Harnessing Endophytic Fungi for Enhancing Growth, Tolerance and Quality of Rose-Scented Geranium (Pelargonium graveolens (L’Hér) Thunb.) Plants under Cadmium Stress: A Biochemical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Minerals Assay
2.3. Oxidative Markers
2.4. Activity of Enzymatic Antioxidants
2.5. Detoxification Related Parameters
2.6. Biological and Medicinal Value
2.6.1. Total Antioxidant Capacity
2.6.2. Phenylalanine Ammonia-Lyase (PAL) Activity and Flavonoids
2.6.3. Vitamins
2.6.4. Essential Oil Analysis
2.7. Statistical Analysis
3. Results
3.1. Fungal Isolation and Identification
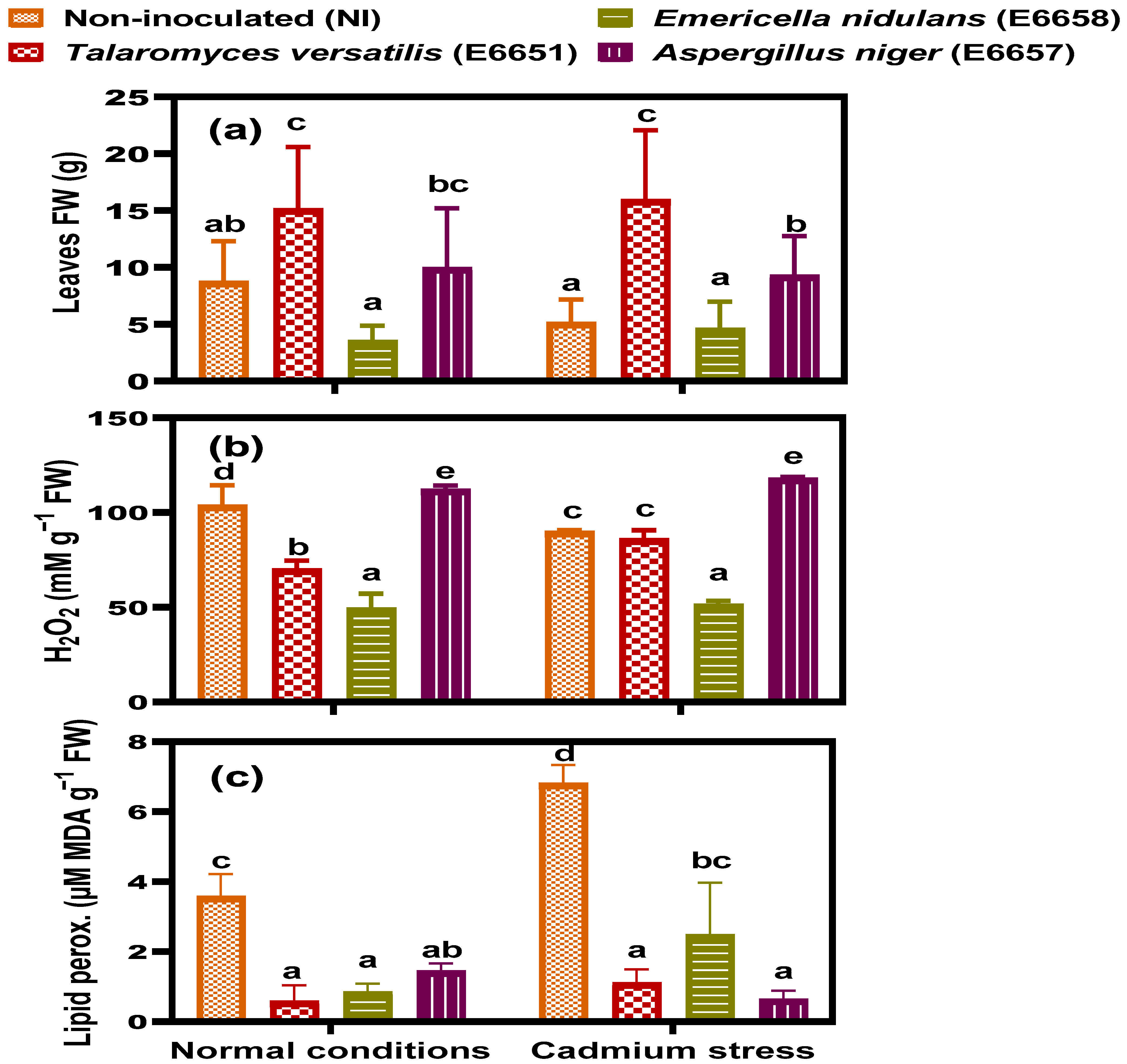
3.2. T. versatilis and A. niger- Inoculation Enhanced Biomass Accumulation
3.3. Endophytic Fungi Alleviated Cd Accumulation and Enhanced Mineral Composition
3.4. Endophytic Fungi Mitigated the Response of Geranium to Cd-Induced Oxidative Stress
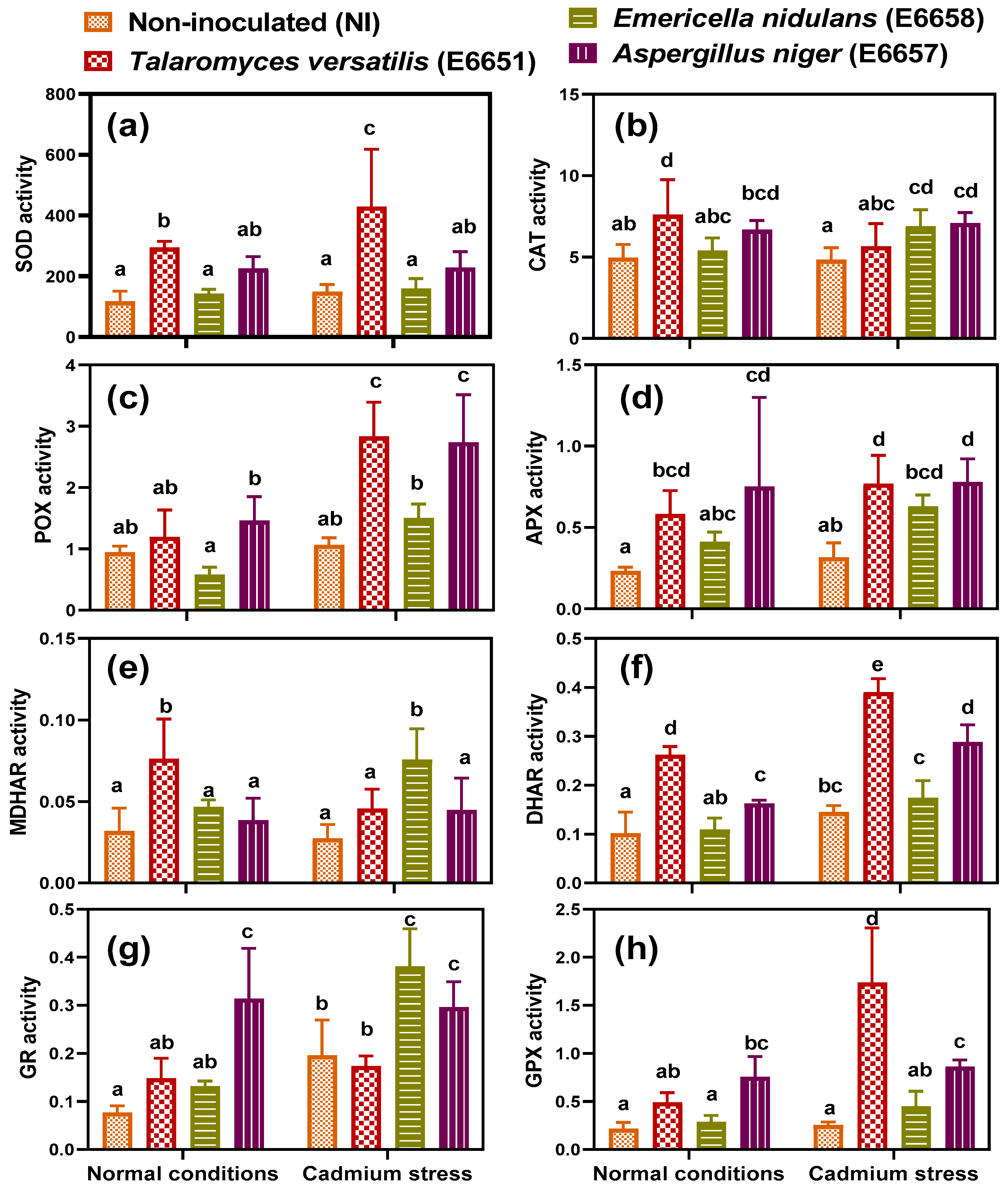
3.5. T. versatilis and A. niger- Inoculation Stimulated Antioxidant Defense under Cd-Stress
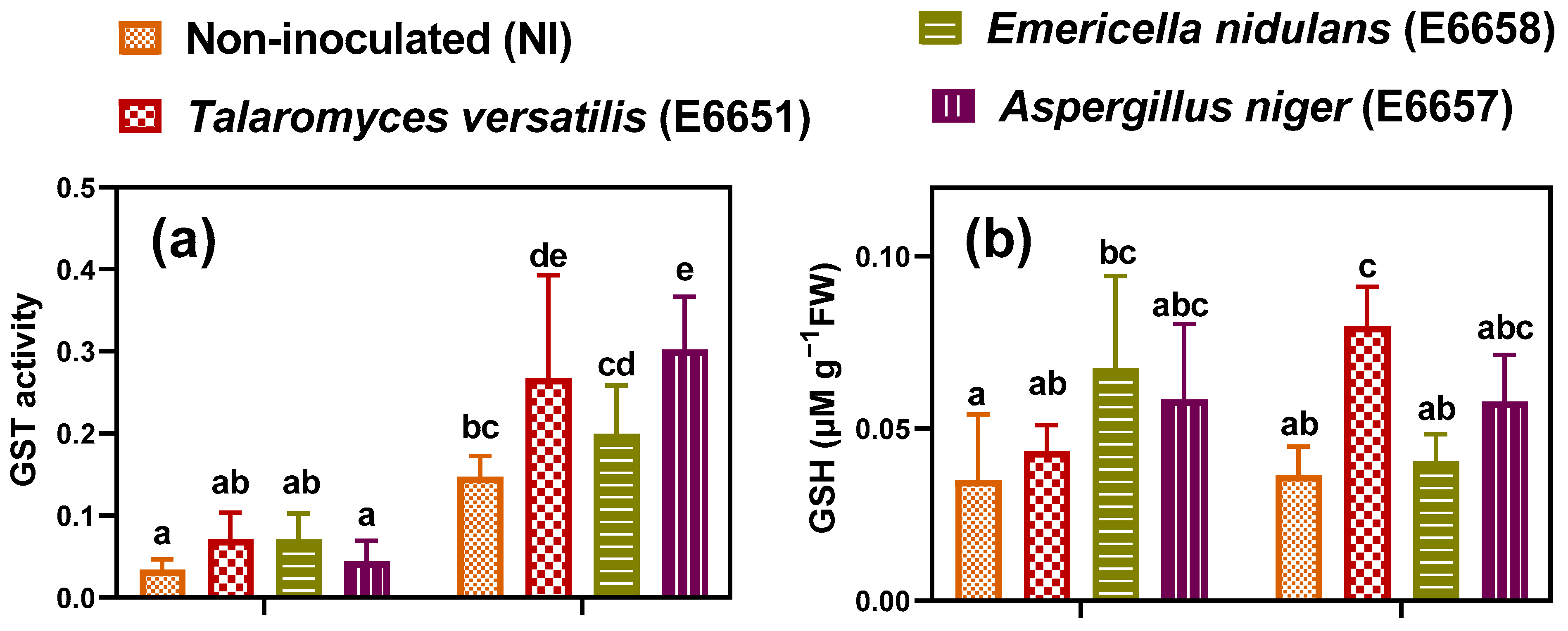
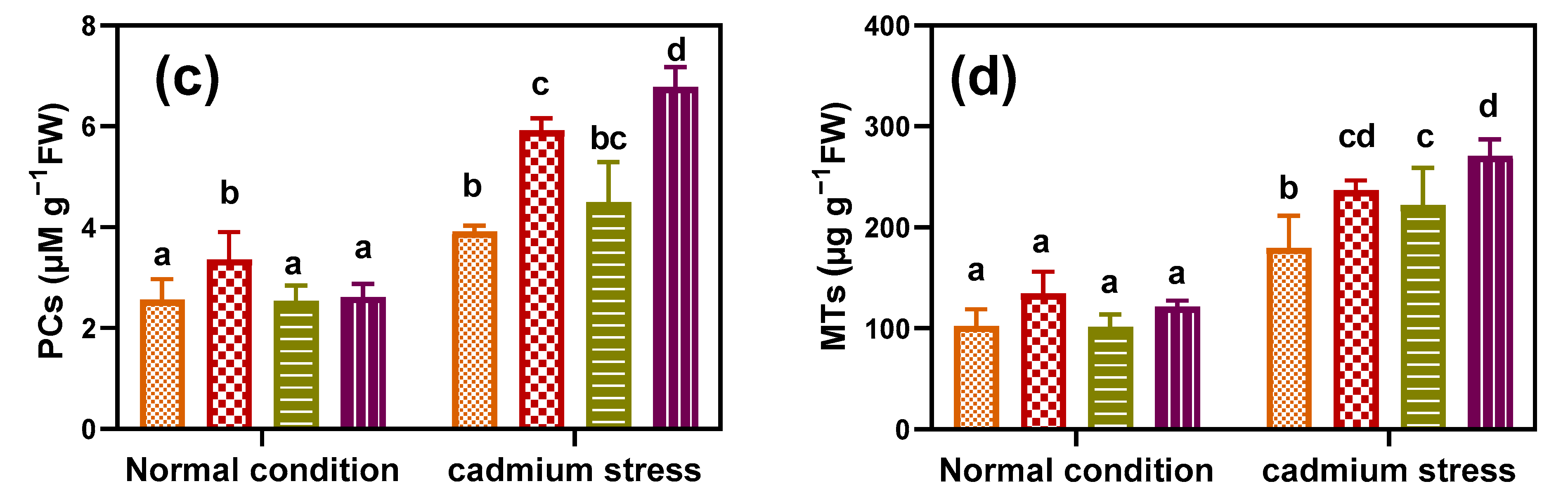
3.6. Inoculation with Fungal Endophytes Reinforced Cd-Detoxification Mechanisms in Geranium
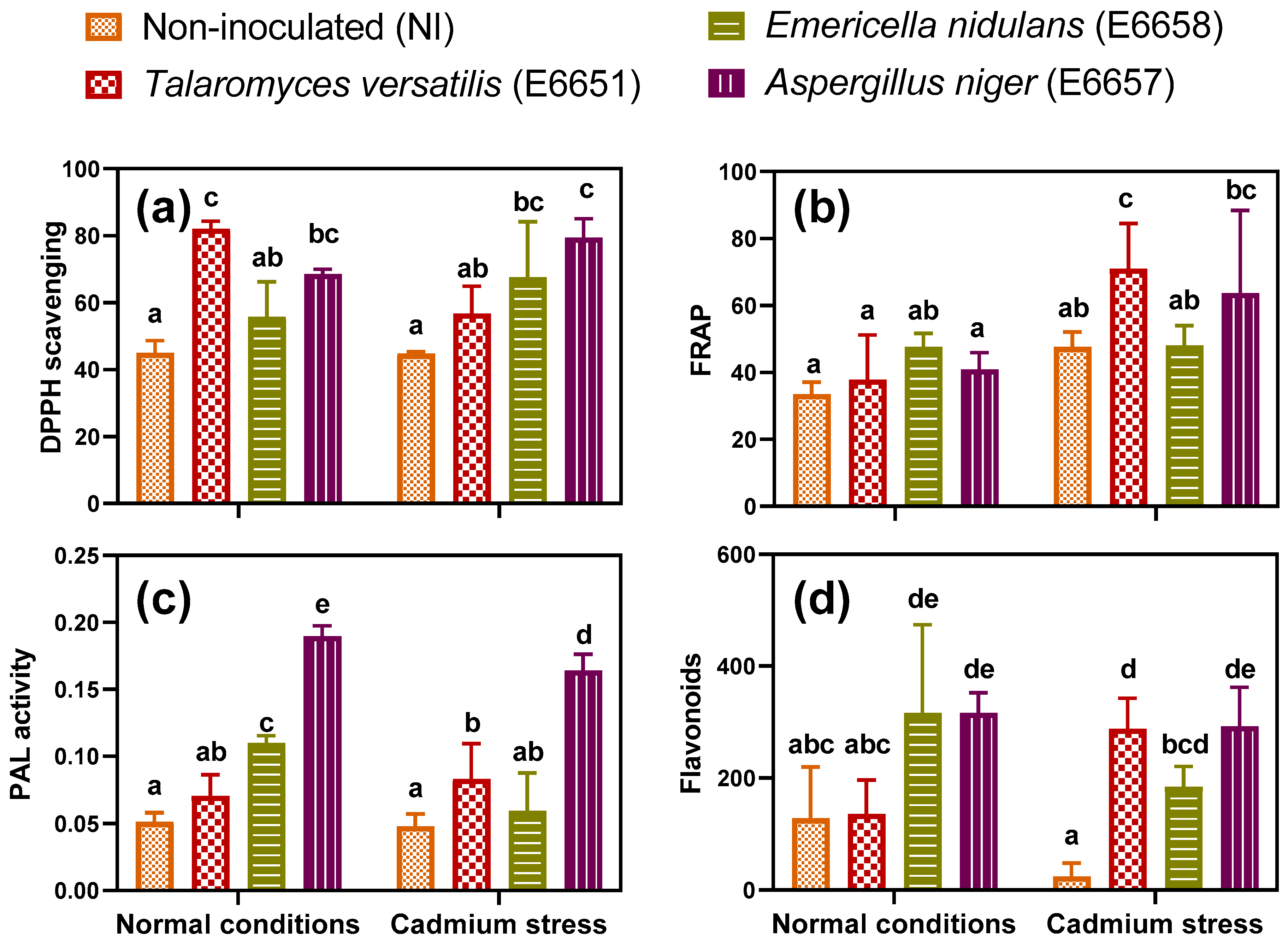
3.7. Biological and Medicinal Value
3.7.1. Inoculation with Endophytes Enhanced Total Antioxidant Capacity (TAC) and Stimulated Phenolic Metabolism
3.7.2. Inoculation with Endophytic Fungi Increased Biological and Medicinal Value of Cd-Stressed Geranium Plants
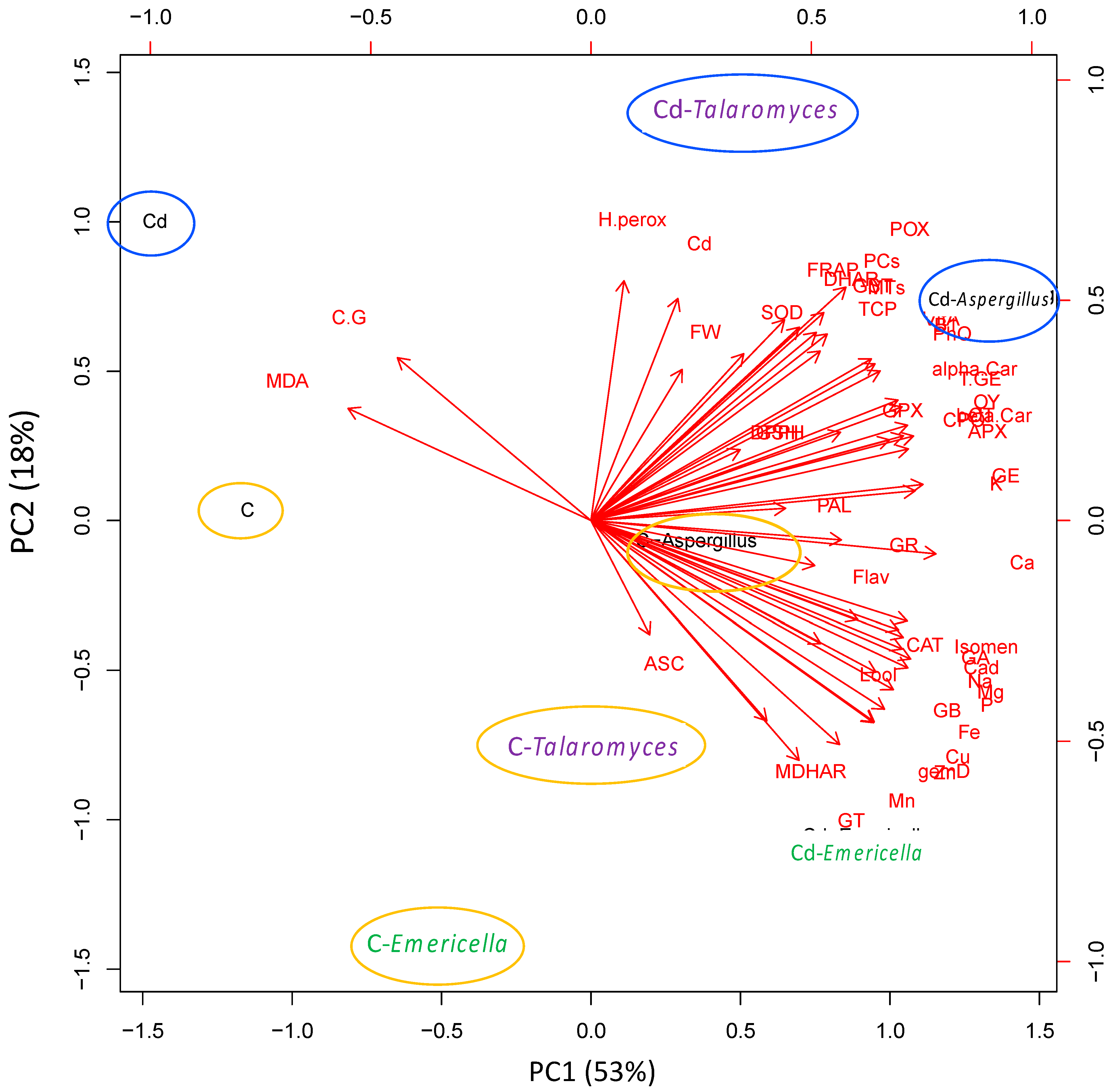
3.8. Principal Component Analysis (PCA) Revealed the Variation in the Mechanisms Modulated by the Different Endophytic Fungi
4. Discussion
4.1. Inoculated Geranium Plants Showed Improved Growth and Mineral Nutrition under Cd-Stress
4.2. Inoculated Geranium Plants Were Less Sensitive to Cd Oxidative Stress
4.3. Improved Cd Detoxification Level Can also Explain Endophytes-Induced Tolerance in Geranium
4.4. Endophyte Increased Medicinal and Economic Values of Geranium Plant by Improving Its Tissue Chemical Composition and Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narnoliya, L.K.; Jadaun, J.S.; Singh, S.P. The Phytochemical Composition, Biological Effects and Biotechnological Approaches to the Production of High-Value Essential Oil from Geranium. In Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer International Publishing: Cham, Switerland, 2019; pp. 327–352. [Google Scholar]
- Blerot, B.; Baudino, S.; Prunier, C.; Demarne, F.; Toulemonde, B.; Caissard, J.-C. Botany, agronomy and biotechnology of Pelargonium used for essential oil production. Phytochem. Rev. 2016, 15, 935–960. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Mohamed, A.A. Survey on medicinal plants and spices used in Beni-Sueif, Upper Egypt. J. Ethnobiol. Ethnomed. 2011, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Marshall, V.; Hamidpour, R. Pelargonium graveolens (Rose Geranium)—A novel therapeutic agent for antibacterial, antioxidant, antifungal and diabetics. Arch. Can. Res. 2017, 5, 1. [Google Scholar] [CrossRef]
- Rao, B.R.R. Chemical Composition and Uses of Indian Rose-Scented Geranium (Pelargonium Species) Essential Oil—A Review. J. Essent. Oil Bear. Plants 2009, 12, 381–394. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Chand, S.; Singh, G.; Rajkumari; Patra, D.D. Performance of rose scented geranium (Pelargonium graveolens) in heavy metal polluted soil vis-à-vis phytoaccumulation of metals. Int. J. Phytoremed. 2016, 18, 754–760. [Google Scholar] [CrossRef]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2020, 1–52. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-Metal-Induced Reactive Oxygen Species: Phytotoxicity and Physicochemical Changes in Plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 232, pp. 1–44. [Google Scholar]
- Fahimirad, S.; Hatami, M. Heavy metal-mediated changes in growth and phytochemicals of edible and medicinal plants. In Medicinal Plants and Environmental Challenges; Springer: Berlin/Heidelberg, Germany, 2017; pp. 189–214. [Google Scholar]
- Luo, J.-S.; Zhang, Z. Mechanisms of cadmium phytoremediation and detoxification in plants. Crop. J. 2021, 9, 521–529. [Google Scholar] [CrossRef]
- Peng, J.-S.; Guan, Y.-H.; Lin, X.-J.; Xu, X.-J.; Xiao, L.; Wang, H.-H.; Meng, S. Comparative understanding of metal hyperaccumulation in plants: A mini-review. Environ. Geochem. Health 2021, 43, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, L.; Duan, Q.; Liu, X.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant Signal. Behav. 2021, 16, 1836884. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Overbeek, L.S.v.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Hussain, J.; Al-Harrasi, A.; Hamayun, M.; Lee, I.-J. Phytohormones enabled endophytic fungal symbiosis improve aluminum phytoextraction in tolerant Solanum lycopersicum: An examples of Penicillium janthinellum LK5 and comparison with exogenous GA3. J. Hazard. Mater. 2015, 295, 70–78. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, O.M.; Mohamed, H.I.; Mogazy, A.M. Biosorption effect of Aspergillus niger and Penicillium chrysosporium for Cd- and Pb-contaminated soil and their physiological effects on Vicia faba L. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Khalvandi, M.; Amerian, M.; Pirdashti, H.; Keramati, S. Does co-inoculation of mycorrhiza and Piriformospora indica fungi enhance the efficiency of chlorophyll fluorescence and essential oil composition in peppermint under irrigation with saline water from the Caspian Sea? PLoS ONE 2021, 16, e0254076. [Google Scholar] [CrossRef]
- Yan, K. Effects of SA and H2O2 Mediated Endophytic Fungal Elicitors on Essential Oil in Suspension Cells of Cinnamomum longepaniculatum. Open Access Libr. J. 2020, 7, 97624. [Google Scholar] [CrossRef]
- Yan, K.; Wei, Q.; Feng, R.; Zhou, W. Transcriptome analysis of the effects of endophytic fungi on the biosynthesis of essential oils in Cinnamomum longepaniculatum. Int. J. Agric. Biol. 2019, 21, 1301–1308. [Google Scholar]
- Wang, Y.; Dai, C.-C.; Zhao, Y.-W.; Peng, Y. Fungal endophyte-induced volatile oil accumulation in Atractylodes lancea plantlets is mediated by nitric oxide, salicylic acid and hydrogen peroxide. Process Biochem. 2011, 46, 730–735. [Google Scholar] [CrossRef]
- Dolatabadi, H.K.; Goltapeh, E.M.; Jaimand, K.; Rohani, N.; Varma, A. Effects of Piriformospora indica and Sebacina vermifera on growth and yield of essential oil in fennel (Foeniculum vulgare) under greenhouse conditions. J. Basic Microbiol. 2011, 51, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, H.K.; Goltapeh, E.M.; Moieni, A.; Jaimand, K.; Sardrood, B.P.; Varma, A. Effect of Piriformospora indica and Sebacina vermifera on plant growth and essential oil yield in Thymus vulgaris in vitro and in vivo experiments. Symbiosis 2011, 53, 29–35. [Google Scholar] [CrossRef]
- Berns, A.E.; Philipp, H.; Narres, H.D.; Burauel, P.; Vereecken, H.; Tappe, W. Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Eur. J. Soil Sci. 2008, 59, 540–550. [Google Scholar] [CrossRef]
- Yasser, M.M.; Marzouk, M.A.; El-Shafey, N.M.; Shaban, S.A. Diversity and Antimicrobial Activity of Endophytic Fungi from the Medicinal Plant Pelargonium graveolens (geranium) in Middle Egypt. Jordan J. Biol. Sci. 2020, 13, 157–205. [Google Scholar]
- AbdElgawad, H.; De Vos, D.; Zinta, G.; Domagalska, M.A.; Beemster, G.T.S.; Asard, H. Grassland species differentially regulate proline concentrations under future climate conditions: An integrated biochemical and modelling approach. New Phytol. 2015, 208, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance, Methods in Molecular Biology (Methods and Protocols); Sunkar, R., Ed.; Humana Press Springer: New York, NY, USA, 2010; Volume 639, pp. 291–297. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.L.; Reid, D.M. Leaf senescence and lipid peroxidation: Effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol. Plant. 1982, 56, 453–457. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods Enzymol.; Lester, P., Ed.; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Kumar, K.B.; Khan, P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv PR 202) leaves during senescence [millets]. Ind. J. Exp. Bot. 1982, 20, 412–416. [Google Scholar]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate-glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. biol. chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Mozer, T.J.; Tiemeier, D.C.; Jaworski, E.G. Purification and characterization of corn glutathione S-transferase. Biochemistry 1983, 22, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- De Knecht, J.A.; Koevoets, P.L.M.; Verkleij, J.A.C.; Ernst, W.H.O. Evidence against a role for phytochelatins in naturally selected increased cadmium tolerance in Silene vulgaris (Moench) Garcke. New Phytol. 1992, 122, 681–688. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Abuelsoud, W.; Hassan, Y.M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Zrieq, R.; Beemster, G.T.S.; Schoenaers, S. An actinomycete strain of Nocardiopsis lucentensis reduces arsenic toxicity in barley and maize. J. Hazard. Mater. 2021, 417, 126055. [Google Scholar] [CrossRef] [PubMed]
- Diopan, V.; Shestivska, V.; Adam, V.; Macek, T.; Mackova, M.; Havel, L.; Kizek, R. Determination of content of metallothionein and low molecular mass stress peptides in transgenic tobacco plants. Plant Cell Tissue Organ Cult. 2008, 94, 291–298. [Google Scholar] [CrossRef]
- De Sousa, A.; AbdElgawad, H.; Asard, H.; Pinto, A.; Soares, C.; Branco-Neves, S.; Braga, T.; Azenha, M.; Selim, S.; Al Jaouni, S.; et al. Metalaxyl Effects on Antioxidant Defenses in Leaves and Roots of Solanum nigrum L. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Camm, E.L.; Towers, G.N. Phenylalanine ammonia lyase. Phytochemistry 1973, 12, 961–973. [Google Scholar] [CrossRef]
- Sayed, M.; Khodary, S.; Ahmed, E.; Hammouda, O.; Hassan, H.; El-Shafey, N. Elicitation of flavonoids by chitosan and salicylic acid in callus of Rumex vesicarius L. Acta Hortic. 2016, 1187, 165–176. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Jakob, E.; Elmadfa, I. Application of a simplified HPLC assay for the determination of phylloquinone (vitamin K1) in animal and plant food items. Food Chem. 1996, 56, 87–91. [Google Scholar] [CrossRef]
- Oostende, C.v.; Widhalm, J.R.; Basset, G.J.C. Detection and quantification of vitamin K1 quinol in leaf tissues. Phytochemistry 2008, 69, 2457–2462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeeb, M.; Ganjali, M.R.; Norouzi, P. Dispersive liquid-liquid microextraction followed by spectrofluorimetry as a simple and accurate technique for determination of thiamine (vitamin B1). Microchim. Acta 2010, 168, 317–324. [Google Scholar] [CrossRef]
- Zinta, G.; AbdElgawad, H.; Domagalska, M.A.; Vergauwen, L.; Knapen, D.; Nijs, I.; Janssens, I.A.; Beemster, G.T.S.; Asard, H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Chang. Biol. 2014, 20, 3670–3685. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Machmudah, S.; Roy, B.C.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of essential oil from geranium (Pelargonium graveolens) with supercritical carbon dioxide. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2006, 81, 167–172. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 37. [Google Scholar] [CrossRef]
- Khan, A.R.; Ullah, I.; Waqas, M.; Park, G.-S.; Khan, A.L.; Hong, S.-J.; Ullah, R.; Jung, B.K.; Park, C.E.; Ur-Rehman, S. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol. Environ. Saf. 2017, 136, 180–188. [Google Scholar] [CrossRef]
- Imelouane, B.; Tahri, M.; Elbastrioui, M.; Aouinti, F.; Elbachiri, A. Mineral contents of some medicinal and aromatic plants growing in eastern Morocco. J. Mater. Environ. Sci 2011, 2, 104–111. [Google Scholar]
- Li, X.; Ma, L.; Li, Y.; Wang, L.; Zhang, L. Endophyte infection enhances accumulation of organic acids and minerals in rice under Pb2+ stress conditions. Ecotoxicol. Environ. Saf. 2019, 174, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-T.; Qiu, Z.-B.; Zhang, X.-W.; Wang, L.-S. Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol. Plant. 2011, 33, 835–842. [Google Scholar] [CrossRef]
- Khan, A.L.; Lee, I.-J. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013, 13, 86. [Google Scholar] [CrossRef]
- Asaf, S.; Hamayun, M.; Khan, A.L.; Waqas, M.; Khan, M.A.; Jan, R.; Lee, I.-J.; Hussain, A. Salt tolerance of Glycine max. L induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiol. Biochem. 2018, 128, 13–23. [Google Scholar]
- Bilal, S.; Khan, A.L.; Shahzad, R.; Asaf, S.; Kang, S.-M.; Lee, I.-J. Endophytic Paecilomyces formosus LHL10 Augments Glycine max L. Adaptation to Ni-Contamination through Affecting Endogenous Phytohormones and Oxidative Stress. Front. Plant Sci. 2017, 8, 870. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Gilani, S.A.; Waqas, M.; Al-Hosni, K.; Al-Khiziri, S.; Kim, Y.-H.; Ali, L.; Kang, S.-M.; Asaf, S.; Shahzad, R.; et al. Endophytes from medicinal plants and their potential for producing indole acetic acid, improving seed germination and mitigating oxidative stress. J. Zhejiang Univ. Sci. B 2017, 18, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Mahmoud, A.; AbdElgawad, H.; Hamed, B.A.; Beemster, G.T.S.; El-Shafey, N.M. Differences in Cadmium Accumulation, Detoxification and Antioxidant Defenses between Contrasting Maize Cultivars Implicate a Role of Superoxide Dismutase in Cd Tolerance. Antioxidants 2021, 10, 1812. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-Transferases: Role in Combating Abiotic Stresses Including Arsenic Detoxification in Plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.; Benavides, M.; Tomaro, M. Involvement of an antioxidant defence system in the adaptive response to heavy metal ions in Helianthus annuus L. cells. Plant Growth Regul. 2002, 36, 267–273. [Google Scholar] [CrossRef]
- Golparyan, F.; Azizi, A.; Soltani, J. Endophytes of Lippia citriodora (Syn. Aloysia triphylla) enhance its growth and antioxidant activity. Eur. J. Plant Pathol. 2018, 152, 759–768. [Google Scholar] [CrossRef]
- Qin, J.; Wu, M.; Liu, H.; Gao, Y.; Ren, A. Endophyte Infection and Methyl Jasmonate Treatment Increased the Resistance of Achnatherum sibiricum to Insect Herbivores Independently. Toxins 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Radwan, U.; El-Zayat, S.; El-Sayed, M.A. The role of the endophytic fungus, Thermomyces lanuginosus, on mitigation of heat stress to its host desert plant Cullen plicata. Biol. Futur. 2019, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Kumar, S.; Pandey, A.; Chand, S. Microbial and chemical sources of phosphorus supply modulate the yield and chemical composition of essential oil of rose-scented geranium (Pelargonium species) in sodic soils. Biol. Fertil. Soils 2012, 48, 117–122. [Google Scholar] [CrossRef]
- Blerot, B.; Martinelli, L.; Prunier, C.; Saint-Marcoux, D.; Legrand, S.; Bony, A.; Sarrabère, L.; Gros, F.; Boyer, N.; Caissard, J.-C.; et al. Functional Analysis of Four Terpene Synthases in Rose-Scented Pelargonium Cultivars (Pelargonium × hybridum) and Evolution of Scent in the Pelargonium Genus. Front. Plant Sci. 2018, 9, 1435. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef]
| Minerals | Normal Conditions | Cadmium Stress | ||||||
|---|---|---|---|---|---|---|---|---|
| NI | T. versatilis (E6651) | E. nidulans (E6658) | A. niger (E6657) | NI | T. versatilis (E6651) | E. nidulans (E6658) | A. niger (E6657) | |
| Cd | 0.000 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 8.351 ± 0.51 c | 8.187 ± 0.62 c | 6.586 ± 0.64 b | 6.352 ± 0.84 b |
| K | 1.440 ± 0.09 bc | 1.36 ± 0.24 bc | 1.14 ± 0.20 ab | 1.94 ± 0.19 d | 0.780 ± 0.05 a | 1.85 ± 0.12 cd | 2.070 ± 0.19 d | 2.270 ± 0.10 d |
| Ca | 0.028 ± 0.00 b | 0.035 ± 0.01 bc | 0.03 ± 0.00 bc | 0.04 ± 0.00 cd | 0.017 ± 0.00 a | 0.04 ± 0.00 cd | 0.047 ± 0.00 d | 0.051 ± 0.00 d |
| Mg | 0.410 ± 0.02 ab | 0.61 ± 0.11 cd | 0.54 ± 0.06 bc | 0.62 ± 0.05 cd | 0.252 ± 0.03 a | 0.53 ± 0.05 bc | 0.850 ± 0.04 e | 0.768 ± 0.09 de |
| P | 2.840 ± 0.23 b | 4.12 ± 0.59 cd | 3.71 ± 0.31 bc | 4.09 ± 0.34 cd | 1.550 ± 0.11 a | 3.58 ± 0.26 bc | 5.370 ± 0.35 d | 4.950 ± 0.54 de |
| Na | 0.378 ± 0.01 a | 0.73 ± 0.14 bc | 0.71 ± 0.08 bc | 0.68 ± 0.05 bc | 0.261 ± 0.03 a | 0.606 ± 0.07 b | 0.75 ± 0.06 bc | 0.876 ± 0.09 c |
| Fe | 0.060 ± 0.01 ab | 0.08 ± 0.0 bcd | 0.08 ± 0.0 bcd | 0.07 ± 0.01 bc | 0.039 ± 0.00 a | 0.067 ± 0.01 b | 0.103 ± 0.01 d | 0.098 ± 0.01 cd |
| Cu | 0.010 ± 0.00 ab | 0.02 ± 0.0 bcd | 0.02 ± 0.0 bcd | 0.016 ± 0.0 bc | 0.008 ± 0.00 a | 0.014 ± 0.0 bc | 0.022 ± 0.00 d | 0.019 ± 0.00 cd |
| Mn | 0.034 ± 0.00 b | 0.06 ± 0.01 cd | 0.053 ± 0.0 cd | 0.052 ± 0.00 c | 0.016 ± 0.00 a | 0.05 ± 0.01 bc | 0.069 ± 0.00 d | 0.046 ± 0.00 bc |
| Zn | 0.060 ± 0.01 b | 0.09 ± 0.02 c | 0.090 ± 0.01 c | 0.089 ± 0.01 c | 0.030 ± 0.00 a | 0.08 ± 0.01 bc | 0.120 ± 0.01 d | 0.095 ± 0.00 cd |
| Normal Conditions | Cadmium Stress | |||||||
|---|---|---|---|---|---|---|---|---|
| NI | T. versatilis (E6651) | E. nidulans (E6658) | A. niger (E6657) | NI | T. versatilis (E6651) | E. nidulans (E6658) | A. niger (E6657) | |
| Vitamins | ||||||||
| β-Cryptoxanthin (Vit. A) | 1.27 ± 0.07 bc | 1.27 ± 0.09 bc | 0.73 ± 0.06 a | 1.48 ± 0.09 cd | 1.05 ± 0.09 b | 1.61 ± 0.14 d | 1.56 ± 0.09 cd | 1.94 ± 0.11 e |
| α-Carotene (Vit A) | 0.79 ± 0.05 bc | 0.89 ± 0.07 cd | 0.54 ± 0.03 a | 0.99 ± 0.05 de | 0.68 ± 0.05 ab | 1.03 ± 0.05 e | 1.06 ± 0.04 de | 1.26 ± 0.06 f |
| β-Carotene (Vit. A) | 0.54 ± 0.01 b | 0.67 ± 0.01 c | 0.43 ± 0.01 a | 0.71 ± 0.01 cd | 0.48 ± 0.00 ab | 0.72 ± 0.01 cd | 0.78 ± 0.01 d | 0.90 ± 0.01 e |
| Thiamine (Vit B) | 1.10 ± 0.06 bc | 1.11 ± 0.08 bc | 0.64 ± 0.05 a | 1.29 ± 0.07 cd | 0.91 ± 0.07 b | 1.39 ± 0.11 d | 1.36 ± 0.07 d | 1.68 ± 0.09 e |
| Ascorbic acid (Vit. C) | 0.18 ± 0.07 a | 0.17 ± 0.01 a | 0.27 ± 0.03 c | 0.17 ± 0.01 a | 0.19 ± 0.00 a | 0.17 ± 0.01 a | 0.21 ± 0.00 ab | 0.25 ± 0.01 bc |
| Tocopherols (Vit. E) | 31.9 ± 0.89 a | 58.1 ± 11.1 ab | 48.3 ± 8.3 ab | 63.7 ± 12.2 b | 56.4 ± 1.3 ab | 66.1 ± 11.7 b | 48.6 ± 4.2 ab | 77.4 ± 14.1 b |
| Phylloquinone (Vit. K) | 0.92 ± 0.05 bc | 0.95 ± 0.07 bc | 0.56 ± 0.04 a | 1.09 ± 0.06 cd | 0.77 ± 0.06 b | 1.17 ± 0.09 d | 1.16 ± 0.06 d | 1.42 ± 0.07 e |
| Essential oil (%) | ||||||||
| Citronellol (1.216) | 17.4 ± 1.0 abc | 16.5 ± 3.0 ab | 13.7 ± 2.0 a | 23.5 ± 2.0 cd | 14.8 ± 1.00 a | 22.0 ± 2 bcd | 25.3 ± 2.0 d | 27.6 ± 1.0 d |
| Trans-geraniol (1.166) | 2.60 ± 0.2 ab | 2.4 ± 0.4 a | 2.20 ± 0.3 a | 3.40 ± 0.3 bc | 2.10 ± 0.10 a | 3.60 ± 0.2 c | 3.50 ± 0.2 c | 3.90 ± 0.5 c |
| Isomenthone (1.144) | 1.30 ± 0.2 a | 2.4 ± 0.6 bcd | 2.3 ± 0.3 abcd | 2.40 ± 0.2 bcd | 1.60 ± 0.2 ab | 2.10 ± 0.3 abc | 3.00 ± 0.2 cd | 3.20 ± 0.3 d |
| Linalool (1.082) | 0.96 ± 0.1 ab | 0.91 ± 0.1 ab | 0.70 ± 0.04 ab | 1.00 ± 0.1 b | 0.60 ± 0.04 a | 0.90 ± 0.1 ab | 1.70 ± 0.2 c | 1.10 ± 0.2 b |
| Geranyl acetate (1.362) | 1.00 ± 0.04 a | 2.0 ± 0.4 bc | 1.90 ± 0.2 bc | 1.90 ± 0.2 bc | 1.00 ± 0.10 a | 1.60 ± 0.2 ab | 2.00 ± 0.2 bc | 2.30 ± 0.2 c |
| γ-cadinene (1.514) | 0.04 ± 0.01 a | 0.08 ± 0.02 bc | 0.07 ± 0.01 bc | 0.08 ± 0.01 bc | 0.05 ± 0.01 ab | 0.06 ± 0.01 ab | 0.1 ± 0.01 c | 0.10 ± 0.01 c |
| Geranyl butyrate (1.561) | 0.69 ± 0.10 a | 0.92 ± 0.2 abc | 0.91 ± 0.07 abc | 0.86 ± 0.07 ab | 0.70 ± 0.06 a | 0.77 ± 0.07 a | 1.20 ± 0.07 c | 1.10 ± 0.2 bc |
| Geranyl tiglate (1.572) | 0.10 ± 0.006 ab | 0.18 ± 0.04 cd | 0.17 ± 0.02 cd | 0.17 ± 0.01 cd | 0.07 ± 0.005 a | 0.15 ± 0.02 bc | 0.21 ± 0.02 d | 0.13 ± 0.02 bc |
| Gemacrene D (1.477) | 0.08 ± 0.01 ab | 0.129 ± 0.02 c | 0.125 ± 0.01 c | 0.12 ± 0.01 c | 0.07 ± 0.00 a | 0.11 ± 0.01 bc | 0.17 ± 0.01 d | 0.132 ± 0.01 cd |
| Caryophyllene oxide (1.583) | 1.80 ± 0.06 a | 1.60 ± 0.3 a | 2.20 ± 0.3 ab | 2.90 ± 0.1 bc | 1.70 ± 0.2 a | 2.80 ± 0.2 bc | 2.70 ± 0.3 bc | 3.30 ± 0.4 c |
| Geraniol (1.246) | 11.4 ± 1.1 a | 11.4 ± 1.8 a | 13.0 ± 1.7 ab | 17.1 ± 0.6 c | 9.20 ± 1.0 a | 16.6 ± 1.0 bc | 18.9 ± 1.1 c | 23.1 ± 1.2 d |
| C/G | 1.53 ± 0.1 bc | 1.43 ± 0.1 bc | 1.04 ± 0.1 a | 1.37 ± 0.9 abc | 1.66 ± 0.2 c | 1.36 ± 0.2 abc | 1.33 ± 0.1 abc | 1.20 ± 0.1 ab |
| Oil content (%) | 0.31 ± 0.02 a | 0.39 ± 0.03 ab | 0.34 ± 0.05 a | 0.49 ± 0.03 bc | 0.39 ± 0.03 ab | 0.53 ± 0.02 cd | 0.62 ± 0.03 de | 0.73 ± 0.07 e |
| Oil yield (mL Plant−1) | 27.3 ± 2.2 b | 59.5 ± 4.8 d | 12.1 ± 1.7 a | 48.9 ± 2.8 c | 20.3 ± 1.6 ab | 84.9 ± 3.2 e | 28.5 ± 1.4 b | 67.7 ± 6.1 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shafey, N.M.; Marzouk, M.A.; Yasser, M.M.; Shaban, S.A.; Beemster, G.T.S.; AbdElgawad, H. Harnessing Endophytic Fungi for Enhancing Growth, Tolerance and Quality of Rose-Scented Geranium (Pelargonium graveolens (L’Hér) Thunb.) Plants under Cadmium Stress: A Biochemical Study. J. Fungi 2021, 7, 1039. https://doi.org/10.3390/jof7121039
El-Shafey NM, Marzouk MA, Yasser MM, Shaban SA, Beemster GTS, AbdElgawad H. Harnessing Endophytic Fungi for Enhancing Growth, Tolerance and Quality of Rose-Scented Geranium (Pelargonium graveolens (L’Hér) Thunb.) Plants under Cadmium Stress: A Biochemical Study. Journal of Fungi. 2021; 7(12):1039. https://doi.org/10.3390/jof7121039
Chicago/Turabian StyleEl-Shafey, Nadia Mohamed, Marym A. Marzouk, Manal M. Yasser, Salwa A. Shaban, Gerrit T.S. Beemster, and Hamada AbdElgawad. 2021. "Harnessing Endophytic Fungi for Enhancing Growth, Tolerance and Quality of Rose-Scented Geranium (Pelargonium graveolens (L’Hér) Thunb.) Plants under Cadmium Stress: A Biochemical Study" Journal of Fungi 7, no. 12: 1039. https://doi.org/10.3390/jof7121039
APA StyleEl-Shafey, N. M., Marzouk, M. A., Yasser, M. M., Shaban, S. A., Beemster, G. T. S., & AbdElgawad, H. (2021). Harnessing Endophytic Fungi for Enhancing Growth, Tolerance and Quality of Rose-Scented Geranium (Pelargonium graveolens (L’Hér) Thunb.) Plants under Cadmium Stress: A Biochemical Study. Journal of Fungi, 7(12), 1039. https://doi.org/10.3390/jof7121039







