Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina
Abstract
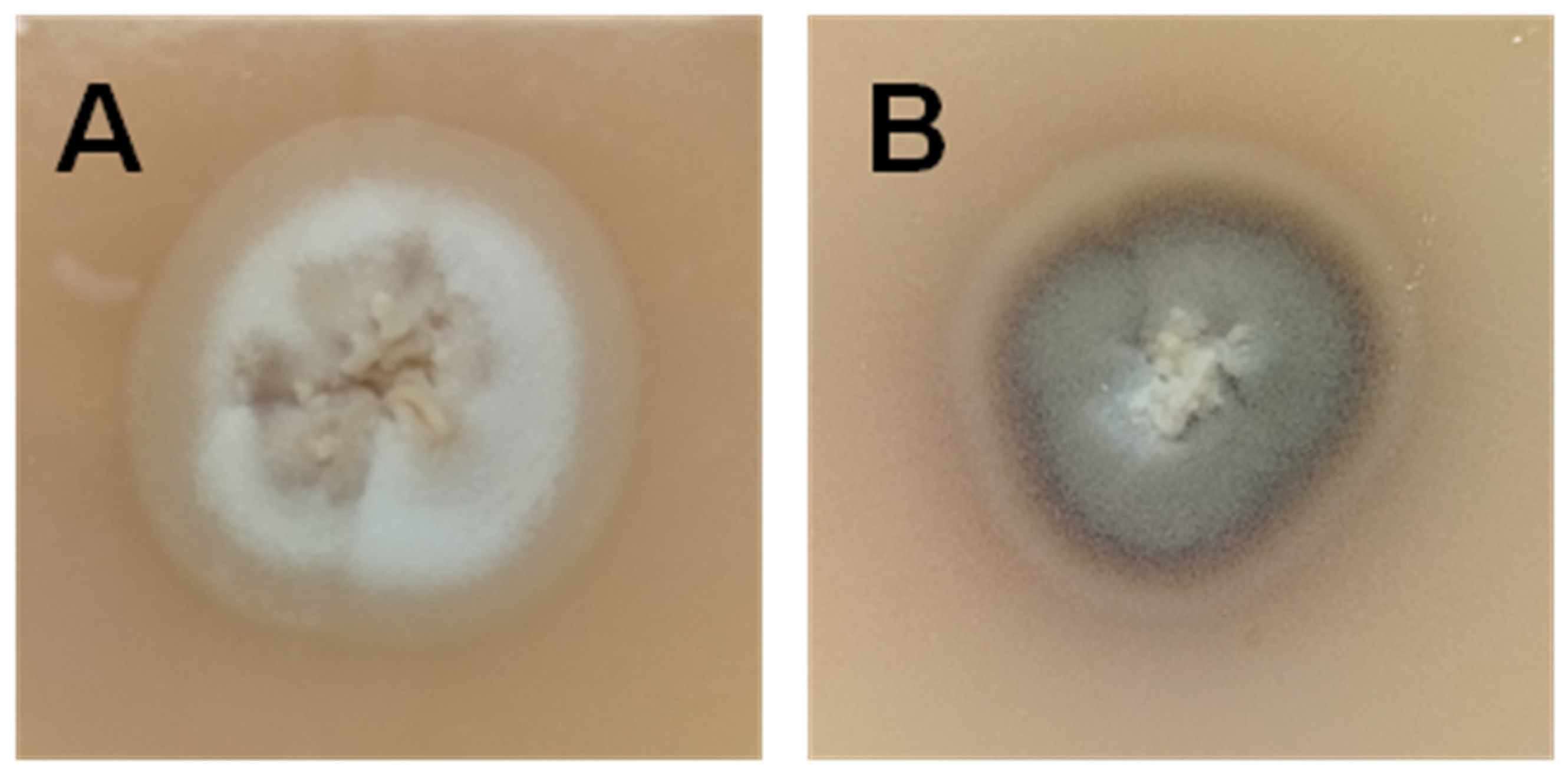
:1. Introduction
2. S. Brasiliensis and Sporotrichosis
3. Sporotrichosis Outbreaks in Argentina and the Hidden Burden: Lessons to Learn About Detecting, Controlling and Preventing Exposure
4. Sporotrichosis: An Indicator of Socio-Environmental Vulnerability?
5. S. Brasiliensis Interactions with the Human Host: Fungal Virulence Factors and Host Immune Response
5.1. Virulence Factors
5.2. Host Immune Response
6. Getting Sporothrichosis on the Map of Different Hosts
6.1. Sporotrichosis in Immunocompetent Patients
6.2. Sporotrichosis in Immunocompromised Patients
6.3. Sporotrichosis in Pregnant Women
6.4. Sporotrichosis in Children
6.5. Sporotrichosis in Cats
6.6. Sporotrichosis in Dogs
7. Implementation of Novel Tools to Improve the Diagnosis of Sporotrichosis
8. Exploring New Antifungal Agents
9. Exploring Vaccine Candidates
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Rossow, J.A.; Queiroz-Telles, F.; Caceres, D.H.; Beer, K.D.; Jackson, B.R.; Pereira, J.G.; Ferreira Gremião, I.D.; Pereira, S.A. A One Health approach to combatting Sporothrix brasiliensis: Narrative review of an emerging zoonotic fungal pathogen in South America. J. Fungi 2020, 6, 247. [Google Scholar] [CrossRef]
- Della Terra, P.P.; Rodrigues, A.M.; Fernandes, G.F.; Nishikaku, A.S.; Burger, E.; de Camargo, Z.P. Exploring virulence and immunogenicity in the emerging pathogen Sporothrix brasiliensis. PLoS Negl. Trop. Dis. 2017, 11, e0005903. [Google Scholar] [CrossRef]
- Gremião, I.D.; Menezes, R.C.; Schubach, T.M.; Figueiredo, A.B.; Cavalcanti, M.C.; Pereira, S.A. Feline sporotrichosis: Epidemiological and clinical aspects. Med. Mycol. 2015, 53, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sporotrichosis Epidemiological Bulletin 001/2018. Secretaria de Estado de Saude do Rio de Janeiro. Available online: http://www.riocomsaude.rj.gov.br/Publico/MostrarArquivo.aspx?C=mgfY3RQJkek%3D (accessed on 27 September 2020).
- Etchecopaz, A.N.; Lanza, N.; Toscanini, M.A.; Devoto, T.B.; Pola, S.J.; Daneri, G.L.; Iovannitti, C.A.; Cuestas, M.L. Sporotrichosis caused by Sporothrix brasiliensis in Argentina: Case report, molecular identification and in vitro susceptibility pattern to antifungal drugs. J. Mycol. Med. 2020, 30, 100908. [Google Scholar] [CrossRef]
- Etchecopaz, A.; Scarpa, M.; Mas, J.; Cuestas, M.L. Sporothrix brasiliensis: A growing hazard in the Northern area of Buenos Aires Province? Rev. Argent. Microbiol. 2020, 52, 350–351. [Google Scholar] [CrossRef]
- Queiroz-Telles, F. How rapid tests can improve the diagnosis of sporotrichosis? 18 th INFOCUS-Web Hall 2020. 17 and 24 October 2020. Available online: https://www.infocuslatam.com.br/home.asp (accessed on 28 October 2020).
- Shenck, B.R. On refractory subcutaneous abscesses caused by a fungus possibly related to the Sporotrichia. Bull. Johns Hopkins Hosp. 1898, 9, 286–290. [Google Scholar]
- Marimon, R.; Cano, J.; Gené, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.M.; de Melo Teixeira, M.; de Hoog, G.S.; Schubach, T.M.; Pereira, S.A.; Fernandes, G.F.; Bezerra, L.M.; Felipe, M.S.; de Camargo, Z.P. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl. Trop. Dis. 2013, 7, e2281. [Google Scholar] [CrossRef] [Green Version]
- Barros, M.B.; de Almeida Paes, R.; Schubach, A.O. Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 2011, 24, 633–654. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hagen, F.; Stielow, B.; Rodrigues, A.M.; Samerpitak, K.; Zhou, X.; Feng, P.; Yang, L.; Chen, M.; Deng, S.; et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia 2015, 35, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macêdo-Sales, P.A.; Souza, L.O.P.; Della-Terra, P.P.; Lozoya-Pérez, N.E.; Machado, R.L.D.; Rocha, E.M.D.S.D.; Lopes-Bezerra, L.M.; Guimarães, A.J.; Rodrigues, A.M.; Mora-Montes, H.M.; et al. Coinfection of domestic felines by distinct Sporothrix brasiliensis in the Brazilian sporotrichosis hyperendemic area. Fungal Genet. Biol. 2020, 140, 103397. [Google Scholar] [CrossRef]
- Oliveira, M.M.E.; Almeida-Paes, R.; Corrêa-Moreira, D.; Borba, C.M.; Menezes, R.C.; Freitas, D.F.S.; do Valle, A.C.F.; Schubach, A.O.; Barros, M.B.L.; Nosanchuk, J.D.; et al. A case of sporotrichosis caused by different Sporothrix brasiliensis strains: Mycological, molecular, and virulence analyses. Mem. Inst. Oswaldo Cruz 2019, 114, e190260. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; de Oliveira, L.C.; Oliveira, M.M.; Gutierrez-Galhardo, M.C.; Nosanchuk, J.D.; Zancopé-Oliveira, R.M. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. Biomed. Res. Int. 2015, 2015, 212308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, R.J.; Morris-Jones, R. Outbreaks of sporotrichosis. Curr. Opin. Infect. Dis. 2008, 21, 119–121. [Google Scholar] [CrossRef]
- Teixeira, M.M.; de Almeida, L.G.; Kubitschek-Barreira, P.; Alves, F.L.; Kioshima, E.S.; Abadio, A.K.; Fernandes, L.; Derengowski, L.S.; Ferreira, K.S.; Souza, R.C.; et al. Comparative genomics of the major fungal agents of human and animal Sporotrichosis: Sporothrix schenckii and Sporothrix brasiliensis. BMC Genomics 2014, 15, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossato, L.; Moreno, L.F.; Jamalian, A.; Stielow, B.; de Almeida, S.R.; de Hoog, S.; Freeke, J. Proteins potentially involved in immune evasion strategies in Sporothrix brasiliensis elucidated by ultra-high-resolution mass spectrometry. mSphere 2018, 3, e00514-17. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.; de Hoog, G.; Zhang, Y.; de Camargo, Z.P. Emerging sporotrichosis is driven by clonal and recombinant Sporothrix species. Emerg. Microbes Infect. 2014, 3, e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida-Paes, R.; de Oliveira, M.M.; Freitas, D.F.; do Valle, A.C.; Zancopé-Oliveira, R.M.; Gutierrez-Galhardo, M.C. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl. Trop. Dis. 2014, 8, e3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gremião, I.D.F.; Oliveira, M.M.E.; Monteiro de Miranda, L.H.; Saraiva Freitas, D.F.; Pereira, S.A. Geographic expansion of Sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, N.; Iachini, R.; Farias, L.; Pozzi, N.; Tiraboschi, I. Esporotricosis; una zoonosis e alerta. In Proceedings of the Infocus Cordoba, Circulo Medico de, Cordoba, Cordoba, Argentina, 5–7 November 2015; p. 11. [Google Scholar]
- Córdoba, S.; Isla, G.; Szusz, W.; Vivot, W.; Hevia, A.; Davel, G.; Canteros, C.E. Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Mycoses 2018, 61, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A.E.; Abad Rizo, M.J.; Porta, J.; Galanternick, L.; Crespi, H.G. Cutaneous sporotrichosis in a cat scratch lesion in a child. Rev. Argent. Dermatol. 1990, 71, 107–109. [Google Scholar]
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef] [Green Version]
- García Duarte, J.M.; Wattiez Acosta, V.R.; Forneron Viera, P.M.L.; Aldama Caballero, A.; Gorostiaga Matiauda, G.A.; Rivelli de Oddone, V.B.; Pereira Brunell, J.G. Esporotricosis transmitida por gato doméstico. Reporte de un caso familiar. Rev. Nac. 2017, 9, 67–76. [Google Scholar]
- Rios, M.E.; Suarez, J.M.D.; Moreno, J.; Vallee, J.; Moreno, J.P. Zoonotic sporotrichosis related to cat contact: First case report from Panama in Central America. Cureus 2018, 10, e2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, R.; Okubo, M.; Siew, H.H.; Kamata, H.; Hasegawa, A. Molecular typing of Sporothrix schenckii isolates from cats in Malaysia. Mycoses 2015, 58, 220–224. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Bosco, S.M.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56 (Suppl. 1), 165–187. [Google Scholar] [CrossRef]
- Lutz, A.; Splendore, A. About a mycosis observed in men and rats. Rev. Med. São Paulo 1907, 21, 433–450. [Google Scholar]
- Lopes-Bezerra, L.M.; Mora-Montes, H.M.; Zhang, Y.; Nino-Vega, G.; Rodrigues, A.M.; de Camargo, Z.P.; de Hoog, S. Sporotrichosis between 1898 and 2017: The evolution of knowledge on a changeable disease and on emerging etiological agents. Med. Mycol. 2018, 56 (Suppl. 1), 126–143. [Google Scholar] [CrossRef] [PubMed]
- Davel, G.; Canteros, C.E. Epidemiological status of mycoses in the Argentine Republic. Rev. Argent. Microbiol. 2007, 39, 28–33. [Google Scholar]
- Negroni, R.; Helou, S. Problemas clínicos en Micología Médica: Problema no. 4. Síndrome linfangítico-nodular [Clinical cases in Medical Mycology]. Rev. Iberoam. Micol. 2003, 20, 71–74. [Google Scholar]
- Iachini, R. Sporotrichosis in a domestic cat. Rev. Argent. Microbiol. 2009, 41, 27. (In Spanish) [Google Scholar]
- Arechavala, A.; Orduna, T.; Maiolo, E.; Mujica, M.T.; Fernanadez, M.; Negroni, R. Esporotricosis diseminada, con compromiso cutáneo y visceral. Rev. Patol. Trop. 2011, 40, 73–84. [Google Scholar]
- Starck, F.; Saponaro, A.E.; Marini, M.A.; Casas, J.G.; Vigovich, F.; Agorio, I. Fixed cutaneous sporotrichosis. Arch. Argent. Dermatol. 2011, 61, 14–17. [Google Scholar]
- Rojas, F.D.; Fernández, M.S.; Lucchelli, J.M.; Lombardi, D.; Malet, J.; Vetrisano, M.E.; Cattana, M.E.; Sosa, M.L.Á.; Giusiano, G. Cavitary pulmonary sporotrichosis: Case report and literature review. Mycopathologia 2017, 182, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Bertera, A.; Rossi, M.V.; García, S.; Tessadro, G.; Aloise, I. Lymphocutaneous sporotrichosis secondary to cat bite. Dermatol. Argent. 2017, 23, 133–135. [Google Scholar]
- Departamento de Epidemiología y Estadística Hospital SAMIC El Calafate. Alerta Epidemiológica. Available online: http://aam.org.ar/src/img_up/18122019.0.pdf (accessed on 3 December 2019).
- Ramírez-Soto, M.C.; Aguilar-Ancori, E.G.; Tirado-Sánchez, A.; Bonifaz, A. Ecological Determinants of sporotrichosis etiological agents. J. Fungi 2018, 4, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macêdo-Sales, P.A.; Souto, S.R.L.S.; Destefani, C.A.; Lucena, R.P.; Machado, R.L.D.; Pinto, M.R.; Rodrigues, A.M.; Lopes-Bezerra, L.M.; Rocha, E.M.S.; Baptista, A.R.S. Domestic feline contribution in the transmission of Sporothrix in Rio de Janeiro State, Brazil: A comparison between infected and non-infected populations. BMC Vet. Res. 2018, 14, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Action for Fund for Fungal Infections (GAFFI). Available online: https://www.gaffi.org/ (accessed on 13 November 2020).
- Horn, S.; Hanula, J.L. Comparison of arthropod prey of redcockaded woodpeckers on the boles of longleaf and loblolly pines. Wildl. Soc. Bull. 2002, 30, 131–138. [Google Scholar]
- Almeida-Paes, R.; Brito-Santos, F.; Oliveira, M.M.E.; Bailão, A.M.; Borges, C.L.; Araújo, G.R.S.; Frases, S.; Soares, C.M.A.; Zancopé-Oliveira, R.M. Interaction with Pantoea agglomerans modulates growth and melanization of Sporothrix brasiliensis and Sporothrix schenckii. Mycopathologia 2019, 184, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Paes, R.; Borba-Santos, L.P.; Rozental, S.; Marco, S.; Zancopé-Oliveira, R.M.; Cunha, M.M. Melanin biosynthesis in pathogenic species of Sporothrix. Fung Biol. Rev. 2017, 31, 50–59. [Google Scholar] [CrossRef]
- Helm, M.; Berman, C. The clinical, therapeutic and epidemiological features of sporotrichosis infection of the mines. In Proceedings of the Transvaal Mine Medical Officers’ Association Sporotrichosis Infection on Mines of the Witwatersrand; The Transvaal Chamber of Mines: Johannesburg, South Africa, 1947. [Google Scholar]
- Alzuguir, C.L.C.; Pereira, S.A.; Magalhães, M.A.F.M.; Almeida-Paes, R.; Freitas, D.F.S.; Oliveira, L.F.A.; Pimentel, M.I.F. Geo-epidemiology and socioeconomic aspects of human sporotrichosis in the municipality of Duque de Caxias, Rio de Janeiro, Brazil, between 2007 and 2016. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 99–106. [Google Scholar] [CrossRef]
- Poester, V.R.; Mattei, A.S.; Madrid, I.M.; Pereira, J.T.B.; Klafke, G.B.; Sanchotene, K.O.; Brandolt, T.M.; Xavier, M.O. Sporotrichosis in Southern Brazil, towards an epidemic? Zoonoses. Public Health 2018, 65, 815–821. [Google Scholar] [CrossRef]
- Castro, R.A.; Kubitschek-Barreira, P.H.; Teixeira, P.A.; Sanches, G.F.; Teixeira, M.M.; Quintella, L.P.; Almeida, S.R.; Costa, R.O.; Camargo, Z.P.; Felipe, M.S.; et al. Differences in cell morphometry, cell wall topography and gp70 expression correlate with the virulence of Sporothrix brasiliensis clinical isolates. PLoS ONE 2013, 8, e75656. [Google Scholar] [CrossRef] [Green Version]
- Mario, D.N.; Schaffer, L.F.; Peroza, L.R.; Jesus, F.P.K.; Denardi, L.B.; Fachinetto, R.; Alves, S.H. Sporothrix brasiliensis produces the highest levels of oxidative stress in a murine model among the species of the Sporothrix schenckii complex. Rev. Soc. Bras. Med. Trop. 2017, 50, 554–557. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Genotyping species of the Sporothrix schenckii complex by PCR-RFLP of calmodulin. Diagn. Microbiol. Infect. Dis. 2014, 78, 383–387. [Google Scholar] [CrossRef]
- Martínez-Álvarez, J.A.; Pérez-García, L.A.; Mellado-Mojica, E.; López, M.G.; Martínez-Duncker, I.; Lópes-Bezerra, L.M.; Mora-Montes, H.M. Sporothrix schenckii sensu stricto and Sporothrix brasiliensis are differentially recognized by human peripheral blood mononuclear cells. Front. Microbiol. 2017, 8, 843. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; de Aguiar, F.R.M.; da Silva, M.L.Q.; de Oliveira, J.S.; de Camargo, Z.P.; Rodrigues, A.M.; Pereira, V.S.; Serpa, R.; Castelo-Branco, D.S.C.M.; Correia, E.E.M.; et al. Antifungal susceptibility of Sporothrix schenckii complex biofilms. Med. Mycol. 2018, 56, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Bezerra, L.M.; Walker, L.A.; Niño-Vega, G.; Mora-Montes, H.M.; Neves, G.W.P.; Villalobos-Duno, H.; Barreto, L.; Garcia, K.; Franco, B.; Martínez-Álvarez, J.A.; et al. Cell walls of the dimorphic fungal pathogens Sporothrix schenckii and Sporothrix brasiliensis exhibit bilaminate structures and sloughing of extensive and intact layers. PLoS Negl. Trop. Dis. 2018, 12, e0006169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Bezerra, L.M. Sporothrix schenckii cell wall peptidorhamnomannans. Front. Microbiol. 2011, 2, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Baca, E.; Toriello, C.; Perez-Torres, A.; Sabanero-Lopez, M.; Villagomez-Castro, J.C.; Lopez-Romero, E. Isolation and some properties of a glycoprotein of 70 kDa (Gp70) from the cell wall of Sporothrix schenckii involved in fungal adherence to dermal extracellular matrix. Med. Mycol. 2009, 47, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.; Fernandes, G.F.; Araujo, L.M.; Della Terra, P.P.; dos Santos, P.O.; Pereira, S.A.; Schubach, T.M.; Burger, E.; Lopes-Bezerra, L.M.; de Camargo, Z.P. Proteomics-based characterization of the humoral immune response in sporotrichosis: Toward discovery of potential diagnostic and vaccine antigens. PLoS Negl. Trop. Dis. 2015, 9, e0004016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portuondo, D.L.; Dores-Silva, P.R.; Ferreira, L.S.; de Oliveira, C.S.; Téllez-Martínez, D.; Marcos, C.M.; de Aguiar Loesch, M.L.; Guzmán, F.; Gava, L.M.; Borges, J.C.; et al. Immunization with recombinant enolase of Sporothrix spp. (rSsEno) confers effective protection against sporotrichosis in mice. Sci. Rep. 2019, 9, 17179. [Google Scholar] [CrossRef] [Green Version]
- Portuondo, D.L.; Batista-Duharte, A.; Ferreira, L.S.; Martínez, D.T.; Polesi, M.C.; Duarte, R.A.; de Paula, E.; Silva, A.C.; Marcos, C.M.; Almeida, A.M.; et al. A cell wall protein-based vaccine candidate induces protective immune response against Sporothrix schenckii infection. Immunobiology 2016, 221, 300–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlos, I.Z.; Sassá, M.F.; da Graça Sgarbi, D.B.; Placeres, M.C.; Maia, D.C. Current research on the immune response to experimental sporotrichosis. Mycopathologia 2009, 168, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.S.; Coelho, A.L.; Lopes Bezerra, L.M.; Barja-Fidalgo, C. Virulence of Sporothrix schenckii conidia and yeast cells, and their susceptibility to nitric oxide. Immunology 2000, 101, 563–569. [Google Scholar] [CrossRef]
- de Toledo Martins, S.; Szwarc, P.; Goldenberg, S.; Alves, L.R. Extracellular vesicles in fungi: Composition and functions. Curr. Top. Microbiol. Immunol. 2019, 422, 45–59. [Google Scholar] [CrossRef]
- Zamith-Miranda, D.; Nimrichter, L.; Rodrigues, M.L.; Nosanchuk, J.D. Fungal extracellular vesicles: Modulating host-pathogen interactions by both the fungus and the host. Microbes. Infect. 2018, 20, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.L.; Freire-de-Lima, C.G.; Nosanchuk, J.D.; Casadevall, A.; Rodrigues, M.L.; Nimrichter, L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 2010, 78, 1601–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, M.A.K.; de Almeida, J.R.F.; Jannuzzi, G.P.; Cronemberger-Andrade, A.; Torrecilhas, A.C.T.; Moretti, N.S.; da Cunha, J.P.C.; de Almeida, S.R.; Ferreira, K.S. Extracellular vesicles from Sporothrix brasiliensis are an important virulence factor that induce an increase in fungal burden in experimental sporotrichosis. Front. Microbiol. 2018, 9, 2286. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; Frases, S.; Fialho Monteiro, P.C.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M.; Nosanchuk, J.D. Growth conditions influence melanization of Brazilian clinical Sporothrix schenckii isolates. Microbes. Infect. 2009, 11, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Paes, R.; Frases, S.; Araújo, G. de S.; de Oliveira, M.M.; Gerfen, G.J.; Nosanchuk, J.D.; Zancopé-Oliveira, R.M. Biosynthesis and functions of a melanoid pigment produced by species of the Sporothrix complex in the presence of L-tyrosine. Appl. Environ. Microbiol. 2012, 78, 8623–8630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, P.A.; De Castro, R.A.; Ferreira, F.R.; Cunha, M.M.; Torres, A.P.; Penha, C.V.; Rozental, S.; Lopes-Bezerra, L.M. L-DOPA accessibility in culture medium increases melanin expression and virulence of Sporothrix schenckii yeast cells. Med. Mycol. 2010, 48, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Paes, R.; Figueiredo-Carvalho, M.H.; Brito-Santosm, F.; Almeida-Silva, F.; Oliveira, M.M.; Zancopé-Oliveira, R.M. Melanins protect Sporothrix brasiliensis and Sporothrix schenckii from the antifungal effects of terbinafine. PLoS ONE 2016, 11, e0152796. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.; Silvana Dos Santos, S.; Ferreira, L.G.; Rogério de Almeida, S. The impact of the absence of Toll-like receptor-2 during Sporothrix brasiliensis infection. J. Med. Microbiol. 2019, 68, 87–94. [Google Scholar] [CrossRef]
- Rossato, L.; Santos, S.S.D.; Ferreira, L.G.; de Almeida, S.R. The importance of Toll-like receptor 4 during experimental Sporothrix brasiliensis infection. Med. Mycol. 2019, 57, 489–495. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G. T-cell subsets and antifungal host defenses. Curr. Fungal Infect. Rep. 2010, 4, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista-Duharte, A.; Téllez-Martínez, D.; Roberto de Andrade, C.; Portuondo, D.L.; Jellmayer, J.A.; Polesi, M.C.; Carlos, I.Z. Sporothrix brasiliensis induces a more severe disease associated with sustained Th17 and regulatory T cells responses than Sporothrix schenckii sensu stricto in mice. Fungal Biol. 2018, 122, 1163–1170. [Google Scholar] [CrossRef] [Green Version]
- Miranda, L.H.M.; Santiago, M.A.; Schubach, T.M.P.; Morgado, F.N.; Pereira, S.A.; Oliveira, R.V.C.; Conceição-Silva, F. Severe feline sporotrichosis associated with an increased population of CD8low cells and a decrease in CD4⁺ cells. Med. Mycol. 2016, 54, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kienzle, N.; Olver, S.; Buttigieg, K.; Groves, P.; Janas, M.L.; Baz, A.; Kelso, A. Progressive differentiation and commitment of CD8+ T cells to a poorly cytolytic CD8low phenotype in the presence of IL-4. J. Immunol. 2005, 174, 2021–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz-Telles, F.; Buccheri, R.; Benard, G. Sporotrichosis in immunocompromised hosts. J. Fungi 2019, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Ramírez Soto, M.C. Sporotrichosis in the ocular adnexa: 21 cases in an endemic area in Peru and review of the literature. Am. J. Ophthalmol. 2016, 162, 173–179.e3. [Google Scholar] [CrossRef] [PubMed]
- Lacerda Filho, A.M.; Cavalcante, C.M.; Da Silva, A.B.; Inácio, C.P.; de Lima-Neto, R.G.; de Andrade, M.C.L.; Magalhães, O.M.C.; Dos Santos, F.A.G.; Neves, R.P. High-virulence cat-transmitted ocular sporotrichosis. Mycopathologia 2019, 184, 547–549. [Google Scholar] [CrossRef]
- Schubach, A.; de Lima Barros, M.B.; Schubach, T.M.; Francesconi-do-Valle, A.C.; Gutierrez-Galhardo, M.C.; Sued, M.; de Matos Salgueiro, M.; Fialho-Monteiro, P.C.; Reis, R.S.; Marzochi, K.B.; et al. Primary conjunctival sporotrichosis: Two cases from a zoonotic epidemic in Rio de Janeiro, Brazil. Cornea 2005, 24, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.M.F.; Moreira, L.M.; Barczewski, B.F.; de Matos, L.X.; de Oliveira, J.B.V.; Pimentel, M.I.F.; Almeida-Paes, R.; Oliveira, M.G.; Pinto, T.C.A.; Lima, N.; et al. Identification by MALDI-TOF MS of Sporothrix brasiliensis isolated from a subconjunctival infiltrative lesion in an immunocompetent patient. Microorganisms 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Vergara, M.L.; de Camargo, Z.P.; Silva, P.F.; Abdalla, M.R.; Sgarbieri, R.N.; Rodrigues, A.M.; dos Santos, K.C.; Barata, C.H.; Ferreira-Paim, K. Disseminated Sporothrix brasiliensis infection with endocardial and ocular involvement in an HIV-infected patient. Am. J. Trop. Med. Hyg. 2012, 86, 477–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Macedo, P.M.; Sztajnbok, D.C.; Camargo, Z.P.; Rodrigues, A.M.; Lopes-Bezerra, L.M.; Bernardes-Engemann, A.R.; Orofino-Costa, R. Dacryocystitis due to Sporothrix brasiliensis: A case report of a successful clinical and serological outcome with low-dose potassium iodide treatment and oculoplastic surgery. Br. J. Dermatol. 2015, 172, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, A.L.; Freitas, D.F.; Valviesse, V.R.; Andrade, H.B.; de Oliveira, M.M.; do Valle, A.C.; Zancope-Oliveira, R.M.; Galhardo, M.C.; Curi, A.L. Multifocal choroiditis in disseminated sporotrichosis in patients with HIV/AIDS. Retin. Cases Brief. Rep. 2017, 11, 67–70. [Google Scholar] [CrossRef]
- Mialski, R.; de Almeida, J.N., Jr.; da Silva, L.H.; Kono, A.; Pinheiro, R.L.; Teixeira, M.J.; Gomes, R.R.; de Queiroz-Telles, F.; Pinto, F.G.; Benard, G. Chronic meningitis and hydrocephalus due to Sporothrix brasiliensis in immunocompetent adults: A challenging entity. Open Forum Infect. Dis. 2018, 5, ofy081. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A.; Bustamante, B.; Chapman, S.W.; Pappas, P.G.; Infectious Diseases Society of America. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Bunce, P.E.; Yang, L.; Chun, S.; Zhang, S.X.; Trinkaus, M.A.; Matukas, L.M. Disseminated sporotrichosis in a patient with hairy cell leukemia treated with amphotericin B and posaconazole. Med. Mycol. 2012, 50, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Paixão, A.G.; Galhardo, M.C.G.; Almeida-Paes, R.; Nunes, E.P.; Gonçalves, M.L.C.; Chequer, G.L.; Lamas, C.D.C. The difficult management of disseminated Sporothrix brasiliensis in a patient with advanced AIDS. AIDS Res. Ther. 2015, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Bonifaz, A.; Tirado-Sánchez, A. Cutaneous disseminated and extracutaneous sporotrichosis: Current status of a complex disease. J. Fungi 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Freitas, D.F.; Lima, M.A.; de Almeida-Paes, R.; Lamas, C.C.; do Valle, A.C.; Oliveira, M.M.; Zancopé-Oliveira, R.M.; Gutierrez-Galhardo, M.C. Sporotrichosis in the central nervous system caused by Sporothrix brasiliensis. Clin. Infect. Dis. 2015, 61, 663–664. [Google Scholar] [CrossRef] [Green Version]
- Eyer-Silva, W.A.; de Azevedo, M.C.V.M.; da Silva, G.A.R.; Basílio-de-Oliveira, R.P.; de Araujo, L.F.; do Lago, I.V.; Pereira, F.C.F.; Fernandes, M.B.T.; Figueiredo-Carvalho, M.H.G.; Souza Rabello, V.B.; et al. Palate ulcer, uvular destruction and nasal septal perforation caused by Sporothrix brasiliensis in an HIV-infected patient. Med. Mycol. Case Rep. 2018, 23, 16–19. [Google Scholar] [CrossRef]
- Lyra, M.R.; Nascimento, M.L.; Varon, A.G.; Pimentel, M.I.; Antonio, L. de F.; Saheki, M.N.; Bedoya-Pacheco, S.J.; Valle, A.C. Immune reconstitution inflammatory syndrome in HIV and sporotrichosis coinfection: Report of two cases and review of the literature. Rev. Soc. Bras. Med. Trop. 2014, 47, 806–809. [Google Scholar] [CrossRef] [Green Version]
- Duani, H.; Palmerston, M.F.; Rosa Júnior, J.F.; Ribeiro, V.T.; Alcântara Neves, P.L. Meningeal and multiorgan disseminated sporotrichosis: A case report and autopsy study. Med. Mycol. Case Rep. 2019, 26, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.O.; Bernardes-Engemann, A.R.; Azulay-Abulafia, L.; Benvenuto, F.; de Neves, M.L.; Lopes-Bezerra, L.M. Sporotrichosis in pregnancy: Case reports of 5 patients in a zoonotic epidemic in Rio de Janeiro, Brazil. Ann. Bras. Dermatol. 2011, 86, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.P.; do Valle, A.C.; Freitas, D.F.; Reis, R.; Galhardo, M.C. Pregnancy during a sporotrichosis epidemic in Rio de Janeiro, Brazil. Int. J. Gynaecol. Obstet. 2012, 117, 294–295. [Google Scholar] [CrossRef]
- Fichman, V.; Valle, A.C.F.D.; de Macedo, P.M.; Freitas, D.F.S.; Oliveira, M.M.E.; Almeida-Paes, R.; Gutierrez-Galhardo, M.C. Cryosurgery for the treatment of cutaneous sporotrichosis in four pregnant women. PLoS Negl. Trop. Dis. 2018, 12, e0006434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, M.B.; Costa, D.L.; Schubach, T.M.; do Valle, A.C.; Lorenzi, N.P.; Teixeira, J.L.; Schubach, A.d.O. Endemic of zoonotic sporotrichosis: Profile of cases in children. Pediatr. Infect. Dis. J. 2008, 27, 246–250. [Google Scholar] [CrossRef]
- Ramírez Soto, M.C. Sporotrichosis among children of a hyperendemic area in Peru: An 8-year retrospective study. Int. J. Dermatol. 2017, 56, 868–872. [Google Scholar] [CrossRef]
- Ramírez Soto, M.C. Sporotrichosis: The story of an endemic region in Peru over 28 years (1985 to 2012). PLoS ONE 2015, 10, e0127924. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Caligiorne, R.B.; Coutinho, D.M.; Gomes, R.R.; Rocha-Silva, F.; Machado, A.S.; Santrer, E.F.R.; Assunção, C.B.; Guimarães, C.F.; Laborne, M.S.; et al. A case of disseminated sporotrichosis caused by Sporothrix brasiliensis. Med. Mycol. Case Rep. 2018, 21, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Vázquez-González, D. Sporotrichosis: An update. G. Ital. Dermatol. Venereol. 2010, 145, 659–673. [Google Scholar] [PubMed]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet Infect. Dis. 2017, 17, e367–e377. [Google Scholar] [CrossRef]
- Lloret, A.; Hartmann, K.; Pennisi, M.G.; Ferrer, L.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; et al. Sporotrichosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2013, 15, 619–623. [Google Scholar] [CrossRef]
- Montenegro, H.; Rodrigues, A.M.; Dias, M.A.; da Silva, E.A.; Bernardi, F.; de Camargo, Z.P. Feline sporotrichosis due to Sporothrix brasiliensis: An emerging animal infection in São Paulo, Brazil. BMC Vet. Res. 2014, 10, 269. [Google Scholar] [CrossRef] [Green Version]
- De Miranda, L.H.M.; Meli, M.; Conceição-Silva, F.; Novacco, M.; Menezes, R.C.; Pereira, S.A.; Sugiarto, S.; Dos Reis, É.G.; Gremião, I.D.F.; Hofmann-Lehmann, R. Co-infection with feline retrovirus is related to changes in immunological parameters of cats with sporotrichosis. PLoS ONE 2018, 13, e0207644. [Google Scholar] [CrossRef] [Green Version]
- Maizels, R.M.; Balic, A.; Gomez-Escobar, N.; Nair, M.; Taylor, M.D.; Allen, J.E. Helminth parasites--masters of regulation. Immunol. Rev. 2004, 201, 89–116. [Google Scholar] [CrossRef]
- Martínez, M.L.; Domínguez, M.G.; Morici, G.E.; Cavia, R.; Montes de Oca, D.P.; Lovera, R.; Schapiro, J.H.; Caracostantogolo, J.L. Morphological and molecular identification of Cysticercus fasciolaris isolated from rodent hosts (Rattus norvegicus) in Buenos Aires province (Argentina). Rev. Argent. Microbiol. 2013, 45, 150–153. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, J.; Huang, H.; Xue, R.; Hu, X.; Li, M.; Zhong, Y.; Yuan, L. Taenia taeniaeformis in rat favors protracted skin lesions caused by Sporothrix schenckii infection: Dectin-1 and IL-17 are dispensable for clearance of this fungus. PLoS ONE 2012, 7, e52514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, E.W.; Borba, C.M.; Pereira, S.A.; Gremião, I.D.F.; Langohr, I.M.; Oliveira, M.M.E.; de Oliveira, R.V.C.; da Cunha, C.R.; Zancopé-Oliveira, R.M.; de Miranda, L.H.M.; et al. Clinical features, fungal load, coinfections, histological skin changes, and itraconazole treatment response of cats with sporotrichosis caused by Sporothrix brasiliensis. Sci. Rep. 2018, 8, 9074. [Google Scholar] [CrossRef] [Green Version]
- Gremião, I.D.; Miranda, L.H.; Reis, E.G.; Rodrigues, A.M.; Pereira, S.A. Zoonotic epidemic of sporotrichosis: Cat to human transmission. PLoS Pathog. 2017, 13, e1006077. [Google Scholar] [CrossRef]
- Schubach, T.M.; Schubach, A.; Okamoto, T.; Barros, M.B.; Figueiredo, F.B.; Cuzzi, T.; Pereira, S.A.; Dos Santos, I.B.; Almeida Paes, R.; Paes Leme, L.R.; et al. Canine sporotrichosis in Rio de Janeiro, Brazil: Clinical presentation, laboratory diagnosis and therapeutic response in 44 cases (1998–2003). Med. Mycol. 2006, 44, 87–92. [Google Scholar] [CrossRef]
- Mascarenhas, M.B.; Lopes, N.L.; Pinto, T.G.; Costa, T.S.; Peixoto, A.P.; Ramadinha, R.R.; Fernandes, J.I. Canine sporotrichosis: Report of 15 advanced cases. Pesqui. Vet. Bras. 2018, 38, 477–481. [Google Scholar] [CrossRef]
- Viana, P.G.; Figueiredo, A.B.F.; Gremião, I.D.F.; de Miranda, L.H.M.; da Silva, A.I.M.; Boechat, J.S.; de Sá Machado, A.C.; de Oliveira, M.M.E.; Pereira, S.A. Successful treatment of canine sporotrichosis with terbinafine: Case reports and literature review. Mycopathologia 2018, 183, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, F.; Li, R.; Gong, J.; Zhao, F. Fast diagnosis of sporotrichosis caused by Sporothrix globosa, Sporothrix schenckii, and Sporothrix brasiliensis based on multiplex real-time PCR. PLoS Negl. Trop. Dis. 2019, 13, e0007219. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, G.F.; Lopes-Bezerra, L.M.; Bernardes-Engemann, A.R.; Schubach, T.M.; Dias, M.A.; Pereira, S.A.; de Camargo, Z.P. Serodiagnosis of sporotrichosis infection in cats by enzyme-linked immunosorbent assay using a specific antigen, SsCBF, and crude exoantigens. Vet. Microbiol. 2011, 147, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Orofino-Costa, R.; Bóia, M.N.; Magalhães, G.A.; Damasco, P.S.; Bernardes-Engemann, A.R.; Benvenuto, F.; Silva, I.C.; Lopes-Bezerra, L.M. Arthritis as a hypersensitivity reaction in a case of sporotrichosis transmitted by a sick cat: Clinical and serological follow up of 13 months. Mycoses 2010, 53, 81–83. [Google Scholar] [CrossRef]
- Santos, C.; Lima, N.; Sampaio, P.; Pais, C. Matrix-assisted laser desorption/ionization time-of-flight intact cell mass spectrometry to detect emerging pathogenic Candida species. Diagn. Microbiol. Infect. Dis. 2011, 71, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.M.; Santos, C.; Sampaio, P.; Romeo, O.; Almeida-Paes, R.; Pais, C.; Lima, N.; Zancopé-Oliveira, R.M. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res. Microbiol. 2015, 166, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Borba-Santos, L.P.; Rodrigues, A.M.; Gagini, T.B.; Fernandes, G.F.; Castro, R.; de Camargo, Z.P.; Nucci, M.; Lopes-Bezerra, L.M.; Ishida, K.; Rozental, S. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med. Mycol. 2015, 53, 178–188. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; Brito-Santos, F.; Figueiredo-Carvalho, M.H.G.; Machado, A.C.S.; Oliveira, M.M.E.; Pereira, S.A.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M. Minimal inhibitory concentration distributions and epidemiological cutoff values of five antifungal agents against Sporothrix brasiliensis. Mem. Inst. Oswaldo Cruz 2017, 112, 376–381. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Abreu, D.P.B.; Almeida-Paes, R.; Brilhante, R.S.N.; Chakrabarti, A.; Chowdhary, A.; Hagen, F.; Córdoba, S.; Gonzalez, G.M.; Govender, N.P.; et al. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob. Agents Chemother. 2017, 61, e01057-17. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Paes, R.; Oliveira, M.M.E.; Freitas, D.F.S.; Valle, A.C.F.D.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M. Refractory sporotrichosis due to Sporothrix brasiliensis in humans appears to be unrelated to in vivo resistance. Med. Mycol. 2017, 55, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Borba-Santos, L.P.; Visbal, G.; Gagini, T.; Rodrigues, A.M.; de Camargo, Z.P.; Lopes-Bezerra, L.M.; Ishida, K.; de Souza, W.; Rozental, S. Δ(24)-Sterol methyltransferase plays an important role in the growth and development of Sporothrix schenckii and Sporothrix brasiliensis. Front. Microbiol. 2016, 7, 311. [Google Scholar] [CrossRef] [Green Version]
- Asquith, C.R.M.; Machado, A.C.S.; de Miranda, L.H.M.; Konstantinova, L.S.; Almeida-Paes, R.; Rakitin, O.A.; Pereira, S.A. Synthesis and identification of pentathiepin-based inhibitors of Sporothrix brasiliensis. Antibiotics 2019, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Garcia Ferreira, P.; Pereira Borba-Santos, L.; Noronha, L.L.; Deckman Nicoletti, C.; de Sá Haddad Queiroz, M.; de Carvalho da Silva, F.; Rozental, S.; Omena Futuro, D.; Francisco Ferreira, V. Synthesis, stability studies, and antifungal evaluation of substituted α- and β-2,3-dihydrofuranaphthoquinones against Sporothrix brasiliensis and Sporothrix schenckii. Molecules 2019, 24, 930. [Google Scholar] [CrossRef] [Green Version]
- Poester, V.R.; Mattei, A.S.; Mendes, J.F.; Klafke, G.B.; Ramis, I.B.; Sanchotene, K.O.; Xavier, M.O. Antifungal activity of diphenyl diselenide alone and in combination with itraconazole against Sporothrix brasiliensis. Med. Mycol. 2019, 57, 328–331. [Google Scholar] [CrossRef]
- Gagini, T.; Borba-Santos, L.P.; Messias Rodrigues, A.; Pires de Camargo, Z.; Rozental, S. Clotrimazole is highly effective in vitro against feline Sporothrix brasiliensis isolates. J. Med. Microbiol. 2017, 66, 1573–1580. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Noronha, L.; Teixeira, R.; Vieira, I.; Borba-Santos, L.P.; Viçosa, A.; de Moraes, M.; Calil-Elias, S.; de Freitas, Z.; da Silva, F.C.; et al. Investigation of a microemulsion containing clotrimazole and itraconazole for transdermal delivery for the treatment of sporotrichosis. J. Pharm. Sci. 2020, 109, 1026–1034. [Google Scholar] [CrossRef]
- Waller, S.B.; Peter, C.M.; Hoffmann, J.F.; Picoli, T.; Osório, L.D.; Chaves, F.; Zani, J.L.; de Faria, R.O.; de Mello, J.R.; Meireles, M.C. Chemical and cytotoxic analyses of brown Brazilian propolis (Apis mellifera) and its in vitro activity against itraconazole-resistant Sporothrix brasiliensis. Microb. Pathog. 2017, 105, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Waller, S.B.; Madrid, I.M.; Silva, A.L.; Dias de Castro, L.L.; Cleff, M.B.; Ferraz, V.; Meireles, M.C.; Zanette, R.; de Mello, J.R. In vitro susceptibility of Sporothrix brasiliensis to essential oils of Lamiaceae Family. Mycopathologia 2016, 181, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Waller, S.B.; Madrid, I.M.; Hoffmann, J.F.; Picoli, T.; Cleff, M.B.; Chaves, F.C.; Faria, R.O.; Meireles, M.C.A.; Braga de Mello, J.R. Chemical composition and cytotoxicity of extracts of marjoram and rosemary and their activity against Sporothrix brasiliensis. J. Med. Microbiol. 2017, 66, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.G.S.; de Melo Guedes, G.M.; Fonseca, X.M.Q.C.; Pereira-Neto, W.A.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.C.; de Aguiar Cordeiro, R.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Antifungal activity of different molecular weight chitosans against planktonic cells and biofilm of Sporothrix brasiliensis. Int. J. Biol. Macromol. 2020, 143, 341–348. [Google Scholar] [CrossRef]
- Poester, V.R.; Munhoz, L.S.; Larwood, D.; Martinez, M.; Stevens, D.A.; Xavier, M.O. Potential use of Nikkomycin Z as an anti- Sporothrix spp. drug. Med. Mycol. 2020, myaa054. [Google Scholar] [CrossRef] [PubMed]
- Borba-Santos, L.P.; Reis de Sá, L.F.; Ramos, J.A.; Rodrigues, A.M.; de Camargo, Z.P.; Rozental, S.; Ferreira-Pereira, A. Tacrolimus increases the effectiveness of itraconazole and fluconazole against Sporothrix spp. Front. Microbiol. 2017, 8, 1759. [Google Scholar] [CrossRef] [Green Version]
- Ishida, K.; Castro, R.A.; Torrado, J.J.; Serrano, D.R.; Borba-Santos, L.P.; Quintella, L.P.; de Souza, W.; Rozental, S.; Lopes-Bezerra, L.M. Efficacy of a poly-aggregated formulation of amphotericin B in treating systemic sporotrichosis caused by Sporothrix brasiliensis. Med. Mycol. 2018, 56, 288–296. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.R.; Santiago, K.L.; Kaihami, G.H.; Maranhão, A.Q.; de Macedo Brígido, M.; de Almeida, S.R. The efficacy of humanized antibody against the Sporothrix antigen, gp70, in promoting phagocytosis and reducing disease burden. Front. Microbiol. 2017, 8, 345. [Google Scholar] [CrossRef] [Green Version]
- Portuondo, D.L.; Batista-Duharte, A.; Ferreira, L.S.; de Andrade, C.R.; Quinello, C.; Téllez-Martínez, D.; de Aguiar Loesch, M.L.; Carlos, I.Z. Comparative efficacy and toxicity of two vaccine candidates against Sporothrix schenckii using either Montanide™ Pet Gel A or aluminum hydroxide adjuvants in mice. Vaccine 2017, 35, 4430–4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, J.R.F.; Jannuzzi, G.P.; Kaihami, G.H.; Breda, L.C.D.; Ferreira, K.S.; de Almeida, S.R. An immunoproteomic approach revealing peptides from Sporothrix brasiliensis that induce a cellular immune response in subcutaneous sporotrichosis. Sci. Rep. 2018, 8, 4192. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals. A Road Map for Neglected Tropical Diseases 2021–2030. Overview. Available online: https://www.who.int/publications/i/item/WHO-UCN-NTD-2020.01 (accessed on 11 February 2021).



| Year | Locality (Province) | Sex | Age (Years) | Source of Infection (Connection) | Clinical Form | Diagnosis |
|---|---|---|---|---|---|---|
| 1986 | N.A. (Misiones) | N.A. | N.A. | N.A. | N.A. | Culture and Molecular |
| 1988 | N.A. (BA) | N.A. | N.A. | Cat | N.A. | Culture and Molecular |
| 1990 | N.A. (BA) | Female | 8 | Cat (Cat owner) | LC | Culture |
| 2011 | N.A. (BA) | N.A. | N.A. | Rat | N.A. | Culture and Molecular |
| 2011 | Paso del Rey (BA) | Male | 67 | Cat (Cat owner) | LC | Culture and Molecular |
| 2011 | Paso del Rey (BA) | Female | 67 | Cat (Cat owner) | FC | Culture and Molecular |
| 2012 | San Miguel (BA) | Male | 35 | Cat (Cat owner) | LC | Culture and Molecular |
| 2012 | Moreno (BA) | Female | 50 | Cat (Cat owner) | LC | Culture and Molecular |
| 2016 | Malvinas Argentinas (BA) | Female | 61 | Cat (Cat owner) | LC | Culture and Molecular |
| 2017 | N.A. (BA) | N.A. | N.A. | N.A. | N.A. | Culture and Molecular |
| 2018 | Los Polvorines (BA) | Female | 3 | Cat (Cat owner) | FC | Culture and Molecular |
| 2018 | Los Polvorines (BA) | Female | 33 | Cat (Veterinarian) | LC | Culture and Molecular |
| 2019 | Tigre (BA) | Female | 30 | Cat (Cat owner) | FC | Culture and Molecular |
| 2019 | Tigre (BA) | Female | 37 | Cat (Veterinarian) | FC | Culture and Molecular |
| 2019 | El Calafate (SC) | N.A. | N.A. | Cat (Catowner) | N.A. | Culture and Molecular |
| 2019 | El Calafate (SC) | N.A. | N.A. | Cat (Cat owner) | N.A. | Culture and Molecular |
| 2019 | El Calafate (SC) | N.A. | N.A. | Cat (Cat owner) | N.A. | Histopathology |
| 2019 | El Calafate (SC) | Female | 32 | Cat (Veterinarian) | N.A. | Histopathology |
| 2019 | N.A. (BA) | Male | N.A. | Cat | LC | Histopathology |
| 2019 | N.A. (BA) | Female | 4 | Cat (Cat owner) | LC | Culture |
| 2019 | Moreno (BA) | Male | 22 | Wood splinter (Workshop worker) | LC | Culture and Molecular |
| Year | Locality/Province | Sex | Age (Years) | Breed | Reproductive Status | Access to Street | FIV/ FELV | Clinical Presentation | Outcome | Zoonotic Transmission to Humans | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1988 | N.A. | N.A. | N.A. | UB | N.A. | Yes | N.A. | Localized | N.A. | Yes | Culture and Molecular |
| 2006 | Tigre/BA | Male | 1.5 | UB | Entire | Yes | N.A. | Disseminated | N.A. | N.A. | Culture |
| 2008 | Paso del Rey/BA | Male | 4 | Siamese | Entire | Yes | N/N | Localized | Recovery after therapy with itraconazole | No | Culture |
| 2011 | Paso del Rey/BA | Male | 2 | UB | Entire | Yes | N/N | Localized | Recovery after therapy with itraconazole | Yes | Culture |
| 2012 | San Miguel/BA | Female | 2 | UB | Entire | Yes | N.A. | Localized | Recovery after therapy with itraconazole | Yes | Cytology |
| San Miguel/BA | Female | 2 | UB | Entire | Yes | N.A. | Disseminated | Euthanasia | Yes | Cytology | |
| Moreno/BA | Male | 2 | UB | Entire | Yes | N.A. | Localized | N.A. | Yes | Culture and Molecular | |
| Paso del Rey/BA | Female | 2 | UB | Entire | Yes | N.A. | Localized | N.A. | No | Cytology | |
| 2013 | Florida/BA | Male | 2 | UB | Neutered | Yes | N/N | Disseminated | Death | No | Culture |
| Grand Burgh/BA | Female | 2 | UB | Neutered | Yes | N/N | Localized | Recovery after therapy with itraconazole | No | Culture | |
| 2016 | San Isidro/BA | Female | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| San Isidro/BA | Female | 2 | UB | N.A. | N.A. | N.A. | N.A. | Recovery after therapy with terbinafine | N.A. | Cytology | |
| Mar del Plata/BA | Female | 2 | Siamese | Neutered | Yes | N/N | Localized | Recovery after therapy with itraconazole | No | Cytology | |
| Los Polvorines/BA | Male | 10 | UB | N.A. | N.A. | N.A. | Disseminated | Death | Yes | Epidemiology and clinic | |
| 2017 | Tigre/BA | Female | 2 | UB | Neutered | Yes | N.A. | Localized | Recovery after therapy with itraconazole | No | Culture and Molecular |
| Tigre/BA | Female | 2 | UB | Neutered | Yes | N.A. | Localized | Recovery after therapy with itraconazole | No | Culture and Molecular | |
| 2018 | Tres de Febrero/BA | Female | 2 | UB | Castrated | Yes | N/N | Localized | Recovery after therapy with itraconazole | Yes | Culture and Molecular |
| 2019 | Tres de Febrero/BA | Male | 4 | UB | Neutered | Yes | P/N | Disseminated | Recovery after therapy with itraconazole | No | Culture and Molecular |
| Tigre/BA | Male | 2 | UB | Neutered | Yes | N.A. | Disseminated | Recovery after therapy with itraconazole | Yes | Culture and Molecular | |
| Ciudad Evita/BA | Male | N.A. | UB | Entire | Yes | N.A. | Localized | N.A. | Yes | Culture and Molecular | |
| El Calafate/SC | Male | N.A. | UB | N.A. | N.A. | N.A. | Disseminated | Death | Yes | Histopathology | |
| El Calafate/SC | Male | N.A. | UB | N.A. | N.A. | N.A. | Disseminated | Death | Yes | Histopathology | |
| El Calafate/SC | Male | N.A. | UB | N.A. | N.A. | N.A. | Disseminated | Death | Yes | Histopathology | |
| El Calafate/SC | Male | N.A. | UB | N.A. | N.A. | N.A. | Disseminated | Death | Yes | Histopathology | |
| El Calafate/SC | Male | N.A. | UB | N.A. | N.A. | N.A. | N.A. | N.A. | No | N.A. | |
| El Calafate/SC | Male | N.A. | UB | N.A. | N.A. | N.A. | N.A. | N.A. | No | N.A. |
| Virulence Factors | Function | Cellular Location |
|---|---|---|
| Thermal dimorphism | Establishment of the infection | - |
| Thermotolerance | Survival and parasitism | - |
| Biofilm | Drug resistance | Extracellular |
| Extracellular cell wall glucanase | Immune evasion and secretion of toxic factors | Extracellular |
| Extracellular vesicles | Drug resistance, cell invasion and pathogenesis | Extracellular |
| 3-carboxymuconate cyclase | Adhesion to extracellular matrix | Cell wall |
| Enolase (2-phospho-d-glycerate hydrolase) | Adhesin | Cell wall |
| Cell wall lipids | Phagocytosis inhibition | Cell wall |
| Melanin | Drug resistance, protection against nitrogen-derived oxidants; phagocytosis resistance; dissemination of internal organ phagocytosis. Penetration into tissues and evasion from immune system | Cell wall |
| Glyceraldehyde-3-phosphate dehydrogenase | Glycolysis and adhesin | Cytosol |
| Progesterone binding protein | Dimorphism | Cytosol |
| Rhamnolipid biosynthesis 3-oxacyl-(acyl-carrier-protein) reductase | Immune modulation, antimicrobial activity, biofilm development, surface motility | Cytosol |
| Hydroxymethylglutaryl-coenzyme A lyase | Immune evasion | Cytosol, endoplasmic reticulum |
| Heat shock 70-kDa protein 1/8 | Immune evasion and secretion of toxic factors | Cytosol, mitochondria, plasma membrane, endoplasmic reticulum and nucleus |
| Mn superoxide dismutase | Immune evasion and secretion of toxic factors | Mitochondria |
| Acetyl-coenzyme A hydrolase | Carbohydrate, lipid and protein metabolism | Mitochondria |
| Aminopeptidase I | Immune evasion and secretion of toxic factors | Vacuole |
| Urease | promotes penetration into tissues and evasion from the immune system | Vacuole |
| Clinical Form | Main Characteristics |
|---|---|
| Cutaneous | Usually appears after a minor trauma that alters the integrity of the epidermis. |
| Fixed | Yeasts remain localized in the subcutaneous tissue. One or few lesions occur at the inoculation site, which are often ulcerated with erythematous edges. Lesions may be ulcerated, verrucous, plaque infiltrated, or tuberous. Without lymphatic involvement. |
| Lymphocutaneous | Yeasts spread to adjacent lymphatic vessels. The primary lesion is usually located on the extremities, especially hands and forearms. The lesion has a papulonodular appearance and may ulcerate and fistulize. Secondary lesions arise along the regional lymphatic channels. Lymph node involvement or systemic symptoms are unusual. The most frequent clinical form (>75% affected patients). |
| Cutaneous-disseminated | Yeasts spread by the hematogenous route. Multiple skin lesions occur at noncontiguous sites. Without extracutaneous involvement. Mainly seen in immunocompromised hosts. |
| Mucosal | May be caused by self-inoculation through fungus-contaminated hands, hematogenous dissemination or conidia inhalation. Preauricular and submandibular lymph node enlargement is frequent. |
| Nasal | Lesions often involve the septum, producing bloody secretions and detachment of crusts. |
| Ocular | May be produced by hematogenous spread or fungal inoculation. Can cause conjunctivitis, episcleritis, uveitis, choroiditis, and retrobulbar lesions, among others. The granulomatous lesion may be accompanied by a serous-purulent discharge, redness, and eyelid edema. |
| Extracutaneous | Observed in patients with acquired immunodeficiency syndrome (AIDS), diabetes, alcoholism, granulomatous diseases, cirrhosis, renal transplantation, malignancies and under corticosteroid or immunosuppressive treatment. |
| Pulmonary | Occurs by inhalation of propagules or hematogenous spread. Usually associated with chronic obstructive pulmonary disease, alcoholism, chronic use of corticosteroids and, immunosuppressive diseases. Presents tuberculosis-like symptoms. |
| Meningeal | Infrequent presentation. Usually associated with immunosuppressive diseases. |
| Osteoarticular | May occur by direct trauma, invasion through a preexisting cutaneous lesion or hematogenous spread. The lesions may vary from small granulomas to large lytic lesions. One or several joints and bones can be involved, as well as tenosynovitis or bursitis. In immunocompetent patients, monoarthritis is more frequent than multiple articular involvement. |
| Sepsis | May occur from a cutaneous focus. |
| Systemic or systemic disseminated | Extremely rare. Always associated with immunosuppressive diseases. |
| Immunoreactive | Rare occurrence, mainly in places with a large number of cases of the disease. Can produce erythema nodosum, erythema multiforme, Sweet’s syndrome, and reactive arthritis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etchecopaz, A.; Toscanini, M.A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.A.; Bendezú, K.; Nusblat, A.D.; Iachini, R.; Cuestas, M.L. Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. J. Fungi 2021, 7, 170. https://doi.org/10.3390/jof7030170
Etchecopaz A, Toscanini MA, Gisbert A, Mas J, Scarpa M, Iovannitti CA, Bendezú K, Nusblat AD, Iachini R, Cuestas ML. Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. Journal of Fungi. 2021; 7(3):170. https://doi.org/10.3390/jof7030170
Chicago/Turabian StyleEtchecopaz, Alejandro, María A. Toscanini, Amelia Gisbert, Javier Mas, Miguel Scarpa, Cristina A. Iovannitti, Karla Bendezú, Alejandro D. Nusblat, Ricardo Iachini, and María L. Cuestas. 2021. "Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina" Journal of Fungi 7, no. 3: 170. https://doi.org/10.3390/jof7030170
APA StyleEtchecopaz, A., Toscanini, M. A., Gisbert, A., Mas, J., Scarpa, M., Iovannitti, C. A., Bendezú, K., Nusblat, A. D., Iachini, R., & Cuestas, M. L. (2021). Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. Journal of Fungi, 7(3), 170. https://doi.org/10.3390/jof7030170






