. Biochemistry 2018, 57, 1190–1200. [Google Scholar] [CrossRef]
- Maurya, A.; Bhattacharya, A.; Khare, S.K. Enzymatic Remediation of Polyethylene Terephthalate (PET)–Based Polymers for Effective Management of Plastic Wastes: An Overview. Front. Bioeng. Biotechnol. 2020, 8, 602325. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Chemical, physical, and biological coordination: An interplay between materials and enzymes as potential platforms for immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Tailoring multipurpose biocatalysts via protein engineering approaches: A review. Catal. Lett. 2019, 149, 2204–2217. [Google Scholar] [CrossRef]
- Kumar, S.; Dangi, A.K.; Shukla, P.; Baishya, D.; Khare, S.K. Thermozymes: Adaptive strategies and tools for their biotechnological applications. Bioresour. Technol. 2019, 278, 372–382. [Google Scholar] [CrossRef]
- Bilal, M.; Nguyen, T.A.; Iqbal, H.M. Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization–a paradigm shift in biocatalyst design. Coord. Chem. Rev. 2020, 422, 213475. [Google Scholar] [CrossRef]
- Jia, R.; Hu, Y.; Liu, L.; Jiang, L.; Huang, H. Chemical modification for improving activity and stability of lipase B from Candida antarctica with imidazolium-functional ionic liquids. Org. Biomol. Chem. 2013, 11, 7192–7198. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, T.; Mizushima, H.; Ohtsuka, J.; Oda, M.; Kawai, F.; Tanokura, M. Structural basis for the Ca2+-enhanced thermostability and activity of PET-degrading cutinase-like enzyme from Saccharomonospora viridis AHK190. Appl. Microbiol. Biotechnol. 2015, 99, 4297–4307. [Google Scholar] [CrossRef]
- Rubingh, D.N. The influence of surfactants on enzyme activity. Curr. Opin. Colloid Interface Sci. 1996, 1, 598–603. [Google Scholar] [CrossRef]
- Zimmermann, W.; Billig, S. Enzymes for the biofunctionalization of poly (ethylene terephthalate). In Biofunctionalization of Polymers and Their Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 97–120. [Google Scholar]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Su, L.; Billig, S.; Zimmermann, W.; Chen, J.; Wu, J. Biochemical characterization of the cutinases from Thermobifida fusca. J. Mol. Catal. B Enzym. 2010, 63, 121–127. [Google Scholar] [CrossRef]
- Espino-Rammer, L.; Ribitsch, D.; Przylucka, A.; Marold, A.; Greimel, K.J.; Herrero Acero, E.; Druzhinina, I.S. Two novel class II hydrophobins from Trichoderma spp. stimulate enzymatic hydrolysis of poly (ethylene terephthalate) when expressed as fusion proteins. Appl. Environ. Microbiol. 2013, 79, 4230–4238. [Google Scholar] [CrossRef] [Green Version]
- Fukuoka, T.; Shinozaki, Y.; Tsuchiya, W.; Suzuki, K.; Watanabe, T.; Yamazaki, T.; Kitamoto, H. Control of enzymatic degradation of biodegradable polymers by treatment with biosurfactants, mannosylerythritol lipids, derived from Pseudozyma spp. yeast strains. Appl. Microbiol. Biotechnol. 2016, 100, 1733–1741. [Google Scholar] [CrossRef]
- Joo, S.; Cho, I.J.; Seo, H.; Son, H.F.; Sagong, H.Y.; Shin, T.J.; Kim, K.J. Structural insight into molecular mechanism of poly (ethylene terephthalate) degradation. Nat. Commun. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaivits, E.; Makris, G.; Topakas, E. Immobilization of a cutinase from Fusarium oxysporum and application in pineapple flavor synthesis. J. Agric. Food Chem. 2017, 65, 3505–3511. [Google Scholar] [CrossRef]
- Su, A.; Shirke, A.; Baik, J.; Zou, Y.; Gross, R. Immobilized cutinases: Preparation, solvent tolerance and thermal stability. Enzym. Microb. Technol. 2018, 116, 33–40. [Google Scholar] [CrossRef]



| PET Packaging Products | Global Consumption in 2020 (Million Tonnes) |
|---|---|
| Water Bottles | 7.02 |
| Carbonated soft drink (CSD) bottles (e.g., Coca Cola, beers) | 7.02 |
| Other drinks (e.g., juices, milk) | 4.86 |
| Other bottles/containers in form of films and sheets | 3.78 |
| Food containers | 2.43 |
| Containers for non-food consumer products (e.g., cosmetics) | 1.62 |
| Hazardous Additives | Chemical Formula | Chemical Structure | Toxic Effects | References |
|---|---|---|---|---|
| Bisphenol A (BPA) |  | Female and male infertility Precocious puberty Breast cancer Prostate cancer Metabolic disorders including polycystic ovary syndrome (PCOS) | [39] | |
| Bis (2-ethylhexyl) phthalate (DEHP) |  | Cancer Reproductive system Stages of development Nerve system Immune system | [40] | |
| Benzyl butyl phthalate (BBP) |  | Decrease in thyroid hormone levels Endocrine system Stages of development Reproductive system | [41] | |
| Lead chromate molybdate sulphate red |  | Cardiovascular system Respiratory system Gastrointestinal-liver Endocrine system Cancer Kidney damage Neurotoxic effects | [42] | |
| Medium-chain chlorinated paraffins (MCCP) |  | Skin dryness Adverse effects on aquatic life | [43] | |
| Triclosan |  | Thyroid hormones Reproductive system Breast cancer | [44] | |
| Dibutyl phthalate (DBP) |  | Effect on kidney Reproductive system Irritation of eyes, nose, throat, and skin | [42] | |
| Diisobutyl phthalate (DiBP) |  | Reproductive system Developmental system Liver Kidney Possible triggering of cancer | [45] | |
| Dicyclohexyl phthalate (DCHP) |  | Reproductive system Cumulative anti-androgenic effect with other phthalates | [46] | |
| Tris(2-chloroethyl)phosphate (TCEP) |  | Possible impairment of fertility Adverse effects on aquatic organisms | [47] | |
| 1,3,5-Tris(oxiran-2-ylmethyl)-1,3,5-triazinane-2,4,6-trione (TGIC) |  | If swallowed If inhaled May cause genetic defects Serious eye damage | [42] | |
| 1,3,5-tris[(2S and 2R)-2,3-epoxypropyl]-1,3,5-triazine-2,4,6- (1H,3H,5H)-trione (β-TGIC) |  | May cause genetic defects Harmful if swallowed Causes serious eye damage May cause damage to organs through prolonged or repeated exposure May cause an allergic skin reaction | [42] | |
| Bisphenol S |  | Obesity Metabolic disorders Possible triggering of cancer Reproductive defects Gestational diabetes Breast cancers | [48] | |
| Benzophenone-3 |  | Allergic reactions Endocrine disruption Hirschsprung’s disease | [49] | |
| Antimony trioxide |  | Possible triggering of lung cancer Reproductive system Kidney, liver, heart | [50] |
| Enzyme | Fungal Strain | PET Source | Percent PET-Degradation (Transformed Products) | Reference |
|---|---|---|---|---|
| Lipase and Cutinase | Aspergillus tamarii and Penicillium crustosum | PET films | TPA | [59] |
| Lipase | Penicillium simplicissimum | Post-consumer (PC)-PET | TPA, MHET and BHET | [60] |
| Cutinase | Fusarium solani | PET waste | 90% conversion into monomers | [61] |
| NR | Aspergillus sp. | Waste Plastic bottles | 22% weight loss after 6 weeks | [62] |
| Cutinase | Fusarium solani | Synthetic PET | EG | [63] |
| Cutinase | Fusarium oxysporum | PET woven fabric | TPA, MHET and BHET | [64] |
| Cutinase | Fusarium oxysporum | PET fabrics | NR | [65] |
| Cutinase | Humicola insolens | PET bottles | TPA, MHET and BHET | [66] |
| Lipase | Candida antarctica | PET bottles | TPA, MHET and BHET | [66] |
| Lipase | Candida rugosa | PET film | NR | [67] |
| Hydrolase | Penicillium funiculosum | PET film | 0.21% weight loss | [68] |
| Lipase | Thermomyces lanuginosus | PET fabrics and films | TPA, BHET, MHET | [69] |
| Cutinase | Fusarium solani | PET fabrics and films | TPA, BHET, MHET, | [69] |
| Cutinase | Humilica insolens | NR | TPA, EG | [70] |
| Cutinase | Fusarium solani | NR | TPA, EG | [70] |
| Cutinase | Fusarium solani | PET fabrics | NR | [71] |
| Cutinase | Fusarium solani | PET fabrics | TPA | [72] |
| Polyesterase | Penicillium citrinum | PET pellets/fabrics | TPA, MHET, BHET and BA | [73] |
| Hydrolase | Fusarium oxysporum LCH I | PET fibers | TPA | [74] |
| Hydrolase | Fusarium solani | PET fibers | TPA | [74] |
| Hydrolase | Fusarium solani | Modified PET fabrics | NR | [75] |
| Cutinase | Fusarium solani | PET film | MHET | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmaditabatabaei, S.; Kyazze, G.; Iqbal, H.M.N.; Keshavarz, T. Fungal Enzymes as Catalytic Tools for Polyethylene Terephthalate (PET) Degradation. J. Fungi 2021, 7, 931. https://doi.org/10.3390/jof7110931
Ahmaditabatabaei S, Kyazze G, Iqbal HMN, Keshavarz T. Fungal Enzymes as Catalytic Tools for Polyethylene Terephthalate (PET) Degradation. Journal of Fungi. 2021; 7(11):931. https://doi.org/10.3390/jof7110931
Chicago/Turabian StyleAhmaditabatabaei, Seyedehazita, Godfrey Kyazze, Hafiz M. N. Iqbal, and Tajalli Keshavarz. 2021. "Fungal Enzymes as Catalytic Tools for Polyethylene Terephthalate (PET) Degradation" Journal of Fungi 7, no. 11: 931. https://doi.org/10.3390/jof7110931
APA StyleAhmaditabatabaei, S., Kyazze, G., Iqbal, H. M. N., & Keshavarz, T. (2021). Fungal Enzymes as Catalytic Tools for Polyethylene Terephthalate (PET) Degradation. Journal of Fungi, 7(11), 931. https://doi.org/10.3390/jof7110931








