Diversity and Evolution of Entomocorticium (Russulales, Peniophoraceae), a Genus of Bark Beetle Mutualists Derived from Free-Living, Wood Rotting Peniophora
Abstract
:1. Introduction
2. Material and Methods
2.1. Fungus Isolation
2.2. Morphological Observations
2.3. Taxa Sampling and Sources
2.4. DNA Extraction, PCR Amplification, and Sequencing
2.5. Phylogenetic Analyses
2.6. Ancestral Character State Reconstruction
2.7. Post-Analyses Graphical Display
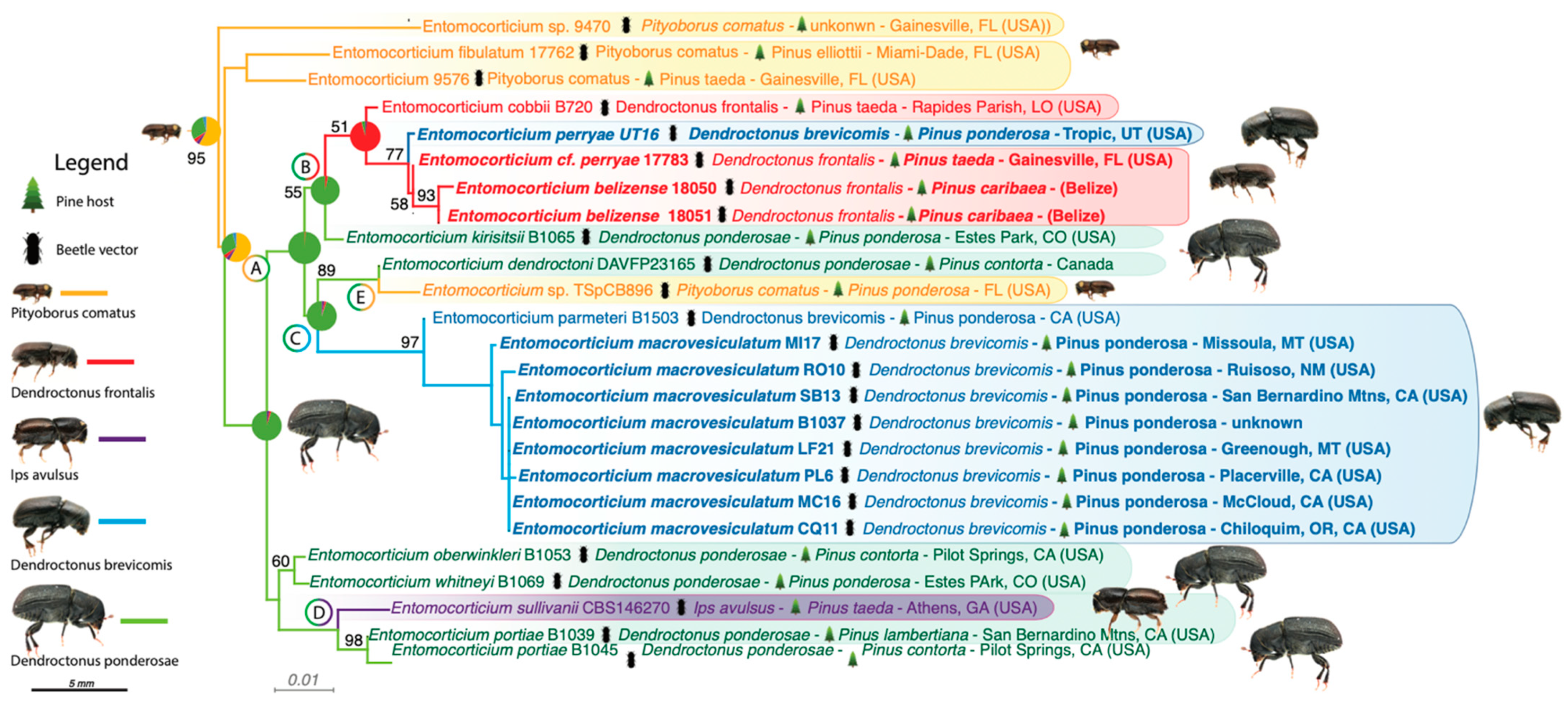
3. Results
Taxonomy
4. Discussion
4.1. How Did Such Relationships Arise?
4.2. Distinct Associations across Bark Beetles and Entomocorticium
4.3. Distinct Functional Traits in Basidiomycota and Ascomycota Associated with Bark Beetles
4.4. Domestication of Entomocorticium by Beetles Facilitated the Loss of Morphological Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aanen, D.K.; Eggleton, P. Fungus-growing termites originated in African rain forest. Curr. Biol. 2005, 15, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Biedermann, P.H.; Vega, F.E. Ecology and evolution of insect–fungus mutualisms. Annu. Rev. Entomol. 2020, 65, 431–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Six, D.L.; Poulsen, M.; Hansen, A.K.; Wingfield, M.J.; Roux, J.; Eggleton, P.; Slippers, B.; Paine, T.D. Anthropogenic effects on insect-microbial symbioses in forest and savanna ecosystems. Symbiosis 2011, 53, 101–121. [Google Scholar] [CrossRef]
- Mueller, U.G.; Gerardo, N.M.; Aanen, D.K.; Six, D.L.; Schultz, T.R. The Evolution of Agriculture in Insects. Ann. Rev. Ecol. Evol. Syst. 2005, 36, 563–595. [Google Scholar] [CrossRef]
- Hulcr, J.; Stelinski, L.L. The ambrosia symbiosis: From evolutionary ecology to practical management. Ann. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke-Grosman, H. Hautdrüsen als träger der pilzsymbiose bei ambrosiakäfern. Zoomorphology 1956, 45, 275–308. [Google Scholar]
- Batra, L.R. Ecology of ambrosia fungi and their dissemination by beetles. Trans. Kans. Acad. Sci. 1963, 66, 213–236. [Google Scholar] [CrossRef]
- Six, D.L. Bark beetle-fungus symbioses. Insect Symbiosis 2003, 1, 97–114. [Google Scholar]
- Gomez, D.F.; Sathyapala, S.; Hulcr, J. Towards Sustainable Forest Management in Central America: Review of Southern Pine Beetle (Dendroctonus frontalis Zimmermann) Outbreaks, Their Causes, and Solutions. Forests 2020, 11, 173. [Google Scholar] [CrossRef] [Green Version]
- Thatcher, R.C.; Searcy, J.L.; Coster, J.E.; Hertel, G.D. The Southern Pine Beetle. USDA, Expanded Southern Pine Beetle Research and Application Program, Forest Service, Science and Education Administration, Pineville, LA. Technical. Bull. 1980, 1631, 265. [Google Scholar]
- Coulson, R.N.; Klepzig, K.D. Southern Pine Beetle II. In General Technical Report, 1st ed.; U.S. Department of Agriculture Forest: Asheville, NC, USA, 2011. [Google Scholar]
- Hofstetter, R.W.; Cronin, J.T.; Klepzig, K.D. Antagonisms, mutualisms, and commensalisms affect outbreak dynamics of the southern pine beetle. Oecologia 2006, 147, 679–691. [Google Scholar] [CrossRef]
- Six, D.L. Ecological and Evolutionary Determinants of Bark Beetle–Fungus Symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitney, H.S.; Bandoni, R.J.; Oberwinkler, F. Entomocorticium dendroctoni gen. et sp. nov. (Basidiomycotina), a possible nutritional symbiote of the mountain pine beetle in lodgepole pine in British Columbia. Can. J. Bot. 1987, 65, 95–102. [Google Scholar] [CrossRef]
- Harrington, T.C.; Batzer, J.C.; McNew, D.L. Corticioid basidiomycetes associated with bark beetles, including seven new Entomocorticium species from North America and Cylindrobasidium ipidophilum, comb. nov. Antonie Van Leeuwenhoek 2021, 114, 561–579. [Google Scholar] [CrossRef]
- Hsiau, P.T.W.; Harrington, T.C. Phylogenetics and adapations of basidiomycetous fungi fed upon by bark beetles (Coleoptera: Scolytidae). Symbiosis 2003, 34, 111–131. [Google Scholar]
- Victor, J.; Zuniga, G. Phylogeny of Dendroctonus bark beetles (Coleoptera: Curculionidae: Scolytinae) inferred from morphological and molecular data. Syst. Entomol. 2016, 41, 162–177. [Google Scholar] [CrossRef]
- Godefroid, M.; Meseguer, A.S.; Sauné, L.; Genson, G.; Streito, J.C.; Rossi, J.P.; Riverón, A.Z.; Mayer, F.; Cruaud, A.; Rasplus, J.Y. Restriction-site associated DNA markers provide new insights into the evolutionary history of the bark beetle genus Dendroctonus. Mol. Phylogenet. Evol. 2019, 139, 106528. [Google Scholar] [CrossRef] [Green Version]
- Whitney, H.S.; Farris, F.H. Maxillary Mycangium in the Mountain Pine Beetle. Science 1970, 167, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L.; Klepzig, K.D. Dendroctonus bark beetles as model systems for studies on symbiosis. Symbiosis 2004, 37, 2077–2232. [Google Scholar]
- Barras, S.J.; Perry, T.J. Fungal symbionts in the prothoracic mycangium of Dendroctonus frontalis (Coleoptera: Scolytidae). Xeitschrift Fur Angewande Entomol. 1972, 71, 95–104. [Google Scholar] [CrossRef]
- Yuceer, C.; Hsu, C.Y.; Erbilgin, N.; Klepzig, K.D. Ultrastructure of the mycangium of the southern pine beetle, Dendroctonus frontalis (Coleoptera: Curculionidae, Scolytinae): Complex morphology for complex interactions. Acta Zool. 2011, 92, 216–224. [Google Scholar] [CrossRef]
- Furniss, M.M.; Woo, J.Y.; Deyrup, M.A.; Atkinson, T.H. Prothoracic mycangium on pine-infesting Pityoborus spp. (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 1987, 80, 692–696. [Google Scholar] [CrossRef]
- Gouger, R.J.; Yearian, W.C.; Wilkinson, R.C. Feeding and reproductive behavior of Ips avulsus. Fla. Entomol. 1975, 58, 221–229. [Google Scholar] [CrossRef]
- Bracewell, R.R.; Six, D.L. Broadscale specificity in a bark beetle-fungal symbiosis: A spatio-temporal analysis of the mycangial fungi of the western pine beetle. Microb. Ecol. 2014, 68, 859–870. [Google Scholar] [CrossRef]
- Wood, S.L. The bark and Ambrosia Beetles of North and Central America (Coleoptera: Scolytidae), a Taxonomic Monograph Volume 6; Brigham Young University: Provo, UT, USA, 1982; pp. 1–1359. [Google Scholar]
- Armendáriz-Toledano, F.; Niño, A.; Sullivan, B.T.; Kirkendall, L.R.; Zúñiga, G. A new species of bark beetle, Dendroctonus mesoamericanus sp. nov. (Curculionidae: Scolytinae), in southern Mexico and Central America. Ann. Entomol. Soc. Am. 2015, 108, 403–414. [Google Scholar] [CrossRef]
- Armendáriz-Toledano, F.; Zúñiga, G. Illustrated key to species of genus Dendroctonus (Coleoptera: Curculionidae) occurring in Mexico and Central America. J. Insect Sci. 2017, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Cui, B.K.; Dai, Y.C. Global diversity and molecular systematics of Wrightoporia s.l. (Russulales, Basidiomycota). Persoonia 2016, 37, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Leal-Dutra, C.A.; Neves, M.A.; Griffith, G.W.; Reck, M.A.; Clasen, L.A.; Dentinger, B.T.M. Reclassification of Parapterulicium Corner (Pterulaceae, Agaricales), contributions to Lachnocladiaceae and Peniophoraceae (Russulales) and introduction of Baltazaria gen. nov. MycoKeys 2018, 37, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 1st ed.; Innis, M.A., Gelfand, D.H., Sninsky, J.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 231–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinform 2006, 22, 2588–2690. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. 2018. Available online: http://www.mesquiteproject.org (accessed on 15 February 2020).
- Huson, H.H.; Scornavacca, C. Dendroscope 3: An Interactive Tool for Rooted Phylogenetic Trees and Networks. Syst. Biol. 2012, 61, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoh, N.; Fukatsu, T. Interkingdom host jumping underground: Phylogenetic analysis of entomoparasitic fungi of the genus Cordyceps. Mol. Biol. Evol. 2000, 17, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, P.; Samuels, G.J. Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with the evidence of interkingdom host jumps and major shifts in ecology. Evolution 2013, 67, 2823–2837. [Google Scholar] [CrossRef] [PubMed]
- Skelton, J.; Jusino, M.A.; Li, Y.; Bateman, C.; Thai, P.H.; Wu, C.; Lindner, D.L.; Hulcr, J. Detecting Symbioses in Complex Communities: The Fungal Symbionts of Bark and Ambrosia Beetles Within Asian Pines. Microb. Ecol. 2018, 76, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Sung, G.H.; Sung, J.M.; Hywel-Jones, N.; White Jr, J.F. Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Mol. Ecol. 2007, 16, 1701–1711. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sink, S.; Libeskind-Hadas, R.; Hulcr, J.; Kasson, M.T.; Ploetz, R.C.; Konkol, J.L.; Ploetz, J.N.; Carrillo, D.; Campbell, A.; et al. Discordant phylogenies suggest repeated host shifts in the Fusarium-Euwallaceae ambrosia beetle mutualism. Fungal Genet. Biol. 2015, 82, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Araújo, J.P.M.; Hughes, D.P. Zombie-ant fungi emerged from non- manipulating, beetle-infecting ancestors. Curr. Biol. 2019, 29, 3735–3738. [Google Scholar] [CrossRef]
- Araújo, J.P.M.; Moriguchi, M.G.; Uchyiama, S.; Kinjo, N.; Matsuura, Y.M. Ophiocordyceps salganeicola, a parasite of social cockroaches in Japan and insights into the evolution of other closely-related Blattodea-associated lineages. IMA Fungus 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Skelton, J.; Johnson, A.J.; Jusino, M.A.; Bateman, C.C.; Li, Y.; Hulcr, J. A selective fungal transport organ (mycangium) maintains coarse phylogenetic congruence between fungus-farming ambrosia beetles and their symbionts. Proc. R. Soc. B 2019, 286, 20182127. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-J.; Breuil, C. Diversity of fungi associated with the mountain pine beetle, Dendroctonus ponderosae, and infected lodgepole pines in British Columbia. Fungal Divers. 2006, 22, 91–105. [Google Scholar]
- Roe, A.R.; James, P.M.A.; Rice, A.V.; Cooke, J.E.K.; Sperling, F.H. Spatial community structure of mountain pine beetle fungal symbionts across a latitudinal gradient. Microb. Ecol. 2011, 62, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Six, D.L.; Bentz, B.J. Temperature determines symbiont abundance in a multipartner bark beetle-fungus ectosymbiosis. Microb. Ecol. 2007, 54, 112–118. [Google Scholar] [CrossRef]
- Bracewell, R.R.; Six, D.L. Experimental evidence of bark beetle adaptation to a fungal symbiont. Ecol. Evol. 2019, 5, 5109–5119. [Google Scholar] [CrossRef]
- Bracewell, R.R.; Vanderpool, D.; Good, J.; Six, D.L. Cascading speciation among mutualists and antagonists in a tree-beetle-fungal interaction. R. Soc. Proc. B 2018, 285, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L.; Elser, J.J. Extreme ecological stoichiometry of a bark beetle-fungus mutualism. Ecol. Entomol. 2019, 44, 543–551. [Google Scholar] [CrossRef]
- Six, D.L. A major symbiont shift supports a major niche shift in a clade of tree-killing bark beetles. Ecol. Entomol. 2020, 45, 190–201. [Google Scholar] [CrossRef]
- Van de Peppel, L.J.; Aanen, D.K.; Biedermann, P.H. Low intraspecific genetic diversity indicates asexuality and vertical transmission in the fungal cultivars of ambrosia beetles. Fungal Ecol. 2018, 32, 57–64. [Google Scholar] [CrossRef]
- Matsuura, Y.; Moriyama, M.; Łukasik, P.; Vanderpool, D.; Tanahashi, M.; Meng, X.Y.; McCutcheon, J.P.; Fukatsu, T. Recurrent symbiont recruitment from fungal parasites in cicadas. Proc. Nat. Acad. Sci. USA 2018, 115, 5970–5979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boidin, J.; Languetin, P.; Gilles, G. Les Peniophoraceae de la zone intertropicale (Basidiomycetes, Aphyllophorales). Bull. Soc. Mycol. Fr. 1991, 107, 91–156. [Google Scholar]
- Andreasen, M.; Hallenberg, N. A taxonomic survey of the Peniophoraceae. Synop. Fungorum 2009, 26, 56–119. [Google Scholar]
- Muller, H.J. The relation of recombination to mutational advance. Mutat. Res. 1964, 1, 2–9. [Google Scholar] [CrossRef]
- Bidochka, M.J.; De Koning, J. Are teleomorphs really necessary? Modelling the potential effects of Muller’s Ratchet on deuteromycetous entomopathogenic fungi. Mycol. Res. 2001, 105, 1014–1019. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Gandhi, K.J.K. Bark Beetle Management, Ecology, and Climate Change; Academic Press: London, UK, 2021; pp. 405–438. [Google Scholar]
- Six, D.L.; Klepzig, K. Context dependency in bark beetle-fungus symbioses revisited: Assessing potential shifts in interaction outcomes against varied genetic, ecological, and evolutionary backgrounds. Front. Microbiol. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Farjon, A. Biodiversity of Pinus (Pinaceae) in Mexico: Speciation and palaeo-endemism. Bot. J. Linn. Soc. 1996, 121, 365–384. [Google Scholar] [CrossRef]





| Species | Voucher (Extype) | Beetle Vector | Tree Host | Isolate Origin | Material Source | Reference |
|---|---|---|---|---|---|---|
| Entomocorticium belizense | 18050 (CBS 148421) | Dendroctonus frontalis | Pinus caribaea | Belize | Mycangium | This study |
| 18051 | Dendroctonus frontalis | Pinus caribaea | Belize | Mycangium | This study | |
| Entomocorticium cobbii | B720 | Dendroctonus frontalis | Pinus taeda | Rapides Parish, LO, USA | Mycangium | Harrington et al. (2021) |
| Entomocorticium dendroctoni | DAVFP 23165 | Dendroctonus ponderosae | Pinus ponderosa | British Columbia, Canada | Pupal Chamber | Whitney et al. (1987) |
| Entomocorticium fibulatum | 17762 (CBS 148418) | Pityoborus comatus | Pinus elliotii | Miami-Dade, FL, USA | Mycangium | This study |
| Entomocorticium perryae | UT16 (CBS 148419) | Dendroctonus brevicomis | Pinus ponderosa | Tropic, UT, USA | Mycangium | This study |
| Entomocorticium kirisitsii | B1065 | Dendroctonus ponderosae | Pinus ponderosa | Estes Park, CO, USA | Pupal Chamber | Harrington et al. (2021) |
| Entomocorticium macrovesiculatum | PL6 | Dendroctonus brevicomis | Pinus ponderosa | Placerville, CA, USA | Mycangium | This study |
| LF21 | Dendroctonus brevicomis | Pinus ponderosa | Greenough, MT, USA | Mycangium | This study, Bracewell and Six (2014) | |
| CQ11 | Dendroctonus brevicomis | Pinus ponderosa | Chiloquim, OR, USA | Mycangium | This study, Bracewell and Six (2014) | |
| MI17 | Dendroctonus brevicomis | Pinus ponderosa | Missoula, MT, USA | Mycangium | This study, Bracewell and Six (2014) | |
| Ro10 | Dendroctonus brevicomis | Pinus ponderosa | Ruisoso, NM, USA | Mycangium | This study, Bracewell and Six (2014) | |
| SB13 | Dendroctonus brevicomis | Pinus ponderosa | San Bernardino Mtns, CA, USA | Mycangium | This study, Bracewell and Six (2014) | |
| MC16 (CBS 148421) | Dendroctonus brevicomis | Pinus ponderosa | McCloud, CA, USA | Mycangium | This study, Bracewell and Six (2014) | |
| Entomocorticium oberwinkleri | B1053 | Dendroctonus ponderosae | Pinus contorta | Pilot Springs, CA, USA | Pupal Chamber | Harrington et al. (2021) |
| Entomocorticium parmeteri | B1503 | Dendroctonus brevicomis | Pinus ponderosa | Tuolumme County, CA, USA | Gallery | Harrington et al. (2021) |
| Entomocorticium cf. perryae | 17783 (CBS 148417) | Dendroctonus frontalis | Pinus taeda | Gainesville, FL, USA | Mycangium | This study |
| Entomocorticium portiae | B1039 | Dendroctonus ponderosae | Pinus lambertiana | Blodgett Res. Forest, CA, USA | Pupal Chamber | Harrington et al. (2021) |
| Entomocorticium portiae | B1060 | Dendroctonus ponderosae | Pinus contorta | San Bernardino Mts., CA, USA | Pupal Chamber | Harrington et al. (2021) |
| Entomocorticium sp. | MMF-4485 | Pitioborus comatus | Pinus ponderosa | Florida | Pupal Chamber | Harrington et al. (2021) |
| Entomocorticium sp. | 9470 | Pityoborus comatus | unknown | Gainesville, FL, USA | Mycangium | This study |
| Entomocorticium sp. | 9576 | Pityoborus comatus | Pinus taeda | Gainesville, FL, USA | Mycangium | This study |
| Entomocorticium sullivanii | B1252 | Ips avulsus | Pinus taeda | Athens, GA, USA | Pupal Chamber | Harrington et al. (2021) |
| Entomocorticium whitneyi | B1069 | Dendroctonus ponderosae | Pinus ponderosa | Estes Park, CO, USA | Pupal Chamber | Harrington et al. (2021) |
| Species | Host | Voucher | SSU | ITS | LSU | TEF | Citation |
|---|---|---|---|---|---|---|---|
| Entomocorticium belizense | Pinus caribaea | 18050 | – | MZ098132 | MZ098117 | – | This study |
| 18051 | – | MZ098133 | MZ098116 | – | This study | ||
| Entomocorticium cobbii | Pinus taeda | B720 | – | MT741707 | MT741692 | – | Harrington et al. (2021) |
| Entomocorticium fibulatum | Pinus elliottii | 17762 | MZ098147 | MZ098135 | MZ098120 | – | This study |
| Entomocorticium perryae | Pinus ponderosa | UT16 | MZ098145 | MZ098123 | MZ098118 | MZ144591 | This study, Bracewell and Six (2014) |
| Entomocorticium kirisitsii | Pinus ponderosa | B1065 | – | MT741714 | MT741699 | – | Harrington et al. (2021) |
| Entomocorticium macrovesiculatum | Pinus ponderosa | MI17 | MZ098143 | MZ098129 | – | MZ144589 | This study, Bracewell and Six (2014) |
| RO10 | MZ098149 | MZ098130 | MZ098108 | MZ144590 | This study, Bracewell and Six (2014) | ||
| SB13 | MZ098141 | MZ098125 | MZ098110 | MZ144586 | This study, Bracewell and Six (2014) | ||
| B1037 | MZ098138 | MZ098124 | MZ098109 | MZ144585 | This study, Bracewell and Six (2014) | ||
| LF21 | MZ098139 | – | MZ098113 | MZ144587 | This study, Bracewell and Six (2014) | ||
| PL6 | MZ098140 | MZ098126 | MZ098114 | MZ144588 | This study | ||
| MC16 | MZ098144 | MZ098128 | MZ098112 | – | This study, Bracewell and Six (2014) | ||
| CQ11 | MZ098142 | MZ098127 | MZ098111 | – | This study, Bracewell and Six (2014) | ||
| Entomocorticium oberwinkleri | Pinus contorta | B1053 | – | MT741712 | MT741697 | – | Harrington et al. (2021) |
| Entomocorticium parmeteri | Pinus ponderosa | B1503 | – | MT741709 | MT741694 | – | Harrington et al. (2021) |
| Entomocorticium cf. perryae | Pinus taeda | 17783 | MZ098146 | MZ098131 | MZ098115 | MZ144592 | This study |
| Entomocorticium portiae | Pinus lambertiana | B1039 | – | MT741710 | MT741695 | – | Harrington et al. (2021) |
| Pinus contorta | B1045 | – | MT741711 | MT741696 | – | Harrington et al. (2021) | |
| Entomocorticium sp. | Pinus taeda | 9576 | MZ098148 | MZ098134 | – | MZ144593 | This study |
| Entomocorticium sp. | Pinus ponderosa | TSpCB896 | – | AF119510 | – | – | Harrington et al. (2021) |
| Entomocorticium sullivanii | Pinus taeda | CBS 146270 | – | MT741715 | MT741700 | – | Harrington et al. (2021) |
| Entomocorticum dendroctoni | Pinus contorta | DAVFP 23165 | – | AF119506 | – | – | Hsiau & Harrington (2003) |
| Entomocorticum whitneyi | Pinus ponderosa | B1069 | – | MT741713 | MT741698 | – | Harrington et al. (2021) |
| Peniophora albobadia | Angiosperms | CBS 329.66 | – | MH858809 | MH870448 | – | Andreasen & Hellenberg (2009) |
| Peniophora aurantiaca | Alnus (Betulaceae) | – | – | Boidin (1994) | |||
| Peniophora bicornis | Pentaclethra (Fabaceae), Musanga (Urticaceae), Anthocleista (Gentianaceae), Casuarina (Casuarinaceae), Acacia (Fabaceae), Acanthophoenyx (Areceae) | He4767 | – | MK588764 | MK588804 | – | Boidin et al. (1991) |
| He3609 | – | MK588763 | MK588803 | – | Boidin et al. (1991) | ||
| Peniophora borbonica | Hypericum (Hypericaceae), Acacia (Fabaceae), Fuchsia (Onagraceae) | He4597 | – | MK588766 | MK588806 | – | Boidin et al. (1991) |
| He4606 | – | MK588765 | MK588805 | – | Boidin et al. (1991) | ||
| Peniophora cinerea | “Angiosperms and Gymnosperms” | B1020 | – | MN475151 | MN475818 | – | Andreasen & Hellenberg (2009) |
| Peniophora crassitunicata | Morinda (Rubiaceae), Schinus (Anacardiaceae), Casuarina (Casuarinaceae), Lantana (Verbenaceae), Tylophora (Apocynaceae), Acanthophoenyx (Arecaceae), Scaevola (Goodeniaceae) | CBS 663.91 | – | MH862292 | MH873972 | – | Boidin et al. (1991) |
| Peniophora duplex | Gymnosperm “similar to P. pini/pseudo-pini” | CBS 286.58 | – | MH857787 | MH869321 | – | Andreasen & Hellenberg (2009) |
| B1022 | – | MN475153 | MN475820 | – | Andreasen & Hellenberg (2009) | ||
| Peniophora eriksonii | Alnus glutinosa (Betulaceae) | CBS 287.58 | – | MH857788 | MH869322 | – | Boidin (1994) |
| Cui11871 | – | MK588771 | MK588811 | – | Boidin (1994) | ||
| Peniophora exima | Abies (Pinaceae) | B1012 | – | MN475159 | MN475826 | – | Boidin (1994) |
| B1011 | – | MN475155 | MN475821 | – | Boidin (1994) | ||
| T523 | – | MK588772 | MK588812 | – | Boidin (1994) | ||
| Peniophora fasticata | Angiosperms | CBS 942.96 | – | MH862624 | – | – | Andreasen & Hellenberg (2009) |
| Peniophora fissilis | Cryptomeria (Cupressaceae), Lantana (Verbenaceae) | CBS 681.91 | MZ233430 | MH862298 | MH873975 | – | Boidin et al. (1991) |
| CBS 684.91 | MZ233431 | MH862299 | MH873976 | – | Boidin et al. (1991) | ||
| Peniophora gabonensis | Pandanus (Pandanaceae) | CBS 673.91 | – | MH862293 | – | – | Andreasen & Hellenberg (2009) |
| Peniophora gilbertsonii | Prosopis juriflora (Fabaceae), Baccharis (Asteraceae), Cercidium (Fabaceae), Condalia (Rhamnaceae), Fouquieria (Fouquieraceae) | CBS 357.95 | – | MH862528 | MH874164 | – | Boidin et al. (1991) |
| CBS 360.95 | – | MH862530 | MH874165 | – | Boidin et al. (1991) | ||
| Peniophora guadelupensis | Leguminosae | CBS 715.91 | – | MH862304 | MH873977 | – | Andreasen & Hellenberg (2009) |
| Peniophora halimi | Atriplex (Amaranthaceae) | CBS 862.84 | – | MH861843 | MH873531 | – | Andreasen & Hellenberg (2009) |
| CBS 860.84 | – | MH861842 | MH873530 | – | Andreasen & Hellenberg (2009) | ||
| Peniophora incarnata | On angiosperms, rarely on Gymnosperms | B1016 | – | MN475156 | MN475822 | – | Andreasen & Hellenberg (2009) |
| CBS 430.72 | – | MH860518 | MH872230 | – | Andreasen & Hellenberg (2009) | ||
| AF506425 | – | AF506425 | – | – | Andreasen & Hellenberg (2009) | ||
| NH10271 | – | AF506425 | – | – | Andreasen & Hellenberg (2009) | ||
| Peniophora junipericola | Juniperus | He2462 | – | MK588773 | MK588813 | – | Boidin (1994) |
| Peniophora laeta | Carpinus (Betulaceae), Ostrya (Betulaceae) | CBS 256.56 | – | MH857617 | MH869165 | – | Andreasen & Hellenberg (2009) |
| CBS 255.56 | – | MH857616 | MH869164 | – | Andreasen & Hellenberg (2009) | ||
| Peniophora laurentii | Populus (Salicaceae), Betula (Betulaceae), Salix (Salicaceae) | CBS 325.73 | – | – | MH872397 | – | Boidin (1994) |
| Peniophora laxitexta | Angiosperms | BAFC 3309 | – | FJ882040 | – | – | Andreasen & Hellenberg (2009) |
| LGMF1159 | – | JX559580 | – | – | Andreasen & Hellenberg (2009) | ||
| BAFC 4687 | – | MN518328 | – | – | Andreasen & Hellenberg (2009) | ||
| Peniophora lilacea | Celtis (Cannabaceae), Staphylea (Staphyleaceae), Alnus (Betulaceae), Gleditsia (Fabaceae), Fraxinus (Olaceae) | CBS 337.66 | – | MH858813 | MH870452 | – | Boidin (1994) |
| CBS 337.66 | – | MH858813 | MH870452 | – | Boidin (1994) | ||
| Peniophora limitata | Fraximus, Syringa, Ligustrum, Phillyrea | CLZhao 5716 | – | MK269148 | – | – | Boidin (1994) |
| Peniophora lycii | Unkonwn | CBS 264.56 | – | MH857624 | MH869169 | – | Andreasen & Hellenberg (2009) |
| CBS 261.56 | – | MH857621 | MH869167 | – | Andreasen & Hellenberg (2009) | ||
| CBS 352.54 | – | MH857357 | MH868899 | – | Andreasen & Hellenberg (2009) | ||
| Peniophora malaiensis | Calophyllum (Calophyllaceae) | CBS 679.91 | – | MH862297 | MH873974 | – | Andreasen & Hellenberg (2009) |
| He4870 | – | MK588775 | MK588815 | – | Andreasen & Hellenberg (2009) | ||
| Peniophora manshurica | Quercus (Fagaceae) | He2956 | – | MK588776 | MK588816 | – | Andreasen and Hellenberg (2009) |
| He3729 | – | MK588777 | MK588817 | – | |||
| Peniophora meridionalis | Quercus, Cistus (Cistaceae), Lentiscus, Eucalyptus (Myrtaceae), Erica (Ericaceae) | CBS 289.58 | – | MH857789 | MH869323 | – | Boidin et al. (1991) |
| Peniophora molesta | Unknown | CBS 678.91 | – | MH862296 | – | – | Andreasen & Hellenberg (2009) |
| CBS 677.91 | – | MH862295 | – | – | Andreasen & Hellenberg (2009) | ||
| CBS 676.91 | – | MH862294 | MH873973 | – | Andreasen & Hellenberg (2009) | ||
| Peniophora monticola | Hypericum (Hypericaceae), Dombeya (Malvaceae) | CBS 649.91 | – | MH862289 | MH873970 | – | Boidin et al. (1991) |
| Peniophora nuda | Angiosperms, rarely Gymnosperms | AFTOL_ID_660 | – | DQ411533 | DQ435788 | – | Andreasen & Hellenberg (2009) |
| Peniophora ovalispora | Acacia (Acaciae), Cryptomeria (Cupressaceae), Fuchsia (Onagraceae), Solanum (Solanaceae), Cyathea (Fern) | CBS 653.91 | – | MH862290 | MH873971 | – | Boidin et al. (1991) |
| Peniophora parvocystidiata | Pinus (Pinaceae) | CBS 716.91 | – | MH862305 | MH873978 | – | Andreasen & Hellenberg (2009) |
| CBS 717.91 | – | MH862306 | MH873979 | – | Andreasen & Hellenberg (2009) | ||
| Peniophora piceae | Abies, Pseudotsuga (Pinaceae) | B1010 | – | MN475158 | MN475825 | – | Boidin (1994) |
| B1009 | – | MN475157 | MN475824 | – | Boidin (1994) | ||
| Peniophora pilatiana | Quercus, Cistus, Nerium, Vitis, Prunus, Pistacia, Olea, Rhammus, Salix, Eucalyptus, Ilex | CBS 269.56 | – | MH857627 | MH869172 | – | Boidin (1994) |
| CBS 265.56 | – | MH857625 | MH869170 | – | Boidin (1994) | ||
| CBS 266.56 | – | MH857626 | MH869171 | – | Boidin (1994) | ||
| Peniophora pini | Pinus sylvestris (Pinaceae) | CBS 272.56 | – | CBS 272.56 | MH869175 | – | Gibson (1960) |
| CBS 273.56 | – | MH857631 | MH869176 | – | Gibson (1960) | ||
| CBS 270.56 | – | MH857628 | MH869173 | – | Gibson (1960) | ||
| CBS 274.56 | – | MH857632 | MH869177 | – | Gibson (1960) | ||
| CBS 414.34 | – | MH855589 | MH867099 | – | Gibson (1960) | ||
| Peniophora pithya | On Gymnosperms (Pinaceae), rarely on Salix | CBS 276.56 | MZ233428 | MH857634 | MH869179 | – | Boidin et al. (1991) |
| B1013 | – | MN475160 | MN475827 | – | |||
| CBS 275.56 | MZ233427 | MH857633 | MH869178 | – | |||
| Peniophora polygonia | Populus (Salicaceae) | He3668 | – | MH669233 | MH669237 | – | Boidin (1994) |
| CBS 404.50 | – | MH856684 | MH868201 | – | Boidin (1994) | ||
| Peniophora proxima | Buxus (Buxaceae) | CBS 406.50 | – | MH856686 | MH868203 | – | Boidin (1994) |
| CBS 405.50 | – | MH856685 | MH868202 | – | |||
| Peniophora pseudo-pini | Pinus, Abies, Pseudotsuga | B1025 | – | MN475164 | MN475830 | – | Gibson (1960) |
| DAOM-30124 | – | MK588784 | MK588824 | – | Gibson (1960) | ||
| B1024 | – | MN475163 | MN475829 | – | Gibson (1960) | ||
| B1007 | – | MN475162 | MN475828 | – | Gibson (1960) | ||
| Peniophora pseudonuda | Quercus, Fagus (Fagaceae) | FCUG 2384 | – | GU322866 | – | – | Boidin (1994) |
| FCUG 2390 | – | GU322865 | – | – | Boidin (1994) | ||
| FCUG 86 | – | GU322867 | – | – | Boidin (1994) | ||
| Peniophora pseudoversicolor | Quercus (Fagaceae) | CBS 125881 | – | MH864303 | MH875753 | – | Boidin (1994) |
| CBS 338.66 | – | MH858814 | MH870453 | – | Boidin (1994) | ||
| Peniophora quercina | Betula, Castanea, Fagus, Salix | CBS 409.50 | – | MH856689 | MH868206 | – | Boidin (1994) |
| CBS 408.50 | – | MH856688 | MH868205 | – | Boidin (1994) | ||
| CBS 407.50 | – | MH856687 | MH868204 | – | Boidin (1994) | ||
| Peniophora reidii | Quercus (Fagaceae), Laurus, Betula, Salix, Fagus, Eucalyptus | CBS 397.83 | – | MH861616 | MH873334 | – | Boidin (1994) |
| Peniophora rufa | Populus tremuloides (Salicaceae) | CBS 351.59 | – | MH857891 | MH869432 | – | Chamuris & Falk (198) |
| B1014 | – | MN475165 | MN475831 | – | Chamuris & Falk (198) | ||
| Peniophora rufomarginata | Quercus, Populus, Tilia and Arbutrus (Ericaceae) | CBS 282.56 | – | MH857640 | MH869184 | – | Andreasen & Hellenberg (2009) |
| CBS 281.56 | – | MH857639 | MH869183 | – | Andreasen & Hellenberg (2009) | ||
| Peniophora septentrionalis | Picea, Abies (Pinaceae) | CBS 294.58 | MZ233429 | MH857791 | MH869325 | – | Andreasen & Hellenberg (2009) |
| Peniophora simulans | Fagus | CBS 875.84 | – | MH861850 | MH873538 | – | Reid (1969) |
| CBS 874.84 | – | MH861849 | MH873537 | – | Reid (1969) | ||
| Peniophora subsalmonea | Mimosaceae | CBS 697.91 | – | MH862303 | – | – | Andreasen & Hellenberg (2009) |
| CBS 696.91 | – | MH862302 | – | – | Andreasen & Hellenberg (2009) | ||
| Peniophora taiwanensis | Angisosperms | Wu 9206 28 | – | MK588793 | MK588833 | – | Andreasen & Hellenberg (2009) |
| Wu 9209 14 | – | MK588794 | MK588834 | – | Andreasen & Hellenberg (2009) | ||
| Peniophora tamaricicola | Tamarix (Tamaricaceae) | CBS 439.62 | – | MH858204 | MH869803 | – | Gilbertson (1975) |
| CBS 441.62 | – | MH858205 | MH869804 | – | Gilbertson (1975) | ||
| CBS 438.62 | – | MH858203 | MH869802 | – | Gilbertson (1975) | ||
| Peniophora versicolor | Salix (Salicaceae), Acer (Sapindaceae), Ostrya (Betulaceae), Celtis (Cannabaceae), Robinia (Fabaceae) and Ceratonia (Fabaceae) | CBS 358.61 | – | MH858082 | MH869651 | – | Boidin (1994) |
| Peniophora violaceolivida | Salicaceae, rarely on “Gymnosperms” | CBS 348.52 | – | MH857077 | MH868613 | – | Andreasen & Hellenberg (2009) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, J.P.M.; Li, Y.; Six, D.; Rajchenberg, M.; Smith, M.E.; Johnson, A.J.; Klepzig, K.D.; Crous, P.W.; Leal-Dutra, C.A.; Skelton, J.; et al. Diversity and Evolution of Entomocorticium (Russulales, Peniophoraceae), a Genus of Bark Beetle Mutualists Derived from Free-Living, Wood Rotting Peniophora. J. Fungi 2021, 7, 1043. https://doi.org/10.3390/jof7121043
Araújo JPM, Li Y, Six D, Rajchenberg M, Smith ME, Johnson AJ, Klepzig KD, Crous PW, Leal-Dutra CA, Skelton J, et al. Diversity and Evolution of Entomocorticium (Russulales, Peniophoraceae), a Genus of Bark Beetle Mutualists Derived from Free-Living, Wood Rotting Peniophora. Journal of Fungi. 2021; 7(12):1043. https://doi.org/10.3390/jof7121043
Chicago/Turabian StyleAraújo, João P. M., You Li, Diana Six, Mario Rajchenberg, Matthew E. Smith, Andrew J. Johnson, Kier D. Klepzig, Pedro W. Crous, Caio A. Leal-Dutra, James Skelton, and et al. 2021. "Diversity and Evolution of Entomocorticium (Russulales, Peniophoraceae), a Genus of Bark Beetle Mutualists Derived from Free-Living, Wood Rotting Peniophora" Journal of Fungi 7, no. 12: 1043. https://doi.org/10.3390/jof7121043
APA StyleAraújo, J. P. M., Li, Y., Six, D., Rajchenberg, M., Smith, M. E., Johnson, A. J., Klepzig, K. D., Crous, P. W., Leal-Dutra, C. A., Skelton, J., Adams, S. N., & Hulcr, J. (2021). Diversity and Evolution of Entomocorticium (Russulales, Peniophoraceae), a Genus of Bark Beetle Mutualists Derived from Free-Living, Wood Rotting Peniophora. Journal of Fungi, 7(12), 1043. https://doi.org/10.3390/jof7121043






