Interactions among Escovopsis, Antagonistic Microfungi Associated with the Fungus-Growing Ant Symbiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nest Collection and Escovopsis Isolation
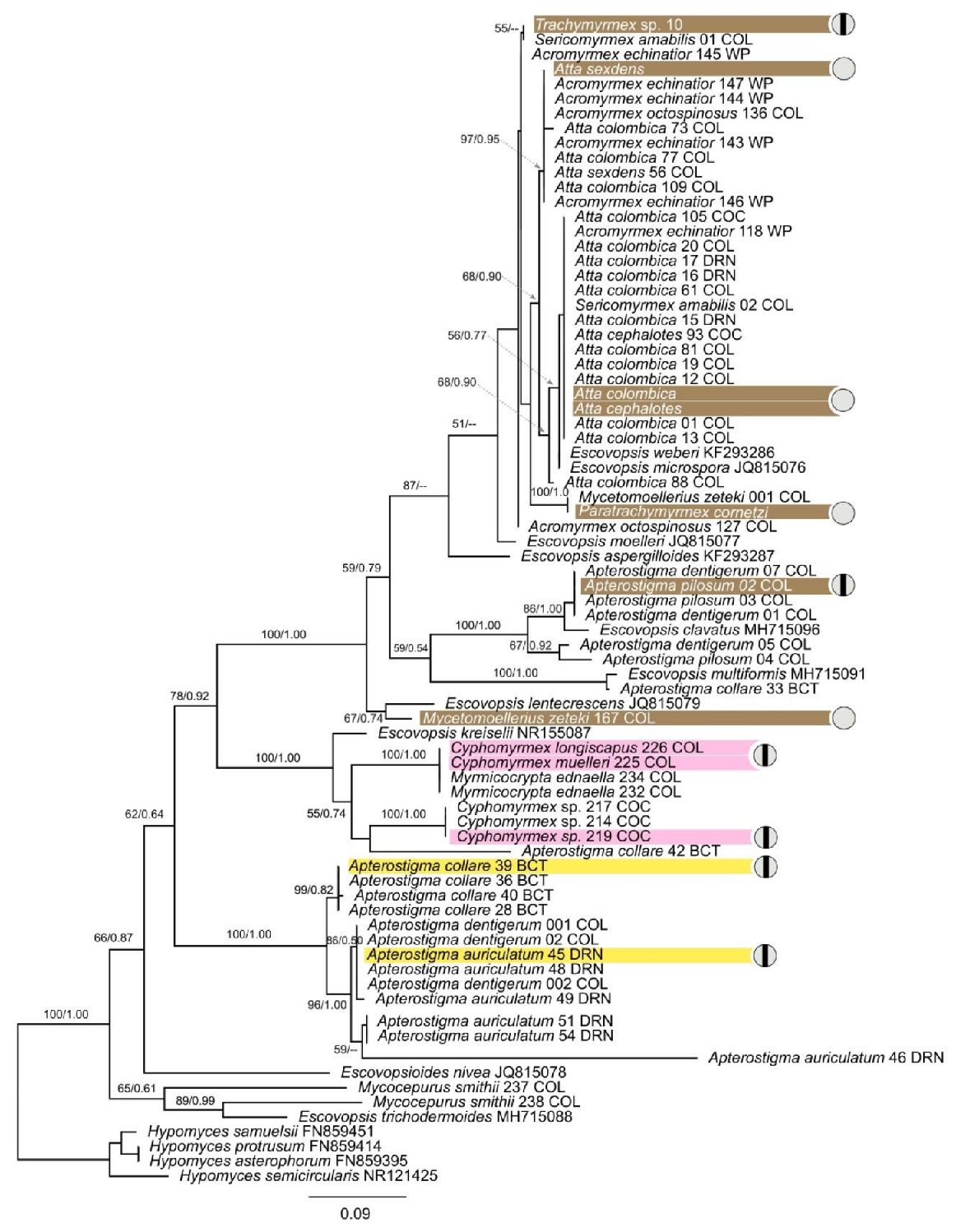
2.2. DNA Extraction, Sequencing and Phylogenetic Analyses
2.3. Evaluation of Escovopsis–Escovopsis Interaction Outcomes
2.3.1. Intraclonal Confrontation Bioassays
2.3.2. Interclonal Confrontation Bioassays
2.3.3. Bioassay Outcome Quantification
2.4. Statistical Analyses
3. Results
3.1. Diversity of Escovopsis Isolates
3.2. Intraclonal Confrontation Bioassays
3.3. Interclonal Confrontation Bioassays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Mendonça, D.M.F.; Caixeta, M.C.S.; Martins, G.L.; Moreira, C.C.; Kloss, T.G.; Elliot, S.L. Low virulence of the fungi Escovopsis and Escovopsioides to a leaf-cutting ant-fungus symbiosis. Front. Microbiol. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Varanda-Haifig, S.S.; Albarici, T.R.; Nunes, P.H.; Haifig, I.; Vieira, P.C.; Rodrigues, A. Nature of the interactions between hypocrealean fungi and the mutualistic fungus of leaf-cutter ants. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2017, 110, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, H.T.; Currie, C.R. Pathogenicity of Escovopsis weberi: The parasite of the attine ant-microbe symbiosis directly consumes the ant-cultivated fungus. Mycologia 2004, 96, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.M.; Gieselmann, M.J.; Martin, J.S. Rectal enzymes of attine ants: Alpha-amylase and chitinase. J. Insect. Physiol. 1973, 19, 1409–1416. [Google Scholar] [CrossRef] [Green Version]
- Chapela, I.H.; Rehner, S.A.; Schultz, T.R.; Mueller, U.G. Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science 1994, 266, 1691–1694. [Google Scholar] [CrossRef]
- Currie, C.R.; Mueller, U.G.; Malloch, D. The agricultural pathology of ant fungus gardens. Proc. Natl. Acad. Sci. USA 1999, 96, 7998–8002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currie, C.R.; Wong, B.; Stuart, A.E.; Schultz, T.R.; Rehner, S.A.; Mueller, U.G.; Sung, G.H.; Spatafora, J.W.; Straus, N.A. Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 2003, 299, 386–388. [Google Scholar] [CrossRef] [Green Version]
- Currie, C.R.; Poulsen, M.; Mendenhall, J.; Boomsma, J.J.; Billen, J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 2006, 311, 81–83. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Marín, H.; Nash, D.R.; Higginbotham, S.; Estrada, C.; van Zweden, J.S.; d’Ettorre, P.; Wcislo, W.T.; Boomsma, J.J. Functional role of phenylacetic acid from metapleural gland secretions in controlling fungal pathogens in evolutionarily derived leaf-cutting ants. Proc. Biol. Sci. 2015, 282, 20150212. [Google Scholar] [CrossRef] [PubMed]
- Gerardo, N.M.; Mueller, U.G.; Price, S.L.; Currie, C.R. Exploiting a mutualism: Parasite specialization on cultivars within the fungus-growing ant symbiosis. Proc. Royal Soc. B 2004, 271, 1791–1798. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, S.S.; Gerardo, N.M. Patterns of specificity of the pathogen Escovopsis across the fungus-growing ant symbiosis. Am. Nat. 2016, 188, 52–65. [Google Scholar] [CrossRef]
- Taerum, S.J.; Cafaro, M.J.; Little, A.E.; Schultz, T.R.; Currie, C.R. Low host-pathogen specificity in the leaf-cutting ant-microbe symbiosis. Proc. Biol. Sci. 2007, 274, 1971–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currie, C.R.; Scott, J.A.; Summerbell, R.C.; Malloch, D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 1999, 398, 701–704. [Google Scholar] [CrossRef]
- Haeder, S.; Wirth, R.; Herz, H.; Spiteller, D. Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 4742–4746. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.C.; Poulsen, M.; Currie, C.R.; Clardy, J. Dentigerumycin: A bacterial mediator of an ant-fungus symbiosis. Nat. Chem. Biol. 2009, 5, 391–393. [Google Scholar] [CrossRef]
- Van Arnam, E.B.; Ruzzini, A.C.; Sit, C.S.; Horn, H.; Pinto-Tomás, A.A.; Currie, C.R.; Clardy, J. Selvamicin, an atypical antifungal polyene from two alternative genomic contexts. Proc. Natl. Acad. Sci. USA 2016, 113, 12940–12945. [Google Scholar] [CrossRef] [Green Version]
- Jutsum, A.R.; Saunders, T.S.; Cherrett, J.M. Intraspecific aggression in the leaf-cutting ant Acromyrmex octospinosus. Anim. Behav. 1979, 27, 839–844. [Google Scholar] [CrossRef]
- Sanhudo, C.E.D.; Izzo, T.; Brandão, C.R.F. Parabiosis between basal fungus-growing ants (Formicidae, Attini). Insect. Soc. 2008, 55, 296–300. [Google Scholar] [CrossRef]
- Poulsen, M.; Boomsma, J.J. Mutualistic fungi control crop diversity in fungus-growing ants. Science 2005, 307, 741–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, S.A.O.; Fernández-Marín, H.; Wcislo, W.T.; Boomsma, J.J. An evaluation of the possible adaptive function of fungal brood covering by attine ants. Evolution 2012, 66, 1966–1975. [Google Scholar] [CrossRef]
- Schoenian, I.; Spiteller, M.; Ghaste, M.; Wirth, R.; Herz, H.; Spiteller, D. Chemical basis of the synergism and antagonism in microbial communities in the nests of leaf-cutting ants. Proc. Natl. Acad. Sci. USA 2011, 108, 1955–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taerum, S.J.; Cafaro, M.J.; Currie, C.R. Presence of multiparasite infections within individual colonies of leaf-cutter ants. Environ. Entomol. 2010, 39, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerardo, N.M.; Mueller, U.G.; Currie, C.R. Complex host-pathogen coevolution in the Apterostigma fungus-growing ant-microbe symbiosis. BMC Evol. Biol. 2006, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Christopher, Y.; Wcislo, W.T.; Martínez-Luis, S.; Hughes, W.O.H.; Gerardo, N.M.; Fernández-Marín, H. Disease management in two sympatric Apterostigma fungus-growing ants for controlling the parasitic fungus Escovopsis. Ecol. Evol. 2021, 11, 6041–6052. [Google Scholar] [CrossRef] [PubMed]
- Viney, M.E.; Graham, A.L. Patterns and processes in parasite co-infection. Adv. Parasitol. 2013, 82, 321–369. [Google Scholar]
- Hochberg, M.E.; Holt, R.D. The coexistence of competing parasites. i. the role of cross-species infection. Am. Nat. 1990, 136, 517–541. [Google Scholar] [CrossRef]
- Mabbott, N.A. The Influence of parasite infections on host immunity to co-infection with other pathogens. Front. Immunol. 2018, 9, 2579. [Google Scholar] [CrossRef] [Green Version]
- Nowak, M.A.; May, R.M. Superinfection and the evolution of parasite virulence. Proc. Biol. Sci. 1994, 255, 81–89. [Google Scholar]
- Mideo, N. Parasite adaptations to within-host competition. Trends. Parasitol. 2009, 25, 261–268. [Google Scholar] [CrossRef]
- Alizon, S.; de Roode, J.C.; Michalakis, Y. Multiple infections and the evolution of virulence. Ecol. Lett. 2013, 16, 556–567. [Google Scholar] [CrossRef] [Green Version]
- Leggett, H.C.; Benmayor, R.; Hodgson, D.J.; Buckling, A. Experimental evolution of adaptive phenotypic plasticity in a parasite. Curr. Biol. 2013, 23, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Bose, J.; Kloesener, M.H.; Schulte, R.D. Multiple-genotype infections and their complex effect on virulence. Zoology 2016, 119, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Vaumourin, E.; Vourch, G.; Gasqui, P.; Vayssier-Taussat, M. The importance of multiparasitism: Examining the consequences of co-infections for human and animal health. Parasit. Vectors. 2015, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.S.; Qu, X.Y.; Zhang, W.Z.; Li, J.; Lv, Z.Y. Infection against infection: Parasite antagonism against parasites, viruses and bacteria. Infect. Dis. Poverty. 2019, 8, 49. [Google Scholar] [CrossRef]
- Susi, H.; Barrès, B.; Vale, P.F.; Laine, A.L. Co-infection alters population dynamics of infectious disease. Nat. Commun. 2015, 6, 5975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Porco, T.C.; Ruan, S. Coinfection dynamics of two diseases in a single host population. J. Math. Anal. Appl. 2016, 442, 171–188. [Google Scholar] [CrossRef] [Green Version]
- Cordero, O.X.; Datta, M.S. Microbial interactions and community assembly at microscales. Curr. Opin. Microbiol. 2016, 31, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Gorter, F.A.; Manhart, M.; Ackermann, M. Understanding the evolution of interspecies interactions in microbial communities. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190256. [Google Scholar] [CrossRef] [Green Version]
- Augustin, J.O.; Groenewald, J.Z.; Nascimento, R.J.; Mizubuti, E.S.G.; Barreto, R.W.; Elliot, S.L.; Evans, H.C. Yet more “weeds” in the garden: Fungal novelties from nests of leaf-cutting ants. PLoS ONE 2013, 8, e82265. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Meirelles, L.A.; Montoya, Q.V.; Solomon, S.E.; Rodrigues, A. New light on the systematics of fungi associated with attine ant gardens and the description of Escovopsis kreiselii sp. nov. PLoS ONE 2015, 10, e0112067. [Google Scholar] [CrossRef] [Green Version]
- Masiulionis, V.E.; Cabello, M.N.; Seifert, K.A.; Rodrigues, A.; Pagnocca, F.C. Escovopsis trichodermoides sp. nov., isolated from a nest of the lower attine ant Mycocepurus goeldii. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 107, 731–740. [Google Scholar] [CrossRef]
- Montoya, Q.V.; Martiarena, M.J.S.; Polezel, D.A.; Kakazu, S.; Rodrigues, A. More pieces to a huge puzzle: Two new Escovopsis species from fungus gardens of attine ants. MycoKeys 2019, 46, 97–118. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, T.R.; Brady, S.G. Major evolutionary transitions in ant agriculture. Proc. Natl. Acad. Sci. USA 2008, 105, 5435–5440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, S.; Rabeling, C.; Sosa-Calvo, J.; Lopes, C.T.; Rodrigues, A.; Vasconcelo, H.; Bacci, M., Jr.; Mueller, U.G.; Schultz, T.R. The molecular phylogenetics of Trachymyrmex forel ants and their fungal cultivars provide insights into the origin and coevolutionary history of ‘higher-attine’ ant agriculture. Syst. Entomol. 2019, 44, 939–956. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Alam, M.Z.; Kabashi, N.A.; Adebayo, O.S. Development of compatible fungal mixed culture for composting process of oil palm industrial waste. Afr. J. Biotechnol. 2011, 10, 18657–18665. [Google Scholar] [CrossRef]
- Morón-Ríos, A.; Gómez-Cornelio, S.; Ortega-Morales, B.O.; De la Rosa-García, S.; Partida-Martínez, L.P.; Quintana, P.; Alayón-Gamboa, J.A.; Cappello-García, S.; González-Gómez, S. Interactions between abundant fungal species influence the fungal community assemblage on limestone. PLoS ONE 2017, 12, e0188443. [Google Scholar] [CrossRef] [Green Version]
- Garland, T., Jr.; Dickerman, A.W.; Janis, C.M.; Jones, J.A. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 1993, 42, 265–292. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. Spatial Analysis of Soybean Plant Height and Plant Canopy Temperature Measured with On-the-Go Tractor Mounted Sensors. In R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Boya, C.A.; Fernández-Marín, H.; Mejía, L.C.; Spadafora, C.; Dorrestein, P.C.; Gutiérrez, M. Imaging mass spectrometry and MS/MS molecular networking reveals chemical interactions among cuticular bacteria and pathogenic fungi associated with fungus-growing ants. Sci. Rep. 2017, 7, 5604. [Google Scholar] [CrossRef] [Green Version]
- Bizarria, R.; Nagamoto, N.S.; Rodrigues, A. Lack of fungal cultivar fidelity and low virulence of Escovopsis trichodermoides. Fungal Ecol. 2020, 45, 100944. [Google Scholar] [CrossRef]
- Marfetán, J.A.; Romero, A.I.; Cafaro, M.J.; Folgarait, P.J. Five new Escovopsis species from Argentina. Mycotaxon 2019, 133, 569–589. [Google Scholar] [CrossRef]
- Kellner, K.; Kardish, M.R.; Seal, J.N.; Linksvayer, T.A.; Mueller, U.G. Symbiont-mediated host-parasite dynamics in a fungus-gardening ant. Microb. Ecol. 2018, 76, 530–543. [Google Scholar] [CrossRef]
- Paoletti, M. Vegetative incompatibility in fungi: From recognition to cell death, whatever does the trick. Fungal Biol. Rev. 2016, 30, 152–162. [Google Scholar] [CrossRef]
- Malik, M.; Vilgalys, R. Somatic Incompatibility in Fungi. In Structure and Dynamics of Fungal Populations; Worrall, J.J., Ed.; Population and Community Biology Series; Springer: Dordrecht, The Netherlands, 1999; p. 25. [Google Scholar]
- Dhodary, B.; Schilg, M.; Wirth, R.; Spiteller, D. Secondary metabolites from Escovopsis weberi and their role in attacking the garden fungus of leaf-cutting ants. Chem. Eur. J. 2018, 24, 4445–4452. [Google Scholar] [CrossRef] [Green Version]
- Heine, D.; Holmes, N.A.; Worsley, S.F.; Santos, A.C.A.; Innocent, T.M.; Scherlach, K.; Patrick, E.H.; Yu, D.W.; Murrell, J.C.; Vieria, P.C.; et al. Chemical warfare between leafcutter ant symbionts and a co-evolved pathogen. Nat. Commun. 2018, 9, 2208. [Google Scholar] [CrossRef]
- Weber, N.A. Gardening Ants, the Attines. In Memoirs of the American Philosophical Society; American Philosophical Society: Philadelphia, PA, USA, 1972; p. 146. [Google Scholar]
- Caten, C.E. Vegetative incompatibility and cytoplasmic infection in fungi. J. Gen. Microbiol. 1972, 72, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Diepeningen, A.D.; Debets, A.J.M.; Hoekstra, R.F. Heterokaryon incompatibility blocks virus transfer among natural isolates of black Aspergilli. Curr. Genet. 1997, 32, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Gerardo, N.M.; Caldera, E.J. Labile associations between fungus-growing ant cultivars and their garden pathogens. ISME J. 2007, 1, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Brglez, A.; Piškur, B.; Ogris, N. In vitro interactions between Eutypella parasitica and some frequently isolated fungi from the wood of the dead branches of young sycamore maple (Acer pseudoplatanus). Forests 2020, 11, 1072. [Google Scholar] [CrossRef]
- Custodio, B.C.; Rodrigues, A. Escovopsis kreiselii specialization to its native hosts in the fungiculture of the lower attine ant Mycetophylax morschi. Antonie Leeuwenhoek Int. J. Mol. Microbiol. 2019, 112, 305–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerardo, N.M.; Jacobs, S.R.; Currie, C.R.; Mueller, U.G. Ancient host-pathogen associations maintained by specificity of chemotaxis and antibiosis. PLoS Biology 2006, 4, e235. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.; Cafaro, M.J.; Erhardt, D.P.; Little, A.E.F.; Gerardo, N.M.; Tebbets, B.; Klein, B.S.; Currie, C.R. Variation in Pseudonocardia antibiotic defence helps govern parasite-induced morbidity in Acromyrmex leaf-cutting ants. Environ. Microbiol. Rep. 2010, 2, 534–540. [Google Scholar] [CrossRef]
- Cafaro, M.J.; Poulsen, M.; Little, A.E.F.; Price, S.L.; Gerardo, N.M.; Wong, B.; Stuart, A.E.; Larget, B.; Abbot, P.; Currie, C.R. Specificity in the symbiotic association between fungus-growing ants and protective Pseudonocardia bacteria. Proc. R. Soc. B Biol. Sci. 2011, 278, 1814–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Marker | Primers | PCR and Sequencing Conditions |
|---|---|---|
| ITS | ITS4- 5′TCCTCCGCTTATTTGATTATTGATC3′ ITS5- 5′GGATATGTATATATATGTCGTATATCATATGG3′ [40] | 3 min of denaturation at 96 °C, 35 cycles consisting of 1 min at 94 °C, 1 min at 55 °C and 2 min at 72 °C [41] |
| LSU | CLAF-5′GCATTATTCATATTATATGCGGATGGAT 3′ CLAR-5′GATCTCCTTGGTCCGTGTTTCAT 3′ [7] | 2 min of denaturation at 95 °C, 40 cycles of 30s at 95 °C, 1 min at 62 °C, 90 s at 72 °C and 5 min of extension at 72 °C [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopher, Y.; Aguilar, C.; Gálvez, D.; Wcislo, W.T.; Gerardo, N.M.; Fernández-Marín, H. Interactions among Escovopsis, Antagonistic Microfungi Associated with the Fungus-Growing Ant Symbiosis. J. Fungi 2021, 7, 1007. https://doi.org/10.3390/jof7121007
Christopher Y, Aguilar C, Gálvez D, Wcislo WT, Gerardo NM, Fernández-Marín H. Interactions among Escovopsis, Antagonistic Microfungi Associated with the Fungus-Growing Ant Symbiosis. Journal of Fungi. 2021; 7(12):1007. https://doi.org/10.3390/jof7121007
Chicago/Turabian StyleChristopher, Yuliana, Celestino Aguilar, Dumas Gálvez, William T. Wcislo, Nicole M. Gerardo, and Hermógenes Fernández-Marín. 2021. "Interactions among Escovopsis, Antagonistic Microfungi Associated with the Fungus-Growing Ant Symbiosis" Journal of Fungi 7, no. 12: 1007. https://doi.org/10.3390/jof7121007
APA StyleChristopher, Y., Aguilar, C., Gálvez, D., Wcislo, W. T., Gerardo, N. M., & Fernández-Marín, H. (2021). Interactions among Escovopsis, Antagonistic Microfungi Associated with the Fungus-Growing Ant Symbiosis. Journal of Fungi, 7(12), 1007. https://doi.org/10.3390/jof7121007






