Comparative Transcriptomics and Gene Knockout Reveal Virulence Factors of Arthrinium phaeospermum in Bambusa pervariabilis × Dendrocalamopsis grandis
Abstract
1. Introduction
2. Materials and Methods
2.1. Meterials
2.2. Methods
2.2.1. Preparation of Transcriptome Sequencing Mycelia
2.2.2. Extraction and Quality Detection of A. phaeospermum RNA
2.2.3. cDNA Preparation and Illumina Sequencing
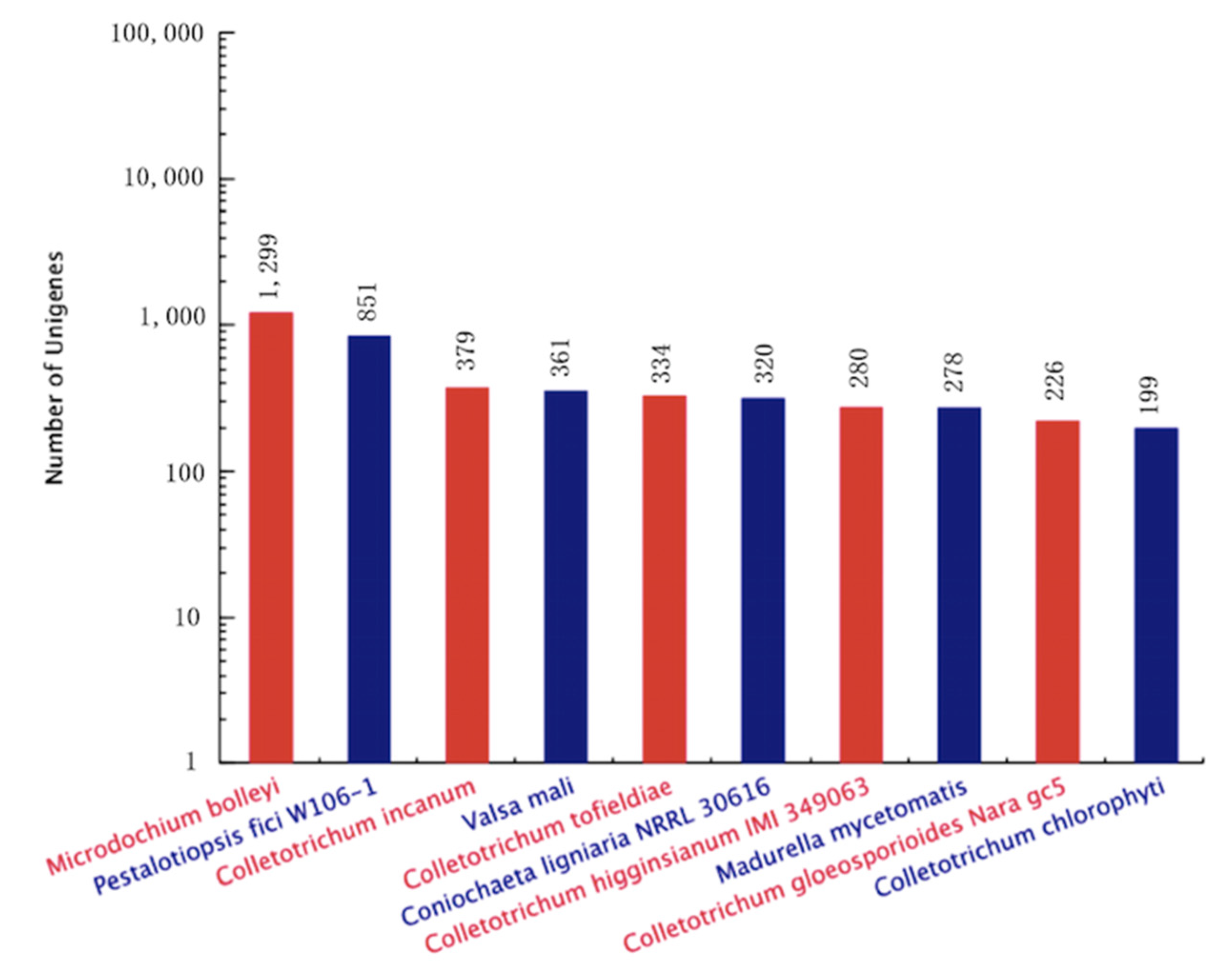
2.2.4. Sequence Data Filtering, De Novo Assembly and Contig Annotation
2.2.5. Analysis of Differentially Expressed Genes under Different Treatment Condition
2.2.6. Real-Time Fluorescence Quantitative PCR Verification
2.2.7. Screening of Antibiotic Types and Concentrations
2.2.8. Construction of Knockout Vector
2.2.9. PEG-Mediated Protoplast Transformation
2.2.10. Phenotype Analysis of Transformant
2.2.11. Pathogenicity Test
branches)/(total branches) × The most serious disease grade] × 100.
2.2.12. Complementation Test of Ctf1β Knockout
3. Results
3.1. Transcriptome Sequencing Results
3.1.1. Growth of Fungi in Different Plant Tissue Culture Condition
3.1.2. RNA Quality Detection
3.1.3. Sequencing Data and DEG Statistics
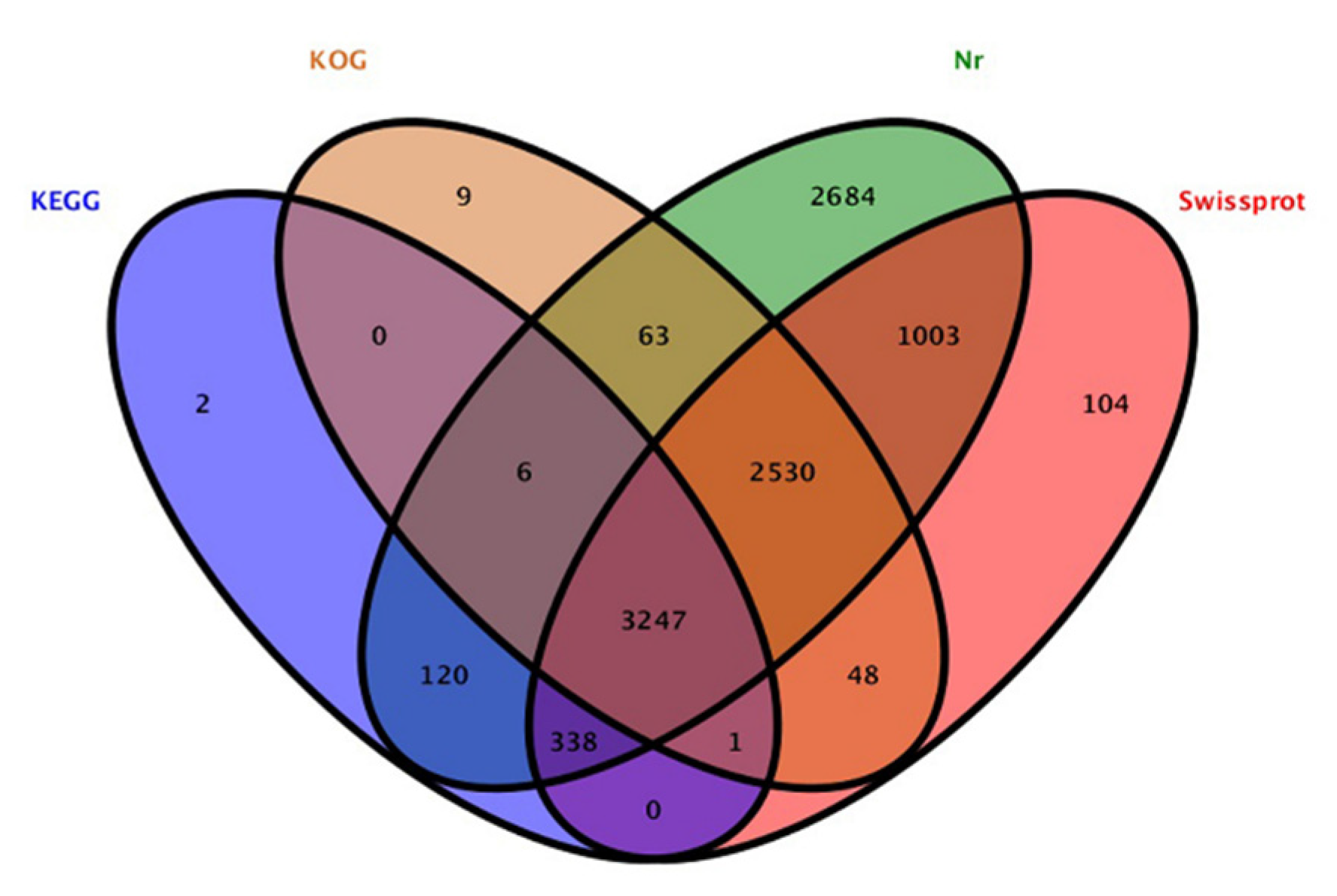
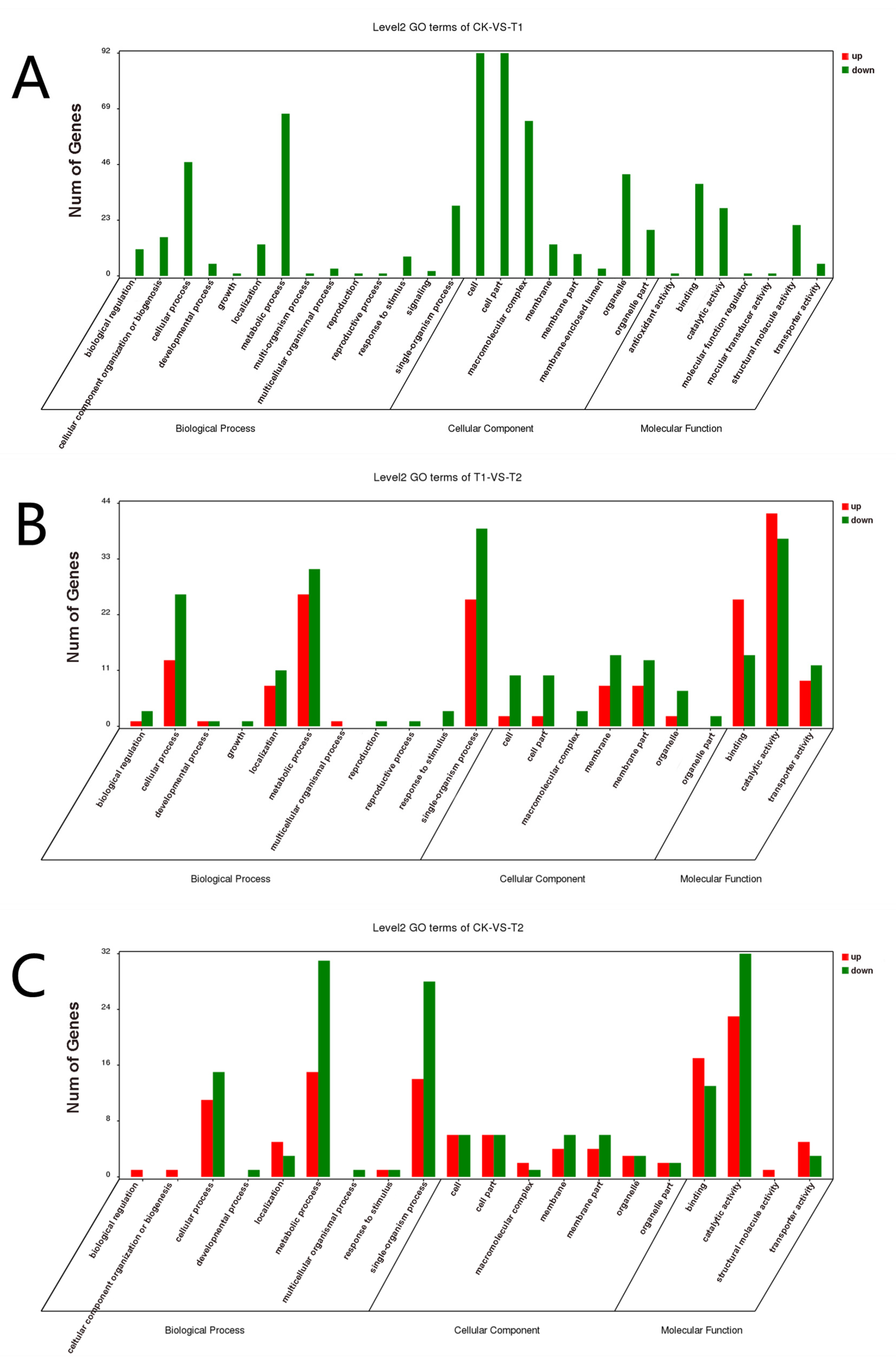
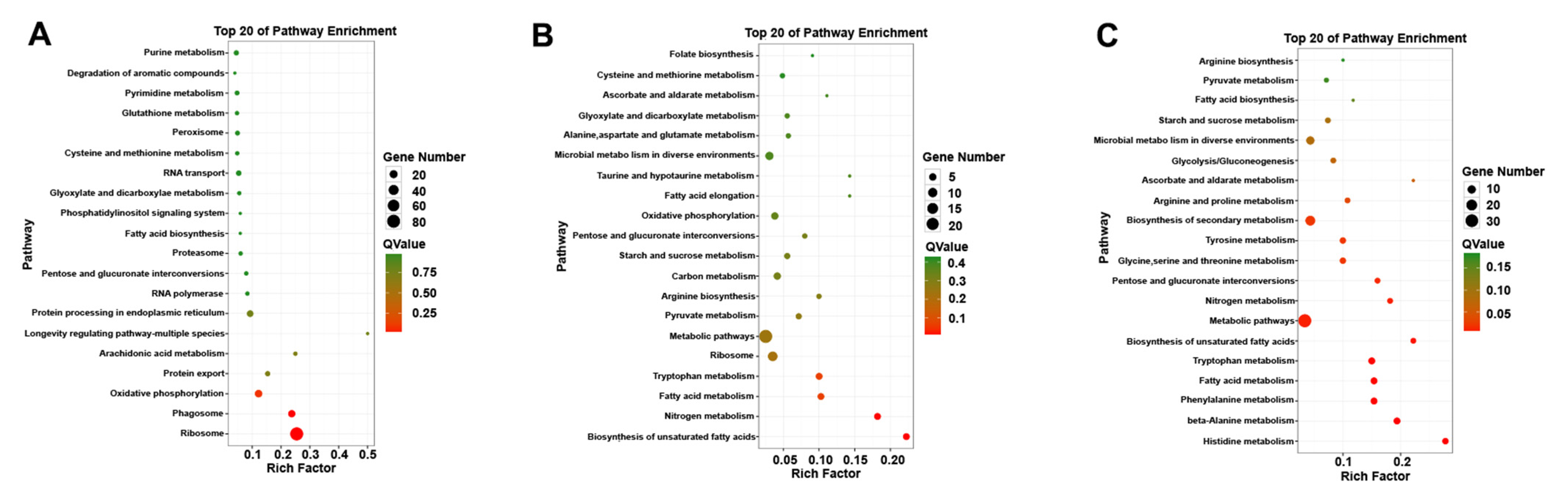
3.2. KEGG and GO Annotation Analysis of DEGs
3.3. Verification by qRT–PCR
3.4. Gene Function Verification Results
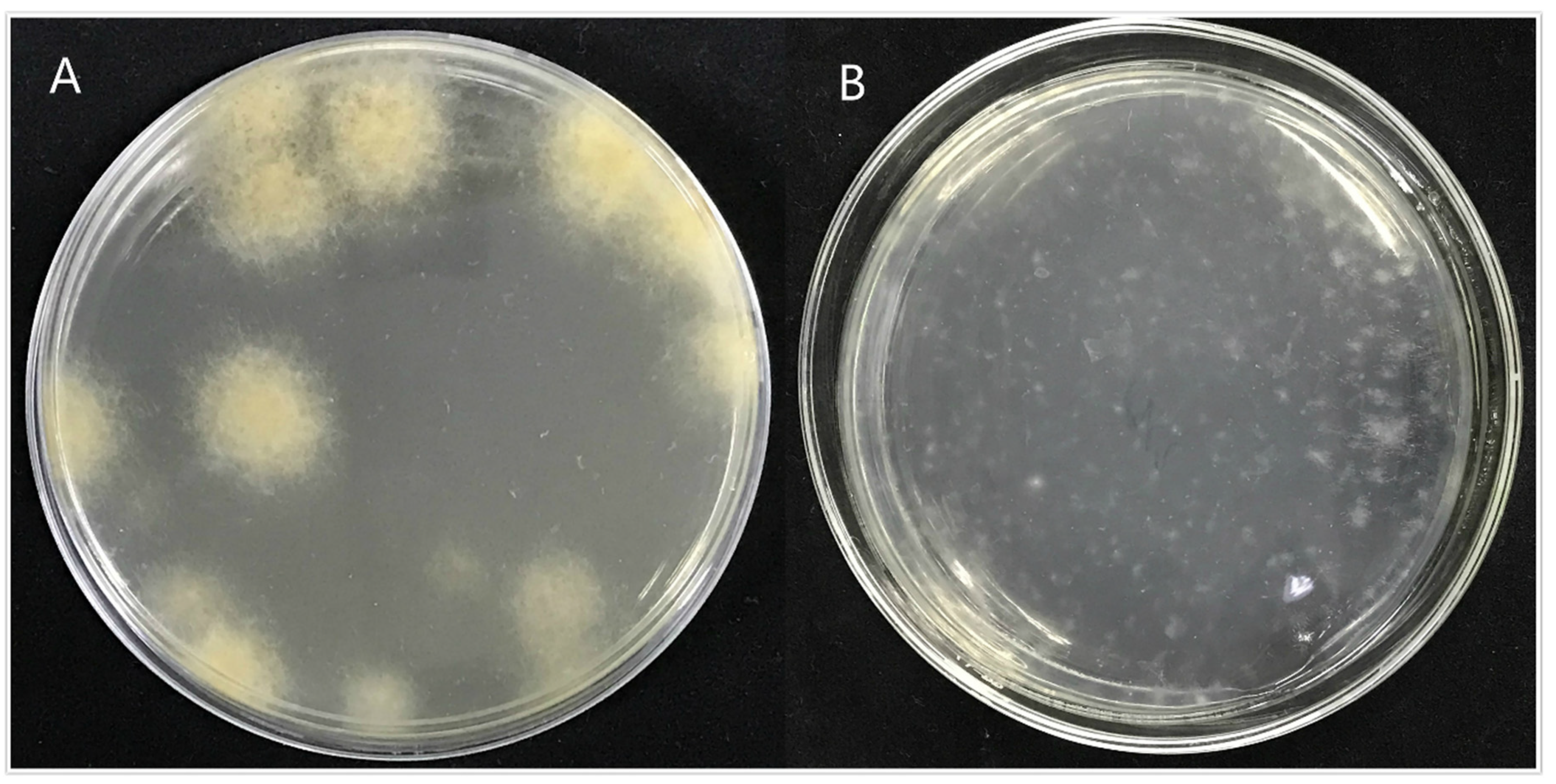
3.4.1. Construction of Knockout Vector and Genetic Transformation
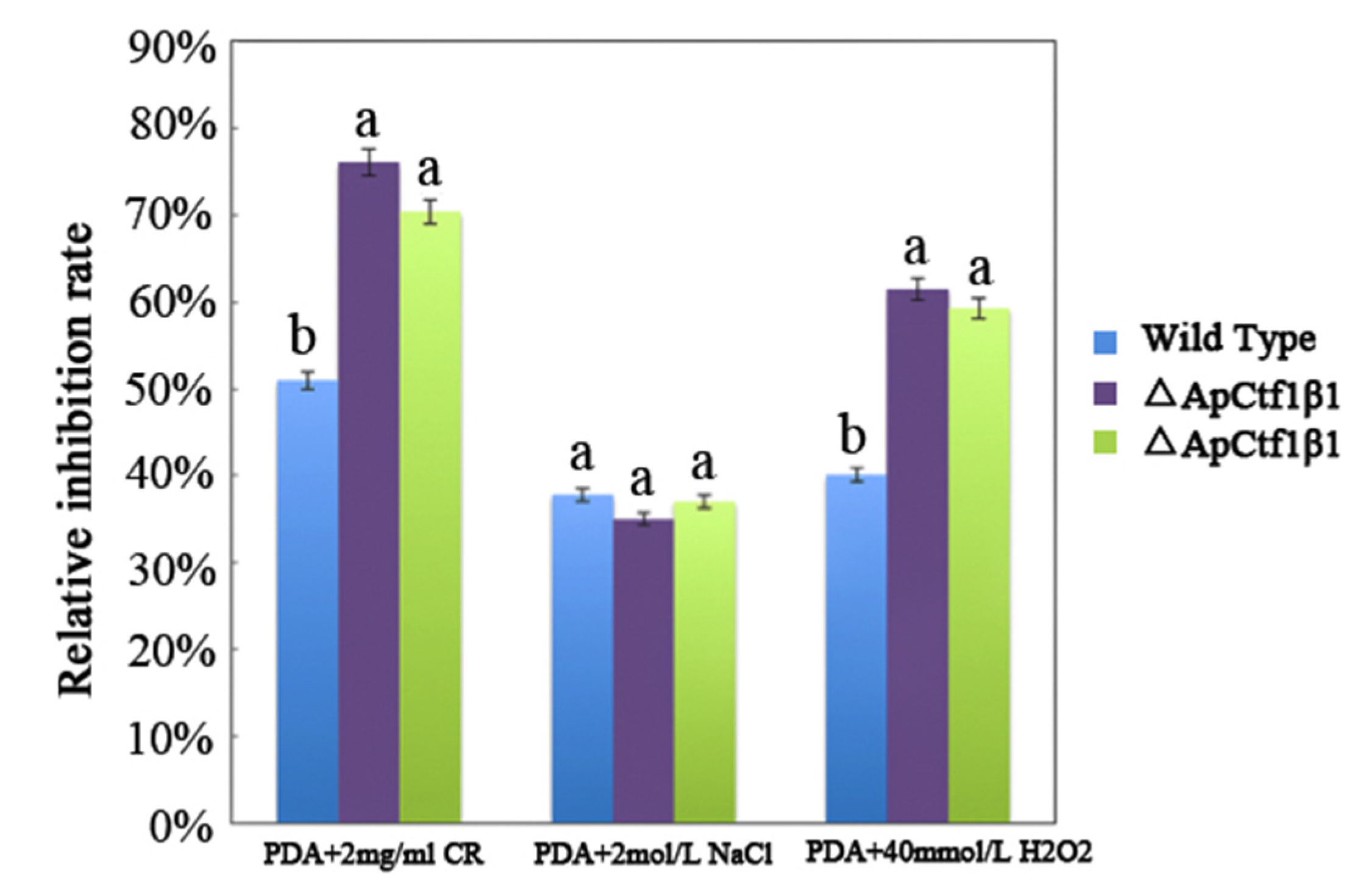
3.4.2. Phenotypic Analysis of Transformant
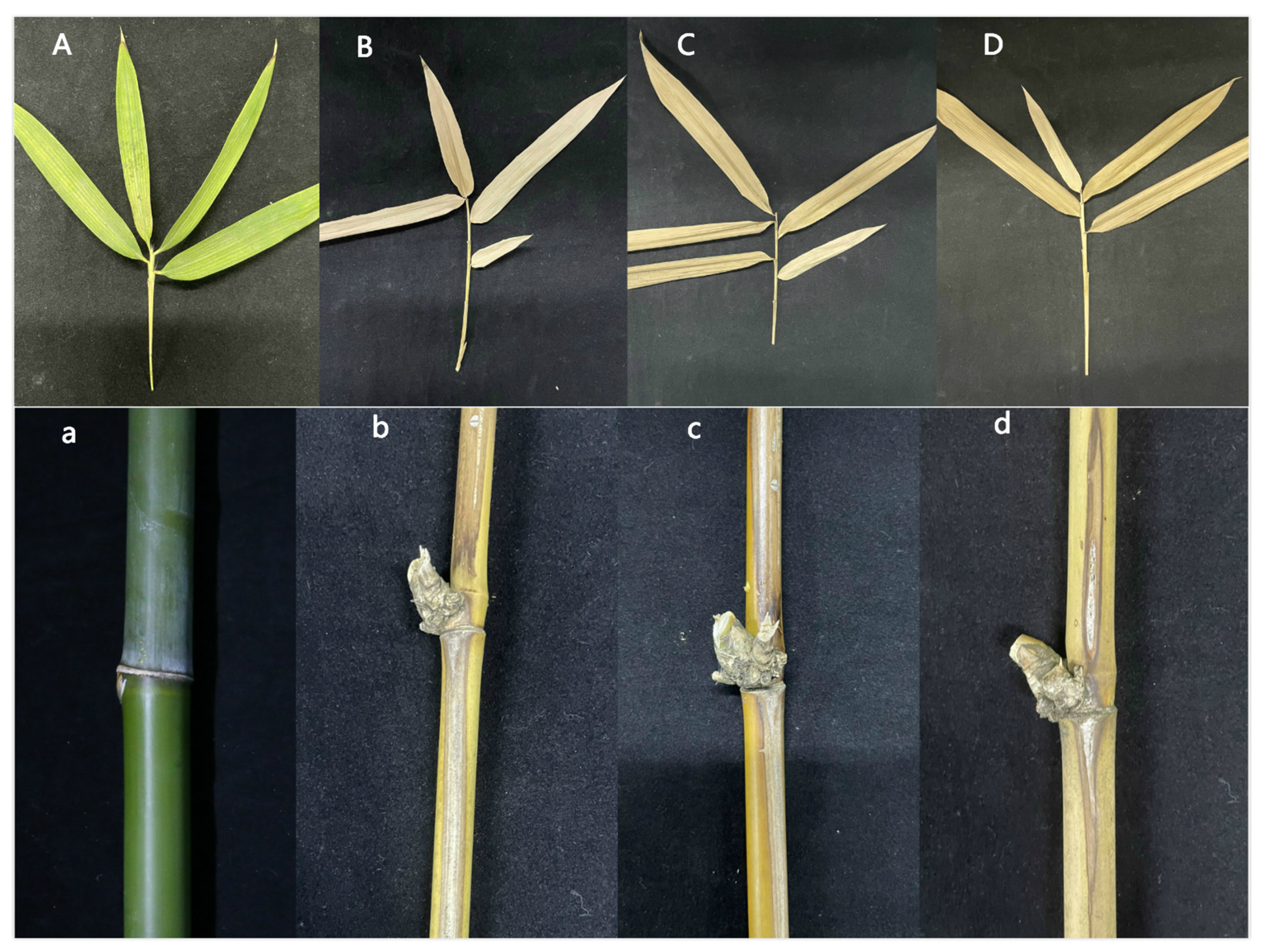
3.4.3. Pathogenicity Test of Transformant
3.4.4. The Results of Complementation Test of Ctf1β Knockout
3.5. Phenotypic Analysis and Pathogenicity Detection of Complementary Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Q. Research Status of B. pervariabilis × D. Daii and Cost-benefit analysis. J. Shandong For. Sci. Technol. 2007, 3, 101–103. [Google Scholar]
- Zhao, M.R.; Huang, J.D.; Yao, J.P.; Li, F.L.; Zhang, X.L. Introduction effect of Bambusa ervariabilis × Dendrocalamopsis daii in dry hot valley of Huaning. For. Investig. Plan. 2004, 29, 24–26. [Google Scholar]
- Li, S.J.; Tang, Y.W.; Fang, X.M.; Qiao, T.M.; Han, S.; Zhu, T.H. Whole-genome sequence of Arthrinium phaeospermum, a globally distributed pathogenic fungus. Genomics 2020, 112, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.H.; Huang, Z.C.; Gao, Q.Z. Pathogen and occurrence regularity of Bambusa ervariabilis × Dendrocalamopsis daii blight. Forest Pest Dis. 2009, 28, 10–12. [Google Scholar]
- Khan, K.R.; Sullia, S.B. Arthrinium phaeospermium var. indicum var. nov., a new market pathogen of cowpea, garden pea and french bean. Acta Bot. Indica 1980, 8, 103–104. [Google Scholar]
- Liu, X.J.; Luo, X.Y.; Li, X.F.; Chen, Q.T. Taxonomic determination of toxigenic Arthrinium strains isolated from poisoning sugarcane. Mycosystema 1988, 7, 221–225. [Google Scholar]
- Xia, L.M.; Zhang, S.X.; Huang, J.H.; Zhang, W.M. Studies on Arthrinium phaeospermum causing moso bamboo foot rot. J. Nanjing Fores. Univ. 1995, 19, 1–7. [Google Scholar]
- Ma, G.L.; Hu, G.L.; Yu, C.Z.; Wu, J.L.; Xu, B.C. Phyllostachys prominens plum shoot wilt pathogenic fungoid and its biological characteristics. Zhejiang For. Coll 2003, 20, 44–48. [Google Scholar]
- Piccolo, S.L.; Mondello, V.; Giambra, S.; Conigliaro, G.; Torta, L.; Burruano, S. Arthrinium phaeospermum, Phoma cladoniicola and Ulocladium consortiale, New Olive Pathogens in Italy. J. Phytopathol. 2014, 162, 258–263. [Google Scholar] [CrossRef]
- Li, B.J.; Liu, P.Q.; Jiang, Y.; Weng, Q.Y.; Chen, Q.H. First Report of Culm Rot Caused by Arthrinium phaeospermum on Phyllostachys viridis in China. Plant Dis. 2016, 100, 10–13. [Google Scholar] [CrossRef]
- Li, S.J.; Qiao, T.M.; Han, S.; Zhu, T.H.; Che, G.N. Effects of Protein Toxin from Arthrinium phaeospermum on Biophysical Characteristic and Respiration of Bambusa pervariabilis × Dendrocalamopsis grandis Mitochondria. Sci. Silvae Sin. 2014, 50, 119–125. [Google Scholar]
- Li, S.J.; Zhu, T.H. Purification of the toxin protein PC from Arthrinium phaeospermum and its effect on the defence enzymes of Bambusa pervariabilis × Dendrocalamopsis grandis varieties. For. Pathol. 2013, 44, 96–106. [Google Scholar] [CrossRef]
- Vijayakumar, E.K.S.; Roy, K.; Chatterjee, S.; Deshmukh, S.K.; Ganguli, B.N.; Fehlhaber, H.W.; Kogler, H. Arthrichitin. A new cell wall active metabolite from Arthrinium phaeospermum. J. Org. Chem. 1996, 61, 6591–6593. [Google Scholar] [CrossRef] [PubMed]
- Bloor, S. Arthrinic acid, a novel antifungal polyhydroxyacid from Arthrinium phaeospermum. J. Antibiot. 2008, 61, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Zhu, T.H. Purification of the toxic protein from Arthrinium phaeospermum and its pathogenicity. Sci. Silvae Sin. 2012, 48, 144–149. [Google Scholar] [CrossRef]
- Li, S.J.; Zhu, T.H.; Zhu, H.M.Y.; Liang, M.; Qiao, T.M.; Han, S.; Che, G. Purification of protein AP-Toxin from Arthrinium phaeospermum causing blight in Bambusa pervariabilis × Dendrocalamopisis grandis and its metabolic effects on four bamboo varieties. Phytopathology 2013, 103, 135–145. [Google Scholar] [CrossRef]
- Qiao, T.M.; Zhu, T.H.; Li, S.J. Colonization of Pseudomonas aeruginosa ZB27 and its control effect on hybrid bamboo blight. Acta Phytophylacica Sin. 2011, 38, 133–138. [Google Scholar] [CrossRef]
- Li, S.J.; Fang, X.M.; Han, S.; Zhu, T.H.; Zhu, H.M.Y. Differential proteome analysis of hybrid Bamboo (Bambusa pervariabilis × Dendrocalamopsis grandis) under fungal stress (Arthrinium phaeospermum). Sci. Rep. 2019, 9, 18681. [Google Scholar] [CrossRef]
- Peng, Q.; Fang, X.M.; Zong, X.Z.; He, Q.Q.; Zhu, T.H.; Han, S.; Li, S.J. Comparative transcriptome analysis of Bambusa pervariabilis× Dendrocalamopsis grandis against Arthrinium phaeospermum under protein AP-toxin induction. Gene 2020, 725, 144–160. [Google Scholar] [CrossRef]
- He, Q.Q.; Fang, X.M.; Zhu, T.H.; Han, S.; Zhu, H.M.Y.; Li, S.J. Differential Proteomics Based on TMT and PRM Reveal the Resistance Response of Bambusa pervariabilis × Dendrocalamopisis grandis Induced by AP-Toxin. Metabolites 2019, 9, 166. [Google Scholar] [CrossRef]
- Ranf, S. Sensing of molecular patterns through cell surface immune receptors. Curr. Opin. Plant Biol. 2007, 38, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Horvath, D.A.A.; Staskawicz, B. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dang, J.L. The Plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Mentlak, T.A.; Kombrink, A.; Shinya, T.; Ryder, L.S.; Otomo, I.; Saitoh, H.; Terauchi, R.; Nishizawa, Y.; Shibuya, N.; Thomma, B.P.H.J.; et al. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell 2012, 24, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shi, S.; Nasir, F. Comparative analysis of the root transcriptomes of cultivated and wild rice varieties in response to Magnaporthe oryzae infection revealed both common and species-specific pathogen responses. Rice 2018, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Windram, O.; Madhou, P.; McHattie, S. Arabidopsis defense against Botrytis cinerea: Chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell 2012, 24, 3530–3557. [Google Scholar] [CrossRef]
- Smith, J.E.; Mengesha, B.; Tang, H.; Mengiste, T.; Bluhm, B.H. Resistance to Botrytis cinerea in Solanum lycopersicoides involves widespread transcriptional reprogramming. BMC Genom. 2014, 15, 334. [Google Scholar] [CrossRef]
- Luo, Z.B.; Li, Y.J.; Mousa, J.; Bruner, S.; Zhang, Y.G.; Pei, Y.; Keyhani, N.O. B bmsn2 acts as a pH dependent negative regulator of secondary metabolite production in the entomopathogenic fungus Beauveria bassiana. Environ. Microbiol. 2015, 17, 1189–1202. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tsunematsu, Y.; Noguchi, H.; Hotta, K.; Watanabe, K. Elucidation of pyranonigrin biosynthetic pathway reveals a mode of tetramic acid, fused γ-pyrone, and exo-methylene formation. Org. Lett. 2015, 17, 4992–4995. [Google Scholar] [CrossRef]
- Fan, L.L.; Fu, K.H.; Yu, C.J.; Li, Y.Y.; Li, Y.Q.; Chen, J. Thc6 protein, isolated from Trichoderma harzianum, can induce maize defense response against Curvularia lunata. J. Basic. Microb. 2015, 55, 591–600. [Google Scholar] [CrossRef]
- Fang, Z.D. Plant Disease Research Methods, 3rd ed; China Agricultural Press: Beijing, China, 1998; pp. 26–27. [Google Scholar]
- Andrade, A.E.; Silva, L.P.; Pereira, J.L.; Noronha, E.F.; Reis, J.F.B.; Bloch, C., Jr.; Dos Santos, M.F.; Domont, G.B.; Franco, O.L.; Mehta, A. In vivo proteome analysis of Xanthomonas campestris pv. campestris in the interaction with the host plant Brassica oleracea. FEMS Microbiol. Lett. 2008, 281, 167–174. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, M.G.; Wang, L.K.; Yao, P.Y.; Ma, L.; Wang, C.T.; Wang, H.; Qiao, G.L.; Hu, B.S.; Fan, J.Q. The Ribosomal Protein RplY Is Required for Pectobacterium carotovorum Virulence and Is Induced by Zantedeschia elliotiana Extract. Phytopahtology 2017, 107, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–169. [Google Scholar] [CrossRef]
- Vogel, H.; Wheat, C.W. Accessing the transcriptome: How to normalize mRNA Pools. Methods Mol. Biol. 2011, 772, 105. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Chlon, T.; Maoqiang, H. Microarray reveals complement components are regulated in the serum-deprived rat retinal ganglion cell line. Mol. Vis. 2007, 13, 293–308. [Google Scholar] [CrossRef]
- Yang, H.; Shi, P.J.; Liu, Y.; Xia, W.; Wang, X.; Cao, H.; Ma, R.; Luo, H.; Bai, Y.; Yao, B. Loop 3 of fungal endoglucanases of glycoside hydrolase family 12 modulates catalytic efficiency. Appl. Environ. Microb. 2016, 83, e03123-16. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.H.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped blasta and PSI blast: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.A.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, 277–280. [Google Scholar] [CrossRef]
- Koonin, E.V.; Fedorova, N.D.; Jackson, J.D.; Jacobset, A.R.; Krylov, D.M.; Makarova, K.S.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; Rao, B.S.; et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004, 5, R7. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Li, J.Q.; Wu, S.F.; Zhu, Y.P.; Zhu, Y.P.; Chen, Y.W.; He, F. Integrated nr database in protein annotation system and its localization. Comp. Eng. 2006, 32, 71–74. [Google Scholar] [CrossRef]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2004, 32, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, S.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3686. [Google Scholar] [CrossRef]
- Ye, J.; Fang, L.; Zheng, H.K.; Zheng, Y.; Chen, J.; Zhang, Z.J.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293-7. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data usingreal-time quantitative PCR and the 2-∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Goswami, R.S. Targeted gene replacement in fungi using a Split-Marker approach. Methods Mol. Biol. 2011, 255–269. [Google Scholar] [CrossRef]
- Luo, F.Y.; Fang, X.M.; Liu, H.; Zhu, T.H.; Han, S.; Peng, Q.; Li, S.J. Differential transcriptome analysis and identification of genes related to resistance to blight in three varieties of Bambusa pervariabilis × Dendrocalamopsis grandis. PeerJ 2021, 9, e12301. [Google Scholar] [CrossRef]
- Logacheva, M.D.; Kasianov, A.S.; Binogradov, D.V.; Samigullin, T.H.; Penin, A.A. De novo sequencing and characterization of floral transcriptome in two species of buckwheat (Fagopyrum). BMC Genom. 2011, 12, 30. [Google Scholar] [CrossRef]
- Zhong, X.; Gu, L.; Wang, H.Z.; Lian, D.H.; Zheng, Y.M.; Zhou, S.; Liu, X. Profile of Ophiocordyceps sinensis transcriptome and differentially expressed genes in three different mycelia, Sclerotium and fruiting body developmental stages. Fungal Biol. 2018, 122, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Alkan, N.; Friedlander, G.; Ment, D.; Prusky, D.; Fluhr, R. Simultaneous transcriptome analysis of Colletotrichum gloeosporioides, and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. New. Phytol. 2015, 205. [Google Scholar] [CrossRef]
- Oh, Y.; Donofrio, N.M.; Pan, H.; Coughlan, S.J.; Brown, D.E.; Meng, S.W.; Mitchell, T.; Dean, R.A. Transcriptome analysis reveals new insight into appressorium formation and function in the rice blast fungus Magnaporthe oryzae. Genome Biol. 2008, 9, R85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Fu, J.J.; Gou, M.Y.; Huai, J.L.; Liu, Y.J.; Jian, M.; Huang, Q.; Guo, X.; Dong, Z.; Wang, H.; et al. Genome-wide transcriptome analysis of two maize inbred lines under drought stress. Plant Mol. Biol. 2010, 72, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Sheng, X.; Yin, Y. Transcriptome analysis of the Taxodium ‘Zhongshanshan 405′ roots in response to salinity stress. Plant Physiol. Biochem. 2016, 100, 156–165. [Google Scholar] [CrossRef]
- Blumwald, E.; Aharon, G.S.; Lam, B.C.H. Early signal transduction pathways in plant–pathogen interactions. Trends Plant Sci. 1998, 3, 342–346. [Google Scholar] [CrossRef]
- Fang, X.P.; Chen, W.Y.; Ma, H.S.; Yu, H.; Wang, S.Z.; Xin, Y. Research progress in plant-pathogen responsive proteins. J. Nuclear Agric. Sci. 2014, 28, 825–832. [Google Scholar] [CrossRef]
- Vlot, A.C.; Klessig, D.F.; Park, S.W. Systemic acquired resistance: The elusive signal(s). Curr. Opin. Plant Biol. 2008, 11, 436–442. [Google Scholar] [CrossRef]
- Braun, E.J.; Howard, R.J. Adhesion of fungal spores and germlings to host plant surfaces. Protoplasma 1994, 181, 202–212. [Google Scholar] [CrossRef]
- Momany, M.; Talbot, N.J. Septins focus cellular growth for host infection by pathogenic fungi. Front. Cell Dev. Biol. 2017, 5. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Epstein, L. Adhesion of fungi to the plant surface: Prerequisite for pathogenesis. Fungal Spore Dis. Initiat. Plants Anim. 1991, 3–23. [Google Scholar] [CrossRef]
- Schafer, W. Molecular mechanisms of fungal pathogenicity to plants. Annu. Rev. Phytopathol. 1994, 32, 461–477. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Enzymatic penetration of the plant cuticle by fungal pathogens. Annu. Rev. Plant Physiol. 1985, 23, 223–250. [Google Scholar] [CrossRef]
- Pascholati, S.F.; Deising, H.; Leiti, B.; Anderson, D. Cutinase and non-specific esterase activities in the conidial mucilage of Colletotrichum graminicola. Physiol. Mol. Plant Pathol. 1993, 42, 37–51. [Google Scholar] [CrossRef]
- Harris, P.V.; Welner, D.; Mcfarland, K.C.; Re, E.; Poulsen, J.C.N.; Brown, K.; Salbo, R.; Ding, H.; Vlasenko, E.; Merino, S.; et al. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase Family 61: Structure and function of a large, Enigmatic Family. Biochemistry 2010, 49, 3305–3316. [Google Scholar] [CrossRef]
- Xu, Z.W.; Escamilla-Trevino, L.L.; Zeng, L.H.; Lalgondar, M.; Bevan, D.R.; Winkel, B.S.J.; Mohamed, A.; Cheng, C.; Shih, M.; Poulton, J.; et al. Functional genomic analysis of Arabidopsis thalianaglycoside hydrolase family 1. Plant Mol. Biol. 2004, 55, 343–367. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, Y.; Yan, Q.; Chen, Z.; Qin, Z.; Jiang, Z. Structural insights into the substrate specificity and transglycosylation activity of a fungal glycoside hydrolase family 5 β-mannosidase. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 2970–2982. [Google Scholar] [CrossRef]
- Waring, P.; Newcombe, N.; Edel, M.; Qin, H.L.; Hui, J.; Sjaarda, A. Cellular uptake and release of the immunomodulating fungal toxin gliotoxin. Toxicon 1994, 32, 491–504. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Wang, N.; Sun, Y.M.; Gu, Z.C.; Wang, R.R.; Saydin, A.; Shen, Q.; Guo, S. Nitrate increased cucumber tolerance to Fusarium wilt by regulating fungal toxin production and distribution. Toxins 2017, 9, 100. [Google Scholar] [CrossRef]
- Hidalgo, P.I.; Poirier, E.; Ullan, R.V.; Piqueras, J.; Meslet-Cladiere, L.; Coton, E.; Coton, M. Penicillium rueforti PR toxin gene cluster characterization. Appl. Microbiol. Biotechnol. 2017, 101, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Dutton, M.F. Fungal toxin. Nature 1970, 225, 1171. [Google Scholar] [CrossRef]
- Rocha, A.L.M.; Pietro, A.D.; Ruiz-Roldan, C.; Roncero, M.I.G. Ctf1, a transcriptional activator of cutinase and lipase genes in Fusarium oxysporum is dispensable for virulence. Mol. Plant Pathol. 2008, 9, 293–304. [Google Scholar] [CrossRef]
- Nawrath, C. Unraveling the complex network of cuticular structure and function. Curr. Opin. Plant Biol. 2006, 9, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Métraux, J. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 274. [Google Scholar] [CrossRef]
- Voisin, D.; Nawrath, C.; Kurdyukov, S.; Franke, R.B.; Reina-Pinto, J.J.; Efremova, N.; Will, I.; Schreiber, L.; Yephremov, A. Dissection of the complex phenotype in cuticular mutants of Arabidopsis reveals a role of SERRATE as a mediator. PLoS Genet. 2009, 5, e1000703. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef]
- Silva, W.O.B.D.; Santi, L.; Correa, A.P.F.; Silva, L.A.D.; Bresciani, F.R.; Schrank, A.; Vainstein, M.H. The entomopathogen Metarhizium anisopliae can modulate the secretion of lipolytic enzymes in response to different substrates including components of arthropod cuticle. Fungal Biol. 2010, 114, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.M.; Flaishman, M.A.; Kolattukudy, P.E. Cutinase Gene Disruption in Fusarium solanif sp pisi Decreases Its Virulenceon Pea. Plant Cell 1994, 6, 935–945. [Google Scholar] [CrossRef][Green Version]
- Voigt, C.A.; Schafer, W.; Salomon, S. A secreted lipase of Fusarium graminearum is a virulence factor required for infection of cereals. Plant J. 2005, 42, 364–375. [Google Scholar] [CrossRef]
- Li, D.X.; Sirakova, T.; Rogers, L.; Ettinger, W.F.; Kolattukudy, P.E. Regulation of Constitutively Expressed and Induced Cutinase Genes by Different Zinc Finger Transcription Factors in Fusarium solani f. sp. pisi (Nectria haematococca). J. Biol. Chem. 2002, 227, 7905–7912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Pan, H.B.; Huang, J.; Yu, X.P. The zinc finger transcription factors Bbctf1α and Bbctf1β regulate the expression of genes involved in lipid degradation and contribute to stress tolerance and virulence in a fungal insect pathogen. Pest Manag. Sci. 2020, 76, 2589–2600. [Google Scholar] [CrossRef]
- Gan, Y.Z.; Zhang, L.S.; Zhang, Z.G.; Dong, S.M.; Li, J.; Wang, Y.; Zheng, X. The LCB 2 subunit of the sphingolip biosynthesis enzyme serine palmitoyltransferase can function as an attenuator of the hypersensitive response and Bax-induced cell death. New Phytol. 2009, 181, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Dangl, J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005, 8, 397–403. [Google Scholar] [CrossRef]
- L’Haridon, F.; Besson-Bard, A.; Binda, M.; Serrano, M.; Abou-Mansour, E.; Balet, F.; Schoonbeek, H.; Hess, S.; Mir, R.; Léon, J.; et al. A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity. PLoS Pathog. 2011, 7, e1002148. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.; Dangl, J.L. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 2005, 37, 1130–1134. [Google Scholar] [CrossRef]
- Zhang, H.J.; Fang, Q.; Zhang, Z.G.; Wang, Y.C.; Zheng, X.B. The role of respiratory burst oxidase homologues in elicitor-induced stomatal closure and hypersensitive response in Nicotiana benthamiana. J. Exp. Bot. 2009, 60, 3109–3122. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yu, R.; Xiong, D.G.; Tian, C.M. A Sge1 homolog in Cytospora chrysosperma governs conidiation, virulence and the expression of putative effectors. Gene 2021, 778, 145474. [Google Scholar] [CrossRef]









| Comparison Group | Gene Name | Log2 Ratio | Description |
|---|---|---|---|
| CK-VS-T1 | Cos1P | 9.956230937 | cytochrome oxidase subunit 1, partial [Brevilegnia unisperma] |
| Ench | −11.38168714 | endochitinase [Amphiamblys sp. WSBS2006] | |
| La4H | −5.228792158 | leukotriene A-4 hydrolase/aminopeptidase | |
| Ench2 | −10.25223177 | endochitinase [Amphiamblys sp. WSBS2006] | |
| HydP | −5.849553363 | hydrolase, partial [Mucor ambiguus] | |
| HydP1 | −5.423936695 | hydrolase, partial [Mucor ambiguus] | |
| CatH | −5.21899521 | cathepsin H [Saprolegnia diclina VS20] | |
| abhyd | −5.16654726 | alpha/beta hydrolase [Magnaporthe oryzae 70–15] | |
| Cal | −5.003422577 | Calmodulin [Phytophthora nicotianae] | |
| Inv | −4.361126768 | Inversin [Phytophthora nicotianae] | |
| CK-VS-T2 | Ctf1b | 1.16472 | Cutinase transcription factor 1 beta |
| Fstfd | 4.66024 | Fungal specific transcription factor domain | |
| E-1,3-bg | 1.60198 | Endo-1,3(4)-beta-glucanase | |
| Gh | 1.926701059 | glycoside hydrolase [Stagonospora sp. SRC1lsM3a] | |
| Ghf43P | 2.046533818 | glycoside hydrolase family 43 protein [Trichoderma atroviride] | |
| Ghf28 | 3.242824341 | glycosyl hydrolase family 28 [Colletotrichum incanum] | |
| Ghf62P | 3.263709993 | glycoside hydrolase family 62 protein [Schizophyllum commune] | |
| Hyd | −9.113389998 | hydrophobin [Trichoderma atroviride IMI 206040] | |
| Pep | −6.728250664 | permease, partial [Mucor ambiguus] | |
| LaipF | −6.542070296 | Low affinity iron permease, Fet4 [Sporothrix insectorum] | |
| T1-VS-T2 | Ctf1b | 1.143966714 | Cutinase transcription factor 1 beta |
| Fstfd | 5.178921385 | Fungal specific transcription factor domain | |
| Gh | 1.674955343 | glycoside hydrolase [Stagonospora sp. SRC1lsM3a] | |
| Ghf62P | 3.240379576 | glycoside hydrolase family 62 protein [Schizophyllum commune] | |
| Ghf43P | 2.798082794 | glycoside hydrolase family 43 protein [Trichoderma atroviride] | |
| Ghf28 | 3.368988569 | glycosyl hydrolase family 28 [Colletotrichum incanum] | |
| HydP | 9.935115927 | hydrolase, partial [Mucor ambiguus] | |
| Pc22g | −3.307249137 | Pc22g21720 [Penicillium rubens Wisconsin 54–1255] | |
| HypP | −3.878218123 | MFS transporter [Purpureocillium lilacinum] | |
| MFSt | −3.222620978 | hypothetical protein NECHADRAFT [Nectria haematococca] |
| Comparison Group | Primer Name | Primer Sequence 5′-3′ |
|---|---|---|
| GAPDH | ATGGAGAAGGCTGGGGCTCATTTGC; GATGACCCTTTTGGCTCCCCCCTGC | |
| CK-VS-T2 | Unigene0001578 | ATGGAGAAGGCTGGGGCTCATTTGC; GATGACCCTTTTGGCTCCCCCCTGC |
| Unigene0004220 | ACGAACAGGATACAGGCGACA; CTGAGGTCCAAATAACGAGATGAA | |
| Unigene0004974 | CAGGCGGTTTGGAACTCGT; TGCCATATCCATCCTGCGTC | |
| Unigene0005793 | GCTACTGGCACGACATCGGAT; TCTAGTGGAGTGACAGCAAACGA | |
| Unigene0005693 | ATGTCGGTGGCTGGGTTGA; CCCCTTTTGAGGCGCTGTAT | |
| Unigene0009373 | TTGAGAATGTCTGGTTGCTGAATG; GCGCAGGTTGAGGAGTTGATGT | |
| Unigene0001633 | CGGCAACTTCCCTGGTAGCT; GCCTGGCACGCTATACACCT | |
| Unigene0004295 | TGCTGCTCTACCCTCGTTCTTG; CAATAGCCGTCTGGCAAAGGA | |
| Unigene0005088 | GCTACAGATCGTCGCCTTGG; GCTAATACCGCATACGCCCTAC | |
| Unigene0008572 | CCTACACTGGTCTCATCGGTTTC; GCTGGTAATCCTGCTCCATAAGA | |
| T1-VS-T2 | Unigene0001578 | ATGGAGAAGGCTGGGGCTCATTTGC; GATGACCCTTTTGGCTCCCCCCTGC |
| Unigene0004220 | ACGAACAGGATACAGGCGACA; CTGAGGTCCAAATAACGAGATGAA | |
| Unigene0005793 | GCTACTGGCACGACATCGGAT; TCTAGTGGAGTGACAGCAAACGA | |
| Unigene0001633 | CGGCAACTTCCCTGGTAGCT; GCCTGGCACGCTATACACCT | |
| Unigene0005693 | ATGTCGGTGGCTGGGTTGA; CCCCTTTTGAGGCGCTGTAT | |
| Unigene0009373 | TTGAGAATGTCTGGTTGCTGAATG; GCGCAGGTTGAGGAGTTGATGT | |
| Unigene0001003 | TCTAAACCCAACTCACGTACCACTT; AACAGGCTGATGACTCCCAAGA | |
| Unigene0005927 | GGCTTTGCCATCCACCACA; GCAATGAAGAGGAGGTAGTCGC | |
| Unigene0007158 | AGGATCGCAATGTGGAAGGC; GGTTGAGGGAAGAGTTTGAGGC | |
| Unigene0007851 | GCAAGAGTCGCACGCAGAA; GCAGGTAAAGCCACAGTCCATAT | |
| CK-VS-T1 | Unigene0006182 | TGCCATAACCCAATACCAAACG; TGTTGAGGTTGCGGTCTGTTAGT |
| Unigene0000037 | GAGTTCAGCCACGCCATCAC; CATTCCCGCTTCGACTTTGTT | |
| Unigene0000618 | AGAGGAGAAGAACGCCGAGGT; CAATTTCGCCCACAAAGAGCT | |
| Unigene0000640 | TCCAGGCAAGAACTACTACGGTC; CCTTCCAGAACCAGAAAGCAGT | |
| Unigene0000947 | GCTTCTTCGCCTCCCACCTA; CGCTGTCTCGACAATGCACC | |
| Unigene0000948 | ATGCTTTCAGCGTTTATCCCTT; CGTGAGACAGTTCGGTCCCTAT | |
| Unigene0005250 | TTCCGCTTTAAGGTGTTCATGG; CAGGTCGCCGTAGTGGTTCA | |
| Unigene0006199 | CTAGGCTCGTCACGTTTGCG; AACTGCTCCGGTTTGATGATATG | |
| Unigene0000783 | TGGCTGATCAACTGACTGAGGAA; GCCCAGTTCCTTGGTGGTGAT | |
| Unigene0004166 | CGAAGCGCCAAGATTAAGGA; CACTGCCCTCACCGTCAATG |
| Primer Name | Primer Sequence 5′-3′ |
|---|---|
| Ctf1β1-5-F | TAGGCCACCATGTTGGGCCCGCTGGCGTGTTGAGCATGA |
| Ctf1β1-5-R | AGTTCAGGCTTTTTCATATCTGGCAGGGCTGTTGGTAGA |
| Ctf1β1-3-F | CGAGGGCAAAGGAATAGAGTATGAAGCACGACCACCAGAT |
| Ctf1β1-3-R | GTGGACTCCTCTTAAAGCTTCGGCGAAAGTGAGTGGATT |
| Ctf1β2-5-F | TTCGGGTTACTTCCCTTCG |
| Ctf1β2-5-R | AGTTCAGGCTTTTTCATATCAGGACGCATTAGTCGAGGC |
| Ctf1β2-3-F | CGAGGGCAAAGGAATAGAGTTTAGGGCGTCATCTCGGTC |
| Ctf1β2-3-R | CCAGCATTCGTCAATATCAAGC |
| Hph-F | GATATGAAAAAGCCTGAACT |
| Hph-R | ACTCTATTCCTTTGCCCTCG |
| Ctf1β1-F | ATGGCCCCAAAAGACGGACTTTTGTCAG |
| Ctf1β1-R | GCGTACGAAGCTTCAGCTG TCAATGTGTGTGGCCAAGGCAAAGCT |
| Ctf1β2-F | ATGGCCGCAGACAACGAAGCTGT |
| Ctf1β2-R | GCGTACGAAGCTTCAGCTG TTACCCCTTTACCATACCCCCGTCC |
| KanMX-F | CAGCTGAAGCTTCGTACGC |
| KanMX-R | GCATAGGCCACTAGTGGATCTG |
| Ctf1β1-Up-F | GCTGGCGTGTTGAGCATGA |
| Ctf1β1-Up-R | CTGACAAAAGTCCGTCTTTTGGGGCCATTGGCAGGGCTGTTGGTAGA |
| Ctf1β1-Down-F | CAGATCCACTAGTGGCCTATGCATGAAGCACGACCACCAGAT |
| Ctf1β1-Down-R | CGGCGAAAGTGAGTGGATT |
| Ctf1β2-Up-F | TTCGGGTTACTTCCCTTCG |
| Ctf1β2-Up-R | ACAGCTTCGTTGTCTGCGGCCATAGGACGCATTAGTCGAGGC |
| Ctf1β2-Down-F | CAGATCCACTAGTGGCCTATGC TTAGGGCGTCATCTCGGTC |
| Ctf1β2-Down-R | CCAGCATTCGTCAATATCAAGC |
| Sample | Clean Reads | GC Content | Reads Lenth | % ≥Q30 |
|---|---|---|---|---|
| CK-1 | 52,922,016 | 56.50% | 150 | 94.95% |
| CK-2 | 50,075,888 | 56.65% | 150 | 94.92% |
| CK-3 | 48,964,936 | 56.68% | 150 | 94.76% |
| T1-1 | 50,112,716 | 56.56% | 150 | 94.87% |
| T1-2 | 58,737,286 | 56.47% | 150 | 94.95% |
| T1-3 | 48,459,904 | 56.39% | 150 | 94.92% |
| T2-1 | 50,093,080 | 56.47% | 150 | 94.96% |
| T2-2 | 66,878,600 | 56.52% | 150 | 94.76% |
| T2-3 | 59,806,800 | 56.46% | 150 | 94.78% |
| Sample | All Reads Number | Unique Mapped Reads | Multiple Mapped Reads | Mapping Ratio |
|---|---|---|---|---|
| CK-1 | 49,948,918 | 42,069,088 | 3,839,542 | 91.91% |
| CK-2 | 48,644,554 | 38,417,922 | 3,768,346 | 86.72% |
| CK-3 | 46,816,432 | 39,365,841 | 3,686,520 | 91.96% |
| T1-1 | 48,309,274 | 40,649,369 | 4,017,201 | 92.46% |
| T1-2 | 55,871,212 | 46,619,707 | 5,052,812 | 92.49% |
| T1-3 | 44,845,488 | 37,341,346 | 3,447,631 | 90.95% |
| T2-1 | 47,835,204 | 40,423,506 | 3,689,078 | 92.22% |
| T2-2 | 63,877,526 | 54,410,080 | 4,332,707 | 91.96% |
| T2-3 | 57,266,814 | 48,703,596 | 4,118,072 | 92.24% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Yan, P.; Guan, M.; Han, S.; Qiao, T.; Lin, T.; Zhu, T.; Li, S. Comparative Transcriptomics and Gene Knockout Reveal Virulence Factors of Arthrinium phaeospermum in Bambusa pervariabilis × Dendrocalamopsis grandis. J. Fungi 2021, 7, 1001. https://doi.org/10.3390/jof7121001
Fang X, Yan P, Guan M, Han S, Qiao T, Lin T, Zhu T, Li S. Comparative Transcriptomics and Gene Knockout Reveal Virulence Factors of Arthrinium phaeospermum in Bambusa pervariabilis × Dendrocalamopsis grandis. Journal of Fungi. 2021; 7(12):1001. https://doi.org/10.3390/jof7121001
Chicago/Turabian StyleFang, Xinmei, Peng Yan, Mingmin Guan, Shan Han, Tianmin Qiao, Tiantian Lin, Tianhui Zhu, and Shujiang Li. 2021. "Comparative Transcriptomics and Gene Knockout Reveal Virulence Factors of Arthrinium phaeospermum in Bambusa pervariabilis × Dendrocalamopsis grandis" Journal of Fungi 7, no. 12: 1001. https://doi.org/10.3390/jof7121001
APA StyleFang, X., Yan, P., Guan, M., Han, S., Qiao, T., Lin, T., Zhu, T., & Li, S. (2021). Comparative Transcriptomics and Gene Knockout Reveal Virulence Factors of Arthrinium phaeospermum in Bambusa pervariabilis × Dendrocalamopsis grandis. Journal of Fungi, 7(12), 1001. https://doi.org/10.3390/jof7121001






