Colonzation of Tobacco Plants by Fungal Entomopathogens and the Effect on Consumption over Diabrotica speciosa (Coleoptera: Chrysomelidae)
Abstract
:1. Introduction
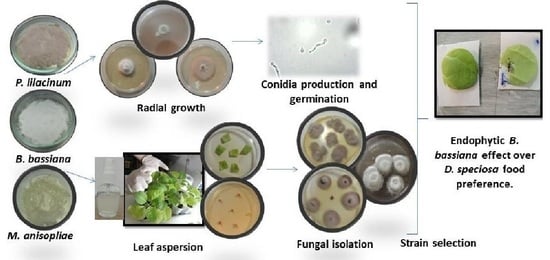
2. Materials and Methods
2.1. Fungal Isolates
2.2. Growth Rate, Production and Germination of Conidia
2.3. Bioassay I: Endophytic Capacity
2.3.1. Plants
2.3.2. Inocula and Leaf Aspersion Technique
2.3.3. Fungal Re-Isolation
2.4. Bioassay II: Feeding Preference
2.4.1. Insects
2.4.2. Choice Test
2.4.3. Data Analysis
3. Results
3.1. Bioassay I
3.2. Bioassay II: Endophytic Effect of B. bassiana on Food Preference of D. speciosa Adults
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corradini, E.; Cuesta, R.; Merello, P.; Sgesso, R.; Gimenez, M.L.; Molfesa, S.; Musco, J.M. Caracterización del Sector Productor Tabacalero en la República Argentina; Universidad Católica Argentina: Buenos Aires, Argentina, 2005; pp. 1–175. [Google Scholar]
- Nelson, P.N.; Burrack, H.J.; Sorenson, C.E. Imidacloprid is Compatible with Control Provided by the Predator Jalysus wickhami Van Duzee (Hemiptera: Berytidae) in flue-cured tobacco (Nicotiana tabacum L.). Crop Prot. 2019, 118, 15–20. [Google Scholar] [CrossRef]
- Maina, U.M.; Galadima, I.B.; Gambo, F.M.; Zakaria, D. A Review on the Use of Entomopathogenic Fungi in the Management of Insect Pests of Field Crops. J. Entomol. Zool. Stud. 2018, 6, 27–32. [Google Scholar]
- Vega, F.E. Insect Pathology and Fungal Endophytes. J. Invertebr. Pathol. 2008, 98, 277–279. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Eliopoulos, P.A. Endophytic Entomopathogenic Fungi: A Valuable Biological Control Tool against Plant Pests. App. Sci. 2020, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Vega, F.E. The Use of Fungal Entomopathogens as Endophytes in Biological Control: A Review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- Vidal, E.; Jaber, L.R. Entomopathogenic Fungi as Endophytes: Plant–Endophyte–Herbivore Interactions and Prospects for Use in Biological Control. Curr. Sci. 2015, 109, 46–54. [Google Scholar]
- Bamisile, B.S.; Senyo Akutse, K.; Dash, C.K.; Qasim, M.; Ramos Aguila, L.C.; Ashraf, H.J.; Wang, L. Effects of Seedling Age on Colonization Patterns of Citrus limon Plants by Endophytic Beauveria bassiana and Metarhizium anisopliae and Their Influence on Seedlings Growth. J. Fungi 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.L.; Pelizza, S.A.; Cabello, M.N.; Stenglein, S.A.; Scorsetti, A.C. Endophytic Colonisation of Tobacco, Corn, Wheat and Soybeans by the Fungal Entomopathogen Beauveria bassiana (Ascomycota, Hypocreales). Biocontrol Sci. Technol. 2015, 25, 475–480. [Google Scholar] [CrossRef]
- García, E.J.; Posadas, J.B.; Perticari, A.; Lecuona, R.E. Metarhizium anisopliae (Metschnikoff) Sorokin Promotes Growth and Has Endophytic Activity in Tomato Plants. Adv. Biol. Res. 2011, 5, 22–27. [Google Scholar]
- Lopez Castillo, D.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The Entomopathogenic Fungal Endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana Negatively Affect Cotton Aphid Reproduction under Both Greenhouse and Field Conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegrucci, N.; Velazquez, M.S.; Russo, M.L.; Vianna, M.F.; Abarca, C.; Scorsetti, A.C. Establishment of the Entomopathogenic Fungus Beauveria bassiana as an Endophyte in Capsicum annuum and Its Effects on the Aphid Pest Myzus persicae (Homoptera: Aphididae). Rev. Biol. Trop. 2020, 68, 1084–1094. [Google Scholar] [CrossRef]
- Vianna, M.F.; Pelizza, S.; Russo, M.L.; Toledo, A.; Mourelos, C.; Scorsetti, A.C. ISSR Markers to Explore Entomopathogenic Fungi Genetic Diversity: Implications for Biological Control of Tobacco Pests. J. Biosci. 2020, 45, 1–11. [Google Scholar] [CrossRef]
- Schapovaloff, M.E.; Alves, L.F.A.; Urrutia, M.I.; Lastra, C.C.L. Ocurrencia Natural de Hongos Entomopatógenos en Suelos Cultivados con Yerba Mate (Ilex paraguariensis St. Hil.) en Misiones, Argentina. Rev. Argent. Microbiol. 2015, 47, 138–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala-Zermeño, M.A.; Gallou, A.; Berlanga-Padilla, A.M.; Serna-Domínguez, M.G.; Arredondo-Bernal, H.C.; Montesinos-Matías, R. Characterisation of Entomopathogenic fungi used in the Biological Control Programme of Diaphorina citri in Mexico. Biocontrol. Sci. Technol. 2015, 25, 1192–1207. [Google Scholar] [CrossRef]
- Butt, T.M.; Goettel, M.S. Bioassays of Entomogenous Fungi. In Bioassays of Entomopathogenic Microbes and Nematodes; Navon, A., Ascher, K.R.S., Eds.; CABI Publishing: New York, NY, USA, 2000; pp. 141–195. [Google Scholar]
- Russo, M.L.; Jaber, L.R.; Scorsetti, A.C.; Vianna, F.; Cabello, M.N.; Pelizza, S.A. Effect of Entomopathogenic Fungi Introduced as Corn Endophytes on the Development, Reproduction, and Food Preference of the Invasive Fall Armyworm Spodoptera frugiperda. J. Pest Sci. 2021, 94, 859–870. [Google Scholar] [CrossRef]
- Napal, G.N.D.; Carpinella, M.C.; Palacios, S.M. Antifeedant Activity of Ethanolic Extract from Flourensia oolepis and Isolation of Pinocembrin as Its Active Principle Compound. Bioresour. Technol. 2009, 100, 3669–3673. [Google Scholar] [CrossRef] [PubMed]
- Gurulingappa, P.; McGee, P.A.; Sword, G. Endophytic Lecanicillium lecanii and Beauveria bassiana Reduce the Survival and Fecundity of Aphis gossypii Following Contact with Conidia and Secondary Metabolites. Crop Prot. 2011, 30, 349–353. [Google Scholar] [CrossRef]
- Bailer, W. Writing ImageJ Plugins. A Tutorial. Version 1.71. 2006. Available online: https://media.ijm.fr/fileadmin/www.ijm.fr/MEDIA/imagerie/fichiers/tut_pluginwb.pdf (accessed on 30 June 2021).
- Petrini, O.; Fisher, P.J. A Comparative Study of Fungal Endophytes in Xylem and Whole Stem of Pinus sylvestris and Fagus sylvatica. Trans. Br. Mycol. Soc. 1987, 91, 233–238. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, Y.C. InfoStat Versión 2011. Grupo InfoStat, FCA; Universidad Nacional de Córdoba: Córdoba, Argentina, 2011; pp. 195–199. Available online: http://www.infostat.com.ar8 (accessed on 13 May 2021).
- Fargues, J.; Goettel, M.S.; Smits, N.; Ouedraogo, A.; Rougier, M. Effect of Temperature on Vegetative Growth of Beauveria bassiana Isolates from Different Origins. Mycologia 1997, 89, 383–392. [Google Scholar] [CrossRef]
- Ouedraogo, A.; Fargues, J.; Goettel, M.S.; Lomer, C.J. Effect of temperature on vegetative growth among isolates of Metarhizium anisopliae and M. flavoviride. Mycopathologia 1997, 137, 37–43. [Google Scholar] [CrossRef]
- Plantey, R.L.; Papura, D.; Couture, C.; Thiéry, D.; Pizzuolo, P.H.; Bertoldi, M.V.; Lucero, G.S. Characterization of Entomopathogenic Fungi from Vineyards in Argentina with Potential as Biological Control Agents Against the European Grapevine Moth Lobesia botrana. BioControl 2019, 64, 501–511. [Google Scholar] [CrossRef]
- Tefera, T.; Vidal, S. Effect of Inoculation Method and Plant Growth Medium on Endophytic Colonization of Sorghum by the Entomopathogenic Fungus Beauveria bassiana. BioControl 2009, 54, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Parsa, S.; Ortiz, V.; Vega, F.E. Establishing Fungal Entomopathogens as Endophytes: Towards Endophytic Biological Control. J. Vis. Exp. 2013, 74, 50360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posada, F.; Aime, M.C.; Peterson, S.W.; Rehner, S.A.; Vega, F.E. Inoculation of Coffee Plants with the Fungal Entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycol. Res. 2007, 111, 748–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyling, N.V.; Thorup-Kristensen, K.; Eilenberg, J. Below and Aboveground Abundance and Distribution of Fungal Entomopathogens in Experimental Conventional and Organic Cropping Systems. Biol. Control 2011, 59, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Sasan, R.K.; Bidochka, M.J. The Insect-Pathogenic Fungus Metarhizium robertsii (Clavicipitaceae) is also an Endophyte that Stimulates Plant Root Development. Am. J. Bot. 2012, 99, 101–107. [Google Scholar] [CrossRef]
- Resquín-Romero, G.; Garrido-Jurado, I.; Delso, C.; Ríos-Moreno, A.; Quesada-Moraga, E. Transient Endophytic Colonizations of Plants Improve the Outcome of Foliar Applications of Mycoinsecticides against Chewing Insects. J. Invertebr. Pathol. 2016, 136, 23–31. [Google Scholar] [CrossRef]
- Batta, Y.A. Efficacy of Endophytic and Applied Metarhizium anisopliae (Metch.) Sorokin (Ascomycota: Hypocreales) against Larvae of Plutella xylostella L. (Yponomeutidae: Lepidoptera) Infesting Brassica napus Plants. Crop Prot. 2013, 44, 128–134. [Google Scholar] [CrossRef]
- Behie, S.W.; Jones, S.J.; Bidochka, M.J. Plant Tissue Localization of the Endophytic Insect Pathogenic Fungi Metarhizium and Beauveria. Fungal Ecol. 2015, 13, 112–119. [Google Scholar] [CrossRef]
- Herrero Asensio, N.H.; Márquez, S.S.; Zabalgogeazcoa, I. Mycovirus Effect on the Endophytic Establishment of the Entomopathogenic Fungus Tolypocladium cylindrosporum in Tomato and Bean Plants. BioControl 2013, 58, 225–232. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal Endophytes Limit Pathogen Damage in a Tropical Tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef] [Green Version]
- Lartey, R.T.; Curl, E.A.; Peterson, C.M.; Harper, J.D. Mycophagous Grazing and Food Preference of Proisotoma minuta (Collembola: Isotomidae) and Onychiurus encarpatus (Collembola: Onychiuridae). Environ. Entomol. 1989, 18, 334337. [Google Scholar] [CrossRef]
- Broza, M.; Pereira, R.M.; Stimac, J.L. The Nonsusceptibility of Soil Collembola to Insect Pathogens and Their Potential as Scavengers of Microbial Pesticides. Pedobiology 2001, 5, 523–534. [Google Scholar] [CrossRef]
- Powell, W.A.; Klingeman, W.E.; Ownley, B.H.; Gwinn, K.D.; Dee, M.; Flanagan, P.C. Endophytic Beauveria bassiana in Tomatoes Yields Mycosis in Tomato Fruitworm Larvae. HortScience 2007, 42, 933. [Google Scholar]
- Cherry, A.J.; Banito, A.; Djegui, D.; Lomer, C. Suppression of the Stem-Borer Sesamia calamistis (Lepidoptera:Noctuidae) in Maize Following Seed Dressing, Topical Application and Stem Injection with African Isolates of Beauveria bassiana. Int. J. Pest Manag. 2004, 50, 67–73. [Google Scholar] [CrossRef]
- Mutune, B.; Ekesi, S.; Niassy, S.; Matiru, V.; Bii, C.; Maniania, N.K. Fungal Endophytes as Promising Tools for the Management of Bean Stem Maggot Ophiomyia phaseoli on Beans Phaseolus vulgaris. J. Pest Sci. 2016, 89, 993–1001. [Google Scholar] [CrossRef]
- Martinuz, A.; Schouten, A.; Menjivar, R.D.; Sikora, R.A. Effectiveness of Systemic Resistance toward Aphis gossypii (Aphididae) as Induced by Combined Applications of the Endophytes Fusarium oxysporum Fo162 and Rhizobium etli G12. Biol. Control 2012, 62, 206–212. [Google Scholar] [CrossRef]
- Leckie, B.M.; Ownley, B.H.; Pereira, R.M.; Klingeman, W.E.; Jones, C.J.; Gwinn, K.D. Mycelia and Spent Fermentation Broth of Beauveria bassiana Incorporated into Synthetic Diets Affect Mortality, Growth and Development of Larval Helicoverpa zea (Lepidoptera: Noctuidae). Biocontrol Sci. Technol. 2008, 18, 697–710. [Google Scholar] [CrossRef]
- Lopez Castillo, D.; Sword, G.A. The Endophytic Fungal Entomopathogens Beauveria bassiana and Purpureocillium lilacinum Enhance the Growth of Cultivated Cotton (Gossypium hirsutum) and Negatively Affect Survival of the Cotton Bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]


| Species | LPSc | ITS | TEF | Origin | Locality | Coordinates | Year of Isolation |
|---|---|---|---|---|---|---|---|
| Beauveria bassiana | 1210 | _ | _ | Soil | Perico, Jujuy | 24°25′14.44″ S/65°01′14.81″ W | 2014 |
| 1211 | MH050803 | _ | Soil | Perico, Jujuy | 24°33′48.63″ S/65°04′58.79″ W | 2014 | |
| 1212 | _ | _ | Soil | Perico, Jujuy | 24°25′14.44″ S/65°01′14.81″ W | 2014 | |
| 1213 | MH050801 | MK047585 | Soil | Perico, Jujuy | 24°25′8.3″ S/65°01′26.8″ W | 2014 | |
| 1214 | MH050799 | _ | Soil | Perico, Jujuy | 24°29′02.6″ S/64°58′21.7″ W | 2014 | |
| 1215 | MH050802 | _ | Soil | Perico, Jujuy | 24°25′8.3″ S/65°01′26.8″ W | 2014 | |
| 1216 | MH050800 | MK015641 | Soil | Perico, Jujuy | 24°29′02.6″ S/64°58′21.7″ W | 2014 | |
| 1217 | MH050798 | _ | Soil | Perico, Jujuy | 24°29′50.8″ S/64°59′19.6″ W | 2014 | |
| 1363 | _ | MK047587 | Soil | Perico, Jujuy | 24°33′37.1″ S/64°54′36.8″ W | 2014 | |
| 1364 | MH050805 | MK047588 | Soil | Perico, Jujuy | 24°33′37.1″ S/64°54′36.8″ W | 2014 | |
| 1365 | _ | _ | Soil | Perico, Jujuy | 24°29′02.6″ S/64°58′21.7″ W | 2014 | |
| 1082 | KJ7722495 | _ | Lepidoptera:Pyralidae | Tres estacas, Chaco | 26°55′27″ S/61°37′36″ O | 2009 | |
| 1098 | KT163259 | _ | Hemiptera:Reduviidae | Tres estacas chaco, Chaco | 26°55′27″ S/61°37′36″ O | 2011 | |
| Lecanicilium lecanii | 1069 | _ | _ | Hemiptera: Aphididae | Concordia, Entre Rios | 31°23′32″ S/58°01′01″ O | 2009 |
| 1367 | MH050808 | _ | Soil | Perico, Jujuy | 24°25′8.3″ S /W 65°01′26.8″ | 2014 | |
| 1368 | MH050804 | _ | Soil | Perico, Jujuy | 24°25′8.3″ S /W 65°01′26.8″ | 2014 | |
| 1369 | MH050806 | _ | Soil | Perico, Jujuy | 24°25′8.3″ S /W 65°01′26.8″ | 2014 | |
| 1370 | MH050807 | MK047586 | Soil | Perico, Jujuy | 24°25′8.3″ S /W 65°01′26.8″ | 2014 | |
| Purpureocillium lilacinum | 1371 | MK110011 | MK047589 | Soil | Perico, Jujuy | 24°33′37.1″ S/64°54′36.8″ W | 2014 |
| 1372 | _ | _ | Soil | Perico, Jujuy | 24°29′50.8″ S/64°59′19.6″ W | 2014 | |
| 1373 | MH050809 | MK047590 | Soil | Perico, Jujuy | 24°29′02.6″ S/64°58′21.7″ W | 2014 | |
| 1374 | MN516739 | MK047591 | Soil | Perico, Jujuy | 24°29′02.6″ S/64°58′21.7″ W | 2014 | |
| Metarhizium anisopliae | 1366 | _ | _ | Soil | Perico, Jujuy | 24°29′02.6″ S/64°58′21.7″ W | 2014 |
| 907 | KT163258 | _ | Hemiptera: Cercopidae | La Plata, Buenos Aires | 34°47′26.45″ S/58°15′09.59″ W | 2004 |
| Strain | Growth Rate (mm/d) | Conidia Germination (%) | Conidia Production (Conidia/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LPSc | 10 °C | 24 °C | 30 °C | 10 °C | 24 °C | 30 °C | 10 °C | 24 °C | 30 °C |
| B. bassiana 1210 | 0.71 ± 0.08 bc | 1.8 ± 0.06 a | 1.39 ± 0.15 ab | 97.8 ± 1.3 bc | 98.6 ± 1.14 b | 98.6 ± 1.14 | 1 × 106 ± 2.29 × 105 fg | 2.82 × 107 ± 2 × 106 c | 1.6 × 107 ± 2.4 × 105 d |
| B. bassiana 1211 | 0.84 ± 0.05 cd | 2.59 ± 0.14 cd | 1.52 ± 0.09 b | 97.8 ± 1.3 bc | 99.2 ± 1.3 b | 98.4 ± 1.52 c | 1 × 105 ± 3.5 × 104 ab | 3.67 × 107 ± 1.1 × 106 cd | 1 × 107 ± 6.2 × 105 c |
| B. bassiana 1212 | 0.58 ± 0.06 a | 2.49 ± 0.06 cd | 2.01 ± 0.06 cd | 96.6 ± 2.61 bc | 97.4 ± 2.88 b | 95.8 ± 2.39 bc | 1 × 105 ± 2.75 × 104 abc | 1.1 × 107 ± 3.7 × 105 b | 3.4 × 106 ± 5.5 × 105 b |
| B. bassiana 1213 | 0.99 ± 0.05 e | 1.66 ± 0.05 a | 1.3 ± 0.1 a | 96.6 ± 2.61 bc | 97.6 ± 2.07 b | 98.6 ± 1.14 c | 1.87 × 106 ± 3.3 × 105 g | 3.4 × 107 ± 7.1 × 105 cd | 2.5 × 107 ± 2.9 × 106 def |
| B. bassiana 1214 | 0.71 ± 0.05 abc | 1.94 ± 0.05 ab | 2.13 ± 0.06 d | 98 ± 1.58 bc | 99.8 ± 0.45 b | 98 ± 1.58 c | 7.1 × 105 ± 1.24 × 105 efg | 2.8 × 107 ± 1.7 × 106 c | 2 × 107 ± 1.5 × 106 de |
| B. bassiana 1215 | 0.75 ± 0.04 bc | 1.87 ± 0.47 a | 1.48 ± 0.07 ab | 99.2 ± 0.84 c | 100 ± 0.00 b | 99 ± 0.71 c | 7 × 104 ± 1.2 × 104 a | 8.1 × 107 ± 5.7 × 106 e | 3.8 × 107 ± 7 × 105 g |
| B. bassiana 1216 | 0.9 ± 0.11 de | 2.61 ± 0.04 d | 2.05 ± 0.11 cd | 95.2 ± 2.28 abc | 97.4 ± 2.41 b | 96.6 ± 2.88 bc | 2.3 × 105 ± 4.6 × 104 bcd | 2 × 106 ± 1.7 × 105 a | 8.5 × 105 ± 9.8 × 104 a |
| B. bassiana 1217 | 0.63 ± 0.02 ab | 2.51 ± 0.09 cd | 2.01 ± 0.06 cd | 97.4 ± 1.82 bc | 96.2 ± 2.77 b | 93.4 ± 3.21 ab | 1.7 × 105 ± 5.15 × 104 abc | 2.8 × 107 ± 1.8 × 106 c | 4.2 × 106 ± 2.9 × 105 b |
| B. bassiana 1363 | 0.74 ± 0.05 bc | 1.63 ± 0.05 a | 1.42 ± 0.08 ab | 89.6 ± 1.14 a | 90.8 ± 0.84 a | 90.6 ± 2.3 a | 3.2 × 105 ± 2.5 × 104 cde | 7.8 × 107 ± 5.2 × 106 e | 3.3 × 107 ± 1.4 × 106 fg |
| B. bassiana 1364 | 0.83 ± 0.06 cd | 1.69 ± 0.14 a | 1.29 ± 0.1 a | 99.4 ± 0.89 c | 99.6 ± 0.55 b | 99 ± 1 c | 5.8 × 105 ± 9 × 104 def | 3.9 × 107 ± 9.6 × 105 d | 2.6 × 107 ± 1 × 106 efg |
| B. bassiana 1365 | 0.7 ± 0.06 ab | 2.26 ± 0.04 bc | 1.9 ± 0.09 c | 92.6 ± 8.26 ab | 96.2 ± 3.03 b | 97.8 ± 1.92 c | 8.4 × 105 ± 1.3 × 105 efg | 2.7 × 107 ± 2 × 106 c | 8.1 × 106 ± 5.7 × 105 c |
| P. lilacinum 1367 | 0.68 ± 0.04 ab | 1.23 ± 0.22 a | 2.1 ± 0.11 a | 98.6 ± 1.14 a | 97.2 ± 1.79 a | 98 ± 1.22 a | 1.3 × 106 ± 1.3 × 105 b | 3.5 × 107 ± 4 × 105 b | 4.1 × 107 ± 3.4 × 106 d |
| P. lilacinum 1368 | 0.89 ± 0.27 b | 1.98 ± 0.76 bc | 2.04 ± 0.38 a | 97.2 ± 1.79 a | 98.6 ± 1.67 a | 98 ± 2.35 a | 1.1 × 106 ± 1.8 × 105 ab | 1.8 × 107 ± 4.4 × 105 a | 3.8 × 107 ± 9.3 × 105 d |
| P. lilacinum 1369 | 0.76 ± 0.07 ab | 2.36 ± 0.09 c | 2.62 ± 0.11 c | 96.2 ± 2.39 a | 97.4 ± 2.07 a | 97.4 ± 2.35 a | 1 × 106 ± 8.5 × 104 ab | 3.2 × 107 ± 8.1 × 105 b | 3.2 × 106 ± 3.6 × 105 a |
| P. lilacinum 1370 | 0.55 ± 0.03 a | 2.18 ± 0.19 bc | 1.94 ± 0.09 b | 98 ± 1.58 a | 97.4 ± 2.7 a | 98.6 ± 1.14 a | 7.6 × 105 ± 5.5 × 104 a | 5.4 × 107 ± 1.1 × 106 c | 1.2 × 107 ± 4.3 × 105 b |
| P. lilacinum 1371 | 0.79 ± 0.06 b | 4.4 ± 0.17 e | 4.31 ± 0.14 d | 98.8 ± 1.3 a | 99.8 ± 0.45 a | 98.8 ± 0.84 a | 1 × 106 ± 5.8 × 104 ab | 1.6 × 107 ± 2.3 × 105 a | 2.2 × 107 ± 6.2 × 105 c |
| P. lilacinum 1372 | 0.76 ± 0.08 ab | 1.73 ± 0.17 abc | 1.85 ± 0.08 ab | 96 ± 2.24 a | 98.6 ± 1.14 a | 96.2 ± 2.28 a | 8.2 × 106 ± 3.4 × 104 a | 5.7 × 107 ± 2 × 106 c | 4.4 × 107 ± 1.1 × 106 d |
| P. lilacinum 1373 | 0.68 ± 0.04 ab | 1.68 ± 0.08 ab | 1.56 ± 0.13 a | 95.8 ± 2.28 a | 96.8 ± 1.92 a | 97.2 ± 2.49 a | 9.2 × 105 ± 9.8 × 104 ab | 3.6 × 107 ± 1.6 × 106 b | 4.9 × 107 ± 9.1 × 105 d |
| P. lilacinum P. lilacinum 1374 | 0.79 ± 0.08 b | 3.34 ± 0.34 d | 2.11 ± 0.04 b | 97.8 ± 1.48 a | 98.2 ± 1.64 a | 98.6 ± 1.52 a | 3.8 × 106 ± 1.5 × 105 c | 5.9 × 107 ± 9.7 × 105 c | 6.8 × 107 ± 4 × 106 e |
| M. anisopliae 1366 | 0.21 ± 0 | 0.23 ± 0.01 | 0.27 ± 0.02 | 98.4 ± 1.51 | 99.2 ± 0.83 | 96.6 ± 2.07 | 2.8 ×10 5 ± 8 × 104 | 5.18 × 107 ± 1.47 × 106 | 1.69 × 107 ± 1.2 × 106 |
| a | b | c | R² | R | ||
|---|---|---|---|---|---|---|
| LPSc1212 | Leaf | 0.6115 | 0.024386 | −0.001133 | 0.560 | 0.763 |
| Root | 0.631875 | −0.014739 | −0.00011 | 0.778 | 0.888 | |
| Stem | 0.223625 | 0.040354 | −0.00139 | 0.445 | 0.688 | |
| Plant | 0.489 | 0.016667 | −0.000878 | 0.340 | 0.592 | |
| LPSc1215 | Leaf | 1.07625 | −0.015679 | 0.000189 | 0.449 | 0.691 |
| Root | 1.1255 | −0.0374 | 0.000449 | 0.671 | 0.829 | |
| Stem | 1.04925 | −0.027836 | 0.000505 | 0.332 | 0.605 | |
| Plant | 1.083667 | −0.026971 | 0.000381 | 0.341 | 0.594 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vianna, F.; Pelizza, S.; Russo, L.; Ferreri, N.; Scorsetti, A.C. Colonzation of Tobacco Plants by Fungal Entomopathogens and the Effect on Consumption over Diabrotica speciosa (Coleoptera: Chrysomelidae). J. Fungi 2021, 7, 1017. https://doi.org/10.3390/jof7121017
Vianna F, Pelizza S, Russo L, Ferreri N, Scorsetti AC. Colonzation of Tobacco Plants by Fungal Entomopathogens and the Effect on Consumption over Diabrotica speciosa (Coleoptera: Chrysomelidae). Journal of Fungi. 2021; 7(12):1017. https://doi.org/10.3390/jof7121017
Chicago/Turabian StyleVianna, Florencia, Sebastian Pelizza, Leticia Russo, Natalia Ferreri, and Ana Clara Scorsetti. 2021. "Colonzation of Tobacco Plants by Fungal Entomopathogens and the Effect on Consumption over Diabrotica speciosa (Coleoptera: Chrysomelidae)" Journal of Fungi 7, no. 12: 1017. https://doi.org/10.3390/jof7121017
APA StyleVianna, F., Pelizza, S., Russo, L., Ferreri, N., & Scorsetti, A. C. (2021). Colonzation of Tobacco Plants by Fungal Entomopathogens and the Effect on Consumption over Diabrotica speciosa (Coleoptera: Chrysomelidae). Journal of Fungi, 7(12), 1017. https://doi.org/10.3390/jof7121017