Detection of Cytokines and Collectins in Bronchoalveolar Fluid Samples of Patients Infected with Histoplasma capsulatum and Pneumocystis jirovecii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Bronchoalveolar Lavage (BAL) Samples
2.3. Compliance with Ethical Standards
2.4. Collectin Determination
2.5. Cytokine Determination
2.6. Statistical Analyses
3. Results
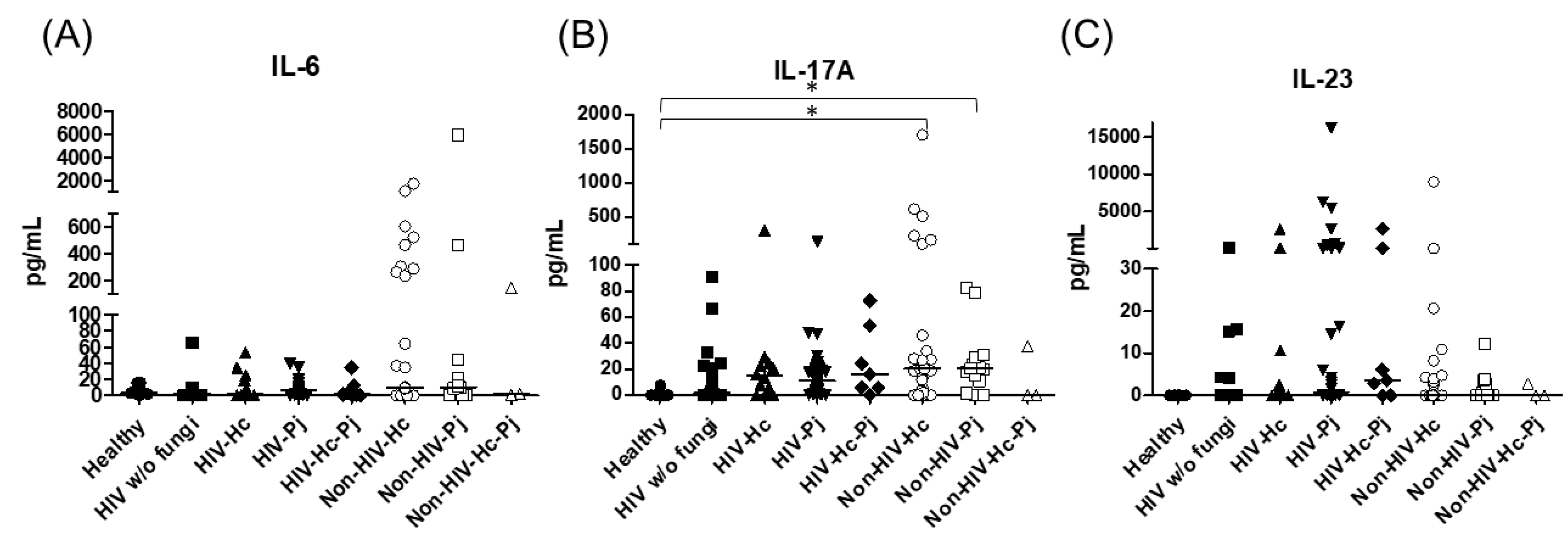
3.1. Surfactant Protein and Cytokine Analyses of All Groups Studied
3.2. Surfactant Protein and Cytokine Analyses Associated with H. capsulatum Infection
3.3. Surfactant Protein and Cytokine Analyses Associated with P. jirovecii Infection
3.4. Surfactant Protein and Cytokine Analyses Associated with the Co-Infection of Both Pathogens
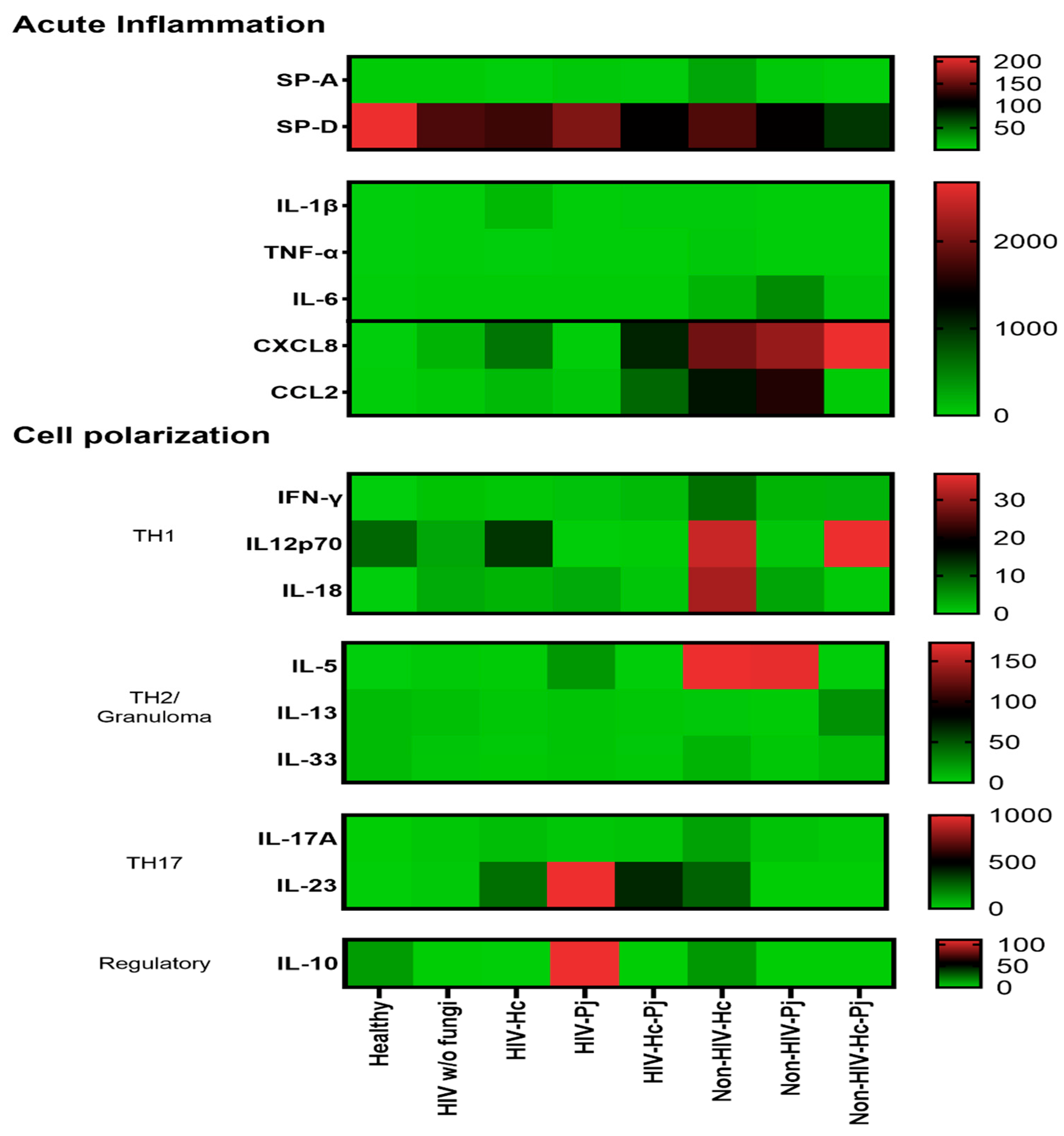
3.5. Surfactant Protein and Cytokine Patterns Using a Heatmap Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-González, A.E.; Aliouat-Denis, C.M.; Ramírez-Bárcenas, J.A.; Demanche, C.; Pottier, M.; Carreto-Binaghi, L.E.; Akbar, H.; Derouiche, S.; Chabé, M.; Aliouat, E.M.; et al. Histoplasma capsulatum and Pneumocystis spp. co-infection in wild bats from Argentina, French Guyana, and Mexico. BMC Microbiol. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veloso, S.S.C.; Ferreiro, L.; Pacheco, S.M.; da Silva, R.R.P.; De Conceição Souza, E.; Machado, G.; Wissmann, G.; Spanamberg, A.; Sanches, E.M.C. Pneumocystis spp. and Histoplasma capsulatum in bats lungs in southern and midwestern regions of Brazil. Acta Sci. Vet. 2014, 42, 1252. (In Portuguese) [Google Scholar]
- Velásquez, G.; Rueda, Z.V.; Vélez, L.A.; Aguirre, D.A.; Gómez-Arias, R.D. Histoplasmosis in AIDS patients. A cohort study in Medellín, Colombia. Infectio 2010, 14, S99–S106. (In Spanish) [Google Scholar]
- Wheat, L.J.; Slama, T.G.; Zeckel, M.L. Histoplasmosis in the acquired immune deficiency syndrome. Am. J. Med. 1985, 78, 203–210. [Google Scholar] [CrossRef]
- Baughman, R.P.; Dohn, M.N.; Frame, P.T. The continuing utility of bronchoalveolar lavage to diagnose opportunistic infection in AIDS patients. Am. J. Med. 1994, 97, 515–522. [Google Scholar] [CrossRef]
- Huber, F.; Nacher, M.; Aznar, C.; Pierre-Demar, M.; El Guedj, M.; Vaz, T.; Vantilcke, V.; Mahamat, A.; Magnien, C.; Chauvet, E.; et al. AIDS-related Histoplasma capsulatum var. capsulatum infection: 25 years’ experience of French Guiana. AIDS 2008, 22, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, S.; Damiani, C.; Virmaux, M.; Schmit, J.L.; Totet, A.; Nevez, G. Photo quiz: A 39-year-old man with human immunodeficiency virus infection presenting with an alveolo-interstitial pulmonary syndrome. J. Clin. Microbiol. 2013, 51, 2809–3165. [Google Scholar] [CrossRef] [Green Version]
- Gago, S.; Esteban, C.; Valero, C.; Zaragoza, O.; Puig de la Bellacasa, J.; Buitrago, M.J. A multiplex real-time PCR assay for identification of Pneumocystis jirovecii, Histoplasma capsulatum, and Cryptococcus neoformans/Cryptococcus gattii in samples from AIDS patients with opportunistic pneumonia. J. Clin. Microbiol. 2014, 52, 1168–1176. [Google Scholar] [CrossRef] [Green Version]
- Carreto-Binaghi, L.E.; Morales-Villarreal, F.R.; García-de la Torre, G.; Vite-Garín, T.; Ramirez, J.A.; Aliouat, E.M.; Martínez-Orozco, J.A.; Taylor, M.L. Histoplasma capsulatum and Pneumocystis jirovecii coinfection in hospitalized HIV and non-HIV patients from a tertiary care hospital in Mexico. Int. J. Infect. Dis. 2019, 86, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AIDS-Defining Conditions. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5710a2.html (accessed on 15 April 2021).
- Tobón, A.M.; Gómez, B.L. Pulmonary Histoplasmosis. Mycopathologia 2021, 186, 697–705. [Google Scholar] [CrossRef]
- Bateman, M.; Oladele, R.; Kolls, J.K. Diagnosing Pneumocystis jirovecii pneumonia: A review of current methods and novel approaches. Med. Mycol. 2020, 58, 1015–1028. [Google Scholar] [CrossRef]
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. N. Am. 2016, 30, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Grasland, A.; Pouchot, J.; Michon, C.; Hertig, A.; Simonpoli, A.M.; Vinceneux, P. Extrapulmonary and disseminated pneumocystosis in AIDS. A review of the literature. Ann. Med. Interne 1997, 148, 177–183. (In French) [Google Scholar]
- Salzer, H.J.F.; Schäfer, G.; Hoenigl, M.; Günther, G.; Hoffmann, C.; Kalsdorf, B.; Alanio, A.; Lange, C. Clinical, diagnostic, and treatment disparities between HIV-infected and non-HIV-infected immunocompromised patients with Pneumocystis jirovecii pneumonia. Respiration 2018, 96, 52–65. [Google Scholar] [CrossRef]
- Tewari, R.; Wheat, L.J.; Ajello, L. Agents of histoplasmosis. In Medical Mycology, Topley & Wilson’s Microbiology and Microbial Infections, 1st ed.; Ajello, L., Hay, R.J., Eds.; Arnold and Oxford University Press: New York, NY, USA, 1998; pp. 373–407. [Google Scholar]
- Akbar, H.; Pinçon, C.; Aliouat-Denis, C.M.; Derouiche, S.; Taylor, M.L.; Pottier, M.; Carreto-Binaghi, L.E.; González-González, A.E.; Courpon, A.; Barriel, V.; et al. Characterizing Pneumocystis in the lungs of bats: Understanding Pneumocystis evolution and the spread of Pneumocystis organisms in mammal populations. Appl. Environ. Microbiol. 2012, 78, 8122–8136. [Google Scholar] [CrossRef] [Green Version]
- Stringer, J.R.; Beard, C.B.; Miller, R.F.; Wakefield, A.E. A new name for Pneumocystis from humans and new perspectives on the host-pathogen relationship. Emerg. Infect. Dis. 2002, 8, 891–896. [Google Scholar] [CrossRef]
- Hauser, P.M.; Cushion, M.T. Is sex necessary for the proliferation and transmission of Pneumocystis? PLoS Pathog. 2018, 14, e1007409. [Google Scholar] [CrossRef]
- Dei-Cas, E.; Jackson, H.; Palluault, F.; Aliouat, E.M.; Hancock, V.; Soulez, B.; Camus, D. Ultrastructural observations on the attachment of Pneumocystis carinii in vitro. J. Protozool. 1991, 38, 205S–207S. [Google Scholar]
- Johansson, J.; Curstedt, T.; Robertson, B. The proteins of the surfactant system. Eur. Respir. J. 1994, 7, 372–391. [Google Scholar] [CrossRef] [Green Version]
- Chroneos, Z.C.; Sever-Chroneos, Z.; Shepherd, V.L. Pulmonary surfactant: An immunological perspective. Cell Physiol. Biochem. 2010, 25, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Hawgood, S.; Clements, J.A. Pulmonary surfactant and its apoproteins. J. Clin. Investig. 1990, 86, 1–6. [Google Scholar] [CrossRef] [Green Version]
- McCormack, F.X.; Gibbons, R.; Ward, S.R.; Kuzmenko, A.; Wu, H.; Deepe, G.S., Jr. Macrophage-independent fungicidal action of the pulmonary collectins. J. Biol. Chem. 2003, 278, 36250–36256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, P.E.; Voelker, D.R.; McCormack, F.X.; Paulsrud, J.R.; Martin, W.J. 120-kD surface glycoprotein of Pneumocystis carinii is a ligand for surfactant protein A. J. Clin. Investig. 1992, 89, 143–149. [Google Scholar] [CrossRef]
- Vuk-Pavlovic, Z.; Standing, J.E.; Crouch, E.C.; Limper, A.H. Carbohydrate recognition domain of surfactant protein D mediates interactions with Pneumocystis carinii glycoprotein A. Am. J. Respir. Cell. Mol. Biol. 2001, 24, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Phelps, D.S.; Umstead, T.M.; Rose, R.M.; Fishman, J.A. Surfactant protein-A levels increase during Pneumocystis carinii pneumonia in the rat. Eur. Respir. J. 1996, 9, 565–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, J.; He, L.; Rong, Z.; Pan, J.; Chen, X.; Morrison, D.C.; Li, X. Alteration of surfactant proteins A and D in bronchoalveolar lavage fluid of Pneumocystis carinii pneumonia. Chin. Med. J. 2001, 114, 1143–1146. [Google Scholar]
- Suárez-Álvarez, R.O.; Sahaza, J.H.; Berzunza-Cruz, M.; Becker, I.; Curiel-Quesada, E.; Pérez-Torres, A.; Reyes-Montes, M.D.R.; Taylor, M.L. Dimorphism and dissemination of Histoplasma capsulatum in the upper respiratory tract after intranasal infection of bats and mice with mycelial propagules. Am. J. Trop. Med. Hyg. 2019, 101, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Inglis, D.O.; Berkes, C.A.; Hocking Murray, D.R.; Sil, A. Conidia but not yeast cells of the fungal pathogen Histoplasma capsulatum trigger a type I interferon innate immune response in murine macrophages. Infect. Immun. 2010, 78, 3871–3882. [Google Scholar] [CrossRef] [Green Version]
- Van Prooyen, N.; Henderson, C.A.; Hocking Murray, D.; Sil, A. CD103+ conventional dendritic cells are critical for TLR7/9-dependent host defense against Histoplasma capsulatum, an endemic fungal pathogen of humans. PLoS Pathog. 2016, 12, e1005749. [Google Scholar] [CrossRef]
- Kroetz, D.N.; Deepe, G.S., Jr. CCR5 dictates the equilibrium of proinflammatory IL-17+ and regulatory Foxp3+ T cells in fungal infection. J. Immunol. 2010, 184, 5224–5231. [Google Scholar] [CrossRef] [Green Version]
- Allendörfer, R.; Brunner, G.D.; Deepe, G.S., Jr. Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J. Immunol. 1999, 162, 7389–7396. [Google Scholar]
- Clemons, K.V.; Darbonne, W.C.; Curnutte, J.T.; Sobel, R.A.; Stevens, D.A. Experimental histoplasmosis in mice treated with anti-murine interferon-gamma antibody and in interferon-gamma gene knockout mice. Microbes Infect. 2000, 2, 997–1001. [Google Scholar] [CrossRef]
- Deepe, G.S., Jr.; Gibbons, R.S. Interleukins 17 and 23 influence the host response to Histoplasma capsulatum. J. Infect. Dis. 2009, 200, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, A.M.; Bullock, W.E.; Taylor, C.L.; Deepe, G.S., Jr. Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect. Immun. 1988, 56, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Deepe, G.S., Jr. Role of CD8+ T cells in host resistance to systemic infection with Histoplasma capsulatum in mice. J. Immunol. 1994, 152, 3491–3500. [Google Scholar]
- Pop, S.M.; Kolls, J.K.; Steele, C. Pneumocystis: Immune recognition and evasion. Int. J. Biochem. Cell. Biol. 2006, 38, 17–22. [Google Scholar] [CrossRef]
- Kelly, M.N.; Shellito, J.E. Current understanding of Pneumocystis immunology. Future Microbiol. 2010, 5, 43–65. [Google Scholar] [CrossRef] [Green Version]
- Hoving, J.C.; Kolls, J.K. New advances in understanding the host immune response to Pneumocystis. Curr. Opin. Microbiol. 2017, 40, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.A. The importance of the innate immune system in controlling HIV infection and disease. Trends Immunol. 2001, 22, 312–316. [Google Scholar] [CrossRef]
- Siegal, F.P.; Fitzgerald-Bocarsly, P.; Holland, B.K.; Shodell, M. Interferon-α generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. AIDS 2001, 15, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Meissner, N.; Rutkowski, M.; Harmsen, A.L.; Han, S.; Harmsen, A.G. Type I interferon signaling and B cells maintain hemopoiesis during Pneumocystis infection of the lung. J. Immunol. 2007, 178, 6604–6615. [Google Scholar] [CrossRef] [Green Version]
- Meissner, N.; Swain, S.; Tighe, M.; Harmsen, A.; Harmsen, A. Role of type I IFNs in pulmonary complications of Pneumocystis murina infection. J. Immunol. 2005, 174, 5462–5471. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.W.; Pryhuber, G.S.; Chess, P.R.; Wang, Z.; Notter, R.H.; Gigliotti, F. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J. Immunol. 2004, 172, 2511–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Havell, E.A.; Moldawer, L.L.; Mcintyre, K.W.; Chizzonite, R.A.; Harmsen, A.G. Interleukin 1: An important mediator of host resistance against Pneumocystis carinii. J. Exp. Med. 1992, 176, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Havell, E.A.; Gigliotti, F.; Harmsen, A.G. Interleukin-6 production in a murine model of Pneumocystis carinii pneumonia: Relation to resistance and inflammatory response. Infect. Immun. 1993, 61, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudner, X.L.; Happel, K.I.; Young, E.A.; Shellito, J.E. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 2007, 75, 3055–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, M.H.; Empey, K.M.; Garvy, B.A. Modulation of proinflammatory responses to Pneumocystis carinii f. sp. muris in neonatal mice by granulocyte-macrophage colony-stimulating factor and IL-4: Role of APCS. J. Immunol. 2005, 174, 441–448. [Google Scholar] [CrossRef]
- Mcallister, F.; Steele, C.; Zheng, M.; Shellito, J.E.; Kolls, J.K. In vitro effector activity of Pneumocystis murina-specific T-cytotoxic-1 CD8+ T cells: Role of granulocyte-macrophage colony-stimulating factor. Infect. Immun. 2005, 73, 7450–7457. [Google Scholar] [CrossRef] [Green Version]
- Ruan, S.; Mckinley, L.; Zheng, M.; Rudner, X.; D’Souza, A.; Kolls, J.K.; Shellito, J.E. Interleukin-12 and host defense against murine Pneumocystis pneumonia. Infect. Immun. 2008, 76, 2130–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, S.; Tate, C.; Lee, J.J.; Ritter, T.; Kolls, J.K.; Shellito, J.E. Local delivery of the viral interleukin-10 gene suppresses tissue inflammation in murine Pneumocystis carinii infection. Infect. Immun. 2002, 70, 6107–6113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, M.H.; Harmsen, A.G.; Garvy, B.A. IL-10 modulates host responses and lung damage induced by Pneumocystis carinii infection. J. Immunol. 2003, 170, 1002–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Horwath, M.C.; Fecher, R.A.; Deepe, G.S., Jr. Histoplasma capsulatum, lung infection and immunity. Future Microbiol. 2015, 10, 967–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddens, T.; Kolls, J.K. Pathological and protective immunity to Pneumocystis infection. Semin. Immunopathol. 2015, 37, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atochina, E.N.; Beck, J.M.; Scanlon, S.T.; Preston, A.M.; Beers, M.F. Pneumocystis carinii pneumonia alters expression and distribution of lung collectins SP-A and SP-D. J. Lab. Clin. Med. 2001, 137, 429–439. [Google Scholar] [CrossRef]
- Schmidt, R.; Markart, P.; Ruppert, C.; Temmesfeld, B.; Nass, R.; Lohmeyer, J.; Seeger, W.; Günther, A. Pulmonary surfactant in patients with Pneumocystis pneumonia and acquired immunodeficiency syndrome. Crit. Care Med. 2006, 34, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Jambo, K.C.; French, N.; Zijlstra, E.; Gordon, S.B. AIDS patients have increased surfactant protein D but normal mannose binding lectin levels in lung fluid. Respir. Res. 2007, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botas, C.; Poulain, F.; Akiyama, J.; Brown, C.; Allen, L.; Goerke, J.; Clements, J.; Carlson, E.; Gillespie, A.M.; Epstein, C.; et al. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc. Natl. Acad. Sci. USA 1998, 95, 11869–11874. [Google Scholar] [CrossRef] [Green Version]
- Haczku, A. Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J. Allergy Clin. Immunol. 2008, 122, 861–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinkerton, J.W.; Kim, R.Y.; Robertson, A.A.B.; Hirota, J.A.; Wood, L.G.; Knight, D.A.; Cooper, M.A.; O’Neill, L.A.J.; Horvat, J.C.; Hansbro, P.M. Inflammasomes in the lung. Mol. Immunol. 2017, 86, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Kroetz, D.N.; Deepe, G.S., Jr. The role of cytokines and chemokines in Histoplasma capsulatum infection. Cytokine 2012, 58, 112–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepe, G.S., Jr.; McGuinness, M. Interleukin-1 and host control of pulmonary histoplasmosis. J. Infect. Dis. 2006, 194, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Deepe, G.S., Jr.; Gibbons, R.S. T cells require tumor necrosis factor-alpha to provide protective immunity in mice infected with Histoplasma capsulatum. J. Infect. Dis. 2006, 193, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Allendörfer, R.; Deepe, G.S., Jr. Blockade of endogenous TNF-alpha exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 1998, 160, 6072–6082. [Google Scholar]
- Jensen, B.N.; Lisse, I.M.; Gerstoft, J.; Borgeskov, S.; Skinhøj, P. Cellular profiles in bronchoalveolar lavage fluid of HIV-infected patients with pulmonary symptoms: Relation to diagnosis and prognosis. AIDS 1991, 5, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.R.; Hashimoto, C.H.; Dickman, P.S.; Foutty, L.F.; Cobb, C.J. Prognostic implications of bronchoalveolar lavage neutrophilia in patients with Pneumocystis carinii pneumonia and AIDS. Am. Rev. Respir. Dis. 1989, 149, 1336–1342. [Google Scholar] [CrossRef]
- Paolini, R.; Bernardini, G.; Molfetta, R.; Santoni, A. NK cells and interferons. Cytokine Growth Factor Rev. 2015, 26, 113–120. [Google Scholar] [CrossRef]
- Sahaza, J.H.; Suárez-Alvarez, R.; Estrada-Bárcenas, D.A.; Pérez-Torres, A.; Taylor, M.L. Profile of cytokines in the lungs of BALB/c mice after intra-nasal infection with Histoplasma capsulatum mycelial propagules. Comp. Immunol. Microbiol. Infect. Dis. 2015, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pottratz, S.T.; Reese, S.; Sheldon, J.L. Pneumocystis carinii induces interleukin 6 production by an alveolar epithelial cell line. Eur. J. Clin. Investig. 1998, 28, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Holmer, S.M.; Evans, K.S.; Asfaw, Y.G.; Saini, D.; Schell, W.A.; Ledford, J.G.; Frothingham, R.; Wright, J.R.; Sempowski, G.D.; Perfect, J.R. Impact of surfactant protein D, interleukin-5, and eosinophilia on cryptococcosis. Infect. Immun. 2014, 82, 683–693. [Google Scholar] [CrossRef] [Green Version]
- Haczku, A.; Cao, Y.; Vass, G.; Kierstein, S.; Nath, P.; Atochina-Vasserman, E.N.; Scanlon, S.T.; Li, L.; Griswold, D.E.; Chung, K.F.; et al. IL-4 and IL-13 form a negative feedback circuit with surfactant protein-D in the allergic airway response. J. Immunol. 2006, 176, 3557–3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Kroetz, D.N.; Tweedle, J.L.; Deepe, G.S., Jr. Type II cytokines impair host defense against an intracellular fungal pathogen by amplifying macrophage generation of IL-33. Mucosal. Immunol. 2015, 8, 380–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heninger, E.; Hogan, L.H.; Karman, J.; Macvilay, S.; Hill, B.; Woods, J.P.; Sandor, M. Characterization of the Histoplasma capsulatum-induced granuloma. J. Immunol. 2006, 177, 3303–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.L.; Algayed, I.A.; Yogev, R.; Chou, P.M.; Scholl, P.R.; Pachman, L.M. Atypical Pneumocystis carinii pneumonia in a child with hyper-IgM syndrome. Pediatr. Pathol. Lab. Med. 1998, 18, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Leroy, X.; Copin, M.C.; Ramon, P.; Jouet, J.P.; Gosselin, B. Nodular granulomatous Pneumocystis carinii pneumonia in a bone marrow transplant recipient. Case report. APMIS 2000, 108, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Oki, Y.; Kami, M.; Kishi, Y.; Ueyama, J.I.; Honma, S.; Sugiyama, T.; Miyakoshi, S.; Kanda, Y.; Morinaga, S.I.; Muto, Y. Pneumocystis carinii pneumonia with an atypical granulomatous response in a patient with chronic lymphocytic leukemia. Leuk. Lymphoma 2001, 41, 435–438. [Google Scholar] [CrossRef]
- Gal, A.A.; Plummer, A.L.; Langston, A.A.; Mansour, K.A. Granulomatous Pneumocystis carinii pneumonia complicating hematopoietic cell transplantation. Pathol. Res. Pract. 2002, 198, 553–558, discussion 559–561. [Google Scholar] [CrossRef]
- Lauffer, L.; Kini, J.A.; Costello, P.; Godleski, J. Granulomatous Pneumocystis carinii pneumonia in a non-AIDS patient: An atypical presentation. J. Thorac. Imaging 2004, 19, 196–199. [Google Scholar] [CrossRef]
- Fang, J.; Viksman, M.Y.; Ebisawa, M.; Bochner, B.S. Increased circulating levels of interleukin-5 in a case of steroid-resistant hypereosinophilic syndrome with ileal involvement. Longcope Firm of the Osler Housestaff. J. Allergy Clin. Immunol. 1994, 94, 129–131. [Google Scholar] [CrossRef]
- Eddens, T.; Elsegeiny, W.; Nelson, M.P.; Horne, W.; Campfield, B.T.; Steele, C.; Kolls, J.K. Eosinophils contribute to early clearance of Pneumocystis murina infection. J. Immunol. 2015, 195, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Ramirez, H.G.; Soto-Dominguez, A.; González, G.M.; Barboza-Quintana, O.; Salinas-Carmona, M.C.; Ceceñas-Falcon, L.A.; Montes-de-Oca-Luna, R.; Arce-Mendoza, A.Y.; Rosas-Taraco, A.G. Inflammatory and anti-inflammatory responses co-exist inside lung granuloma of fatal cases of coccidioidomycosis: A pilot report. Mycopathologia 2018, 183, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Fecher, R.A.; Horwath, M.C.; Friedrich, D.; Rupp, J.; Deepe, G.S., Jr. Inverse correlation between IL-10 and HIF-1α in macrophages infected with Histoplasma capsulatum. J. Immunol. 2016, 197, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Benfield, T.L.; Lundgren, B.; Shelhamer, J.H.; Lundgren, J.D. Pneumocystis carinii major surface glycoprotein induces interleukin-8 and monocyte chemoattractant protein-1 release from a human alveolar epithelial cell line. Eur. J. Clin. Investig. 1999, 29, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, W.A.; Deepe, G.S., Jr. The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J. Immunol. 2009, 183, 1964–1974. [Google Scholar] [CrossRef] [Green Version]
- Gorski, S.A.; Lawrence, M.G.; Hinkelman, A.; Spano, M.M.; Steinke, J.W.; Borish, L.; Teague, W.G.; Braciale, T.J. Expression of IL-5 receptor alpha by murine and human lung neutrophils. PLoS ONE 2019, 15, e02211-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Group | Number of Individuals |
|---|---|
| Healthy volunteers (Healthy, control) | 8 |
| HIV-positive without the studied fungi (HIV w/o fungi, control) | 19 |
| HIV-positive with histoplasmosis (HIV-Hc) | 12 |
| HIV-positive with pneumocystosis (HIV-Pj) | 32 |
| HIV-positive with co-infection (HIV-Hc-Pj) | 7 |
| HIV-negative with histoplasmosis (Non-HIV-Hc) | 35 |
| HIV-negative with pneumocystosis (Non-HIV-Pj) | 15 |
| HIV-negative with co-infection (Non-HIV-Hc-Pj) | 3 |
| TOTAL | 131 |
| Group of Individuals | SP-A | SP-D |
|---|---|---|
| Healthy, control | 1.654 (1.452–1.966) | 217.0 (147.4–284.8) |
| HIV w/o fungi, control | 1.593 (0–2.243) | 140.3 (53.91–228.2) |
| HIV-Hc | 0 (0–0.4048) | 139.6 (48.28–192.9) |
| HIV-Pj | 2.077 (1.538–3.505) | 87.01 (46.15–196.2) |
| HIV-Hc-Pj | 2.105 (1.351–3.602) | 123.0 (35.95–147.5) |
| Non-HIV-Hc | 2.425 (0–3.785) | 79.96 (58.36–253.9) |
| Non-HIV-Pj | 2.889 (0–4.175) | 58.91 (47.04–219.7) |
| Non-HIV-Hc-Pj | 0 (0–1.859) | 0 (0–227.1) |
| Cytokines | Healthy (Control) | HIV w/o Fungi (Control) | HIV-Hc | HIV-Pj | HIV-Hc-Pj | Non-HIV-Hc | Non-HV-Pj | Non-HIV-Hc-Pj |
|---|---|---|---|---|---|---|---|---|
| IL-1β | Undetectable | 1.03 (0–2.58) | 2.90 (0–3.58) | 0 (0–0.15) | 2.56 (0–5.22) | 3.01 (0–8.74) | 0 (0–3.27) | 2.34 (0.74–15.53) |
| TNF-α | Undetectable | 0 (0–0) | Undetectable | 0 (0–0) | 0 (0–0.25) | 0 (0–1.09) | 0 (0–0) | 0.04 (0–14.18) |
| IFN-γ | Undetectable | 0 (0–0.99) | 0 (0–0) | 0 (0–0) | 0 (0–3.87) | 3.62 (0–3.82) | 3.66 (0–3.74) | 0 (0–7.49) |
| IL-12p70 | 0 (0–23.15) | 0 (0–0.01) | 0 (0–0.20) | 0 (0–0.03) | 0 (0–0.57) | 0 (0–0.20) | 0 (0–0.17) | 0.06 (0–110.50) |
| IL-18 | Undetectable | 0 (0–0.33) | 0 (0–0) | 0 (0–5.22) | 0 (0–1.62) | 0.55 (0–4.10) | 0.71 (0–8.12) | 0 (0–1.21) |
| IL-6 | 3.38 (0–12.80) | 2.02 (0–9.09) | 1.47 (0–23.70) | 6.80 (0–9.91) | 1.41 (0–12.54) | 9.13 (0.88–232.00) | 10.09 (0–21.07) | 1.77 (0–140.80) |
| IL-17 | 0.03 (0–0.64) | 1.80 (0–22.69) | 14.83 (1.53–24.25) | 11.25 (3.58–21.49) | 15.93 (5.71–53.56) | 20.39 (3.59–28.15) | 20.65 (10.91–28.87) | 0 (0–37.99) |
| IL-23 | Undetectable | 0 (0–4.11) | 0 (0–8.64) | 0.77 (0–54.78) | 3.61 (0–120.10) | 0 (0–3.18) | 0 (0–2.84) | 0.65 (0–2.79) |
| IL-33 | 7.80 (7.78–7.82) | 0.11 (0–7.81) | 0.04 (0–5.97) | 0.39(0–7.82) | 0.16 (0–7.77) | 0 (0–5.04) | 0 (0–0) | 0.36 (0–22.73) |
| IL-5 | Undetectable | 0 (0–0.09) | 0 (0–0) | 0 (0–0.95) | 0.03 (0–0.07) | 0.00 (0–2.31) | 3.68 (0–29.91) | 0 (0–0.08) |
| IL-13 | 7.80 (7.78–7.82) | 1.58 (1.12–7.83) | 1.59 (1.56–6.24) | 1.56 (0–7.82) | 1.53 (0.16–7.77) | 0.57 (0–1.58) | 0 (0–1.57) | 1.53 (0.36–74.67) |
| IL-10 | 0 (0–26.67) | 0 (0–0) | Undetectable | 0 (0–0) | 0 (0–0.12) | 0 (0–0) | 0 (0–0) | 0 (0–0.01) |
| CXCL8 | Undetectable | 2.29 (0–140.60) | 16.33 (0–699.90) | 1.94 (0–2.29) | 1.35 (0–319.40) | 101.20 (2.28–4018.00) | 2.40 (2.17–4749.00) | 916.60 (46.52–7072.00) |
| CCL2 | 4.47 (3.99–5.04) | 5.35 (0.79–13.73) | 6.47 (0.46–88.25) | 4.16 (0–9.94) | 6.69 (3.69–1700.00) | 9.03 (0–650.90) | 0 (0–118.10) | 11.35 (10.40–23.73) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreto-Binaghi, L.E.; Tenorio, E.P.; Morales-Villarreal, F.R.; Aliouat, E.M.; Zenteno, E.; Martínez-Orozco, J.-A.; Taylor, M.-L. Detection of Cytokines and Collectins in Bronchoalveolar Fluid Samples of Patients Infected with Histoplasma capsulatum and Pneumocystis jirovecii. J. Fungi 2021, 7, 938. https://doi.org/10.3390/jof7110938
Carreto-Binaghi LE, Tenorio EP, Morales-Villarreal FR, Aliouat EM, Zenteno E, Martínez-Orozco J-A, Taylor M-L. Detection of Cytokines and Collectins in Bronchoalveolar Fluid Samples of Patients Infected with Histoplasma capsulatum and Pneumocystis jirovecii. Journal of Fungi. 2021; 7(11):938. https://doi.org/10.3390/jof7110938
Chicago/Turabian StyleCarreto-Binaghi, Laura E., Eda P. Tenorio, Fernando R. Morales-Villarreal, El Moukhtar Aliouat, Edgar Zenteno, José-Arturo Martínez-Orozco, and Maria-Lucia Taylor. 2021. "Detection of Cytokines and Collectins in Bronchoalveolar Fluid Samples of Patients Infected with Histoplasma capsulatum and Pneumocystis jirovecii" Journal of Fungi 7, no. 11: 938. https://doi.org/10.3390/jof7110938
APA StyleCarreto-Binaghi, L. E., Tenorio, E. P., Morales-Villarreal, F. R., Aliouat, E. M., Zenteno, E., Martínez-Orozco, J.-A., & Taylor, M.-L. (2021). Detection of Cytokines and Collectins in Bronchoalveolar Fluid Samples of Patients Infected with Histoplasma capsulatum and Pneumocystis jirovecii. Journal of Fungi, 7(11), 938. https://doi.org/10.3390/jof7110938






