Diversity and N2O Production Potential of Fungi in an Oceanic Oxygen Minimum Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Seawater Filtration
2.2. DNA Extraction
2.3. Sequencing and Analysis of the ITS2 Region
2.4. Analysis of Fungal Diversity and Function from Metagenomes
2.5. Measurements of Potential N2O Production Rates
3. Results
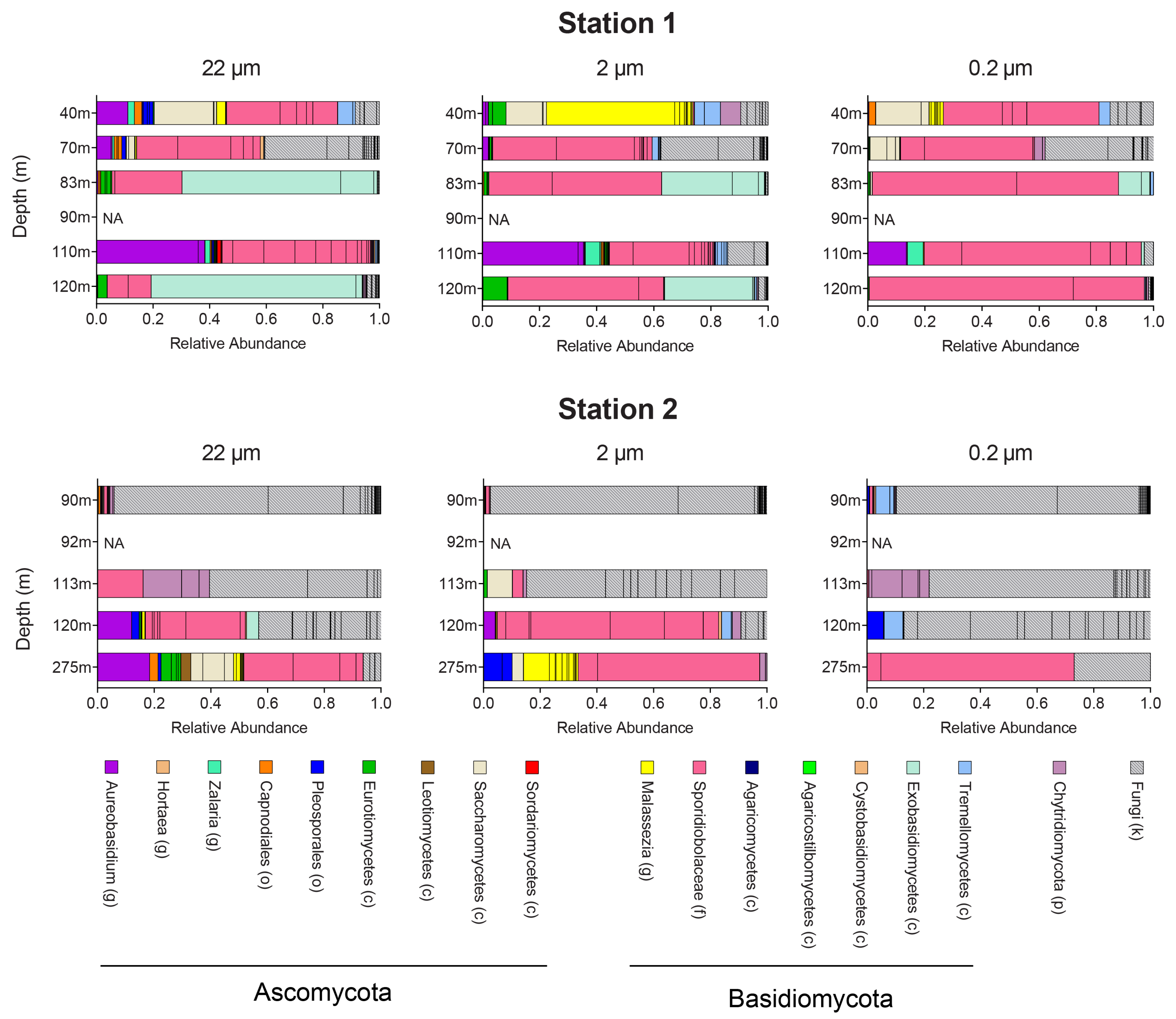
3.1. Fungal Diversity Assessed by Sequencing the ITS2 Region
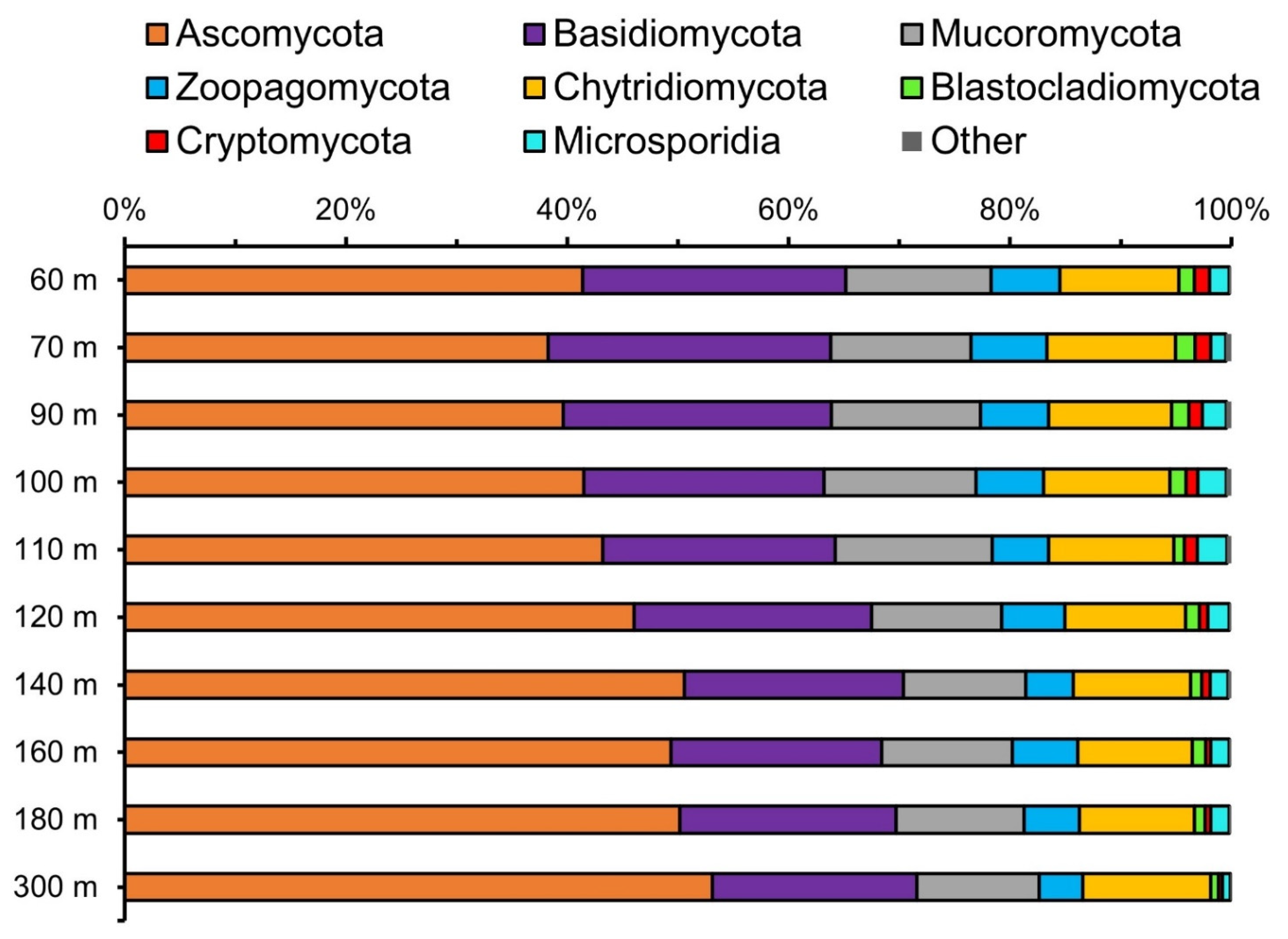
3.2. Fungal Diversity Assessed from Metagenomes
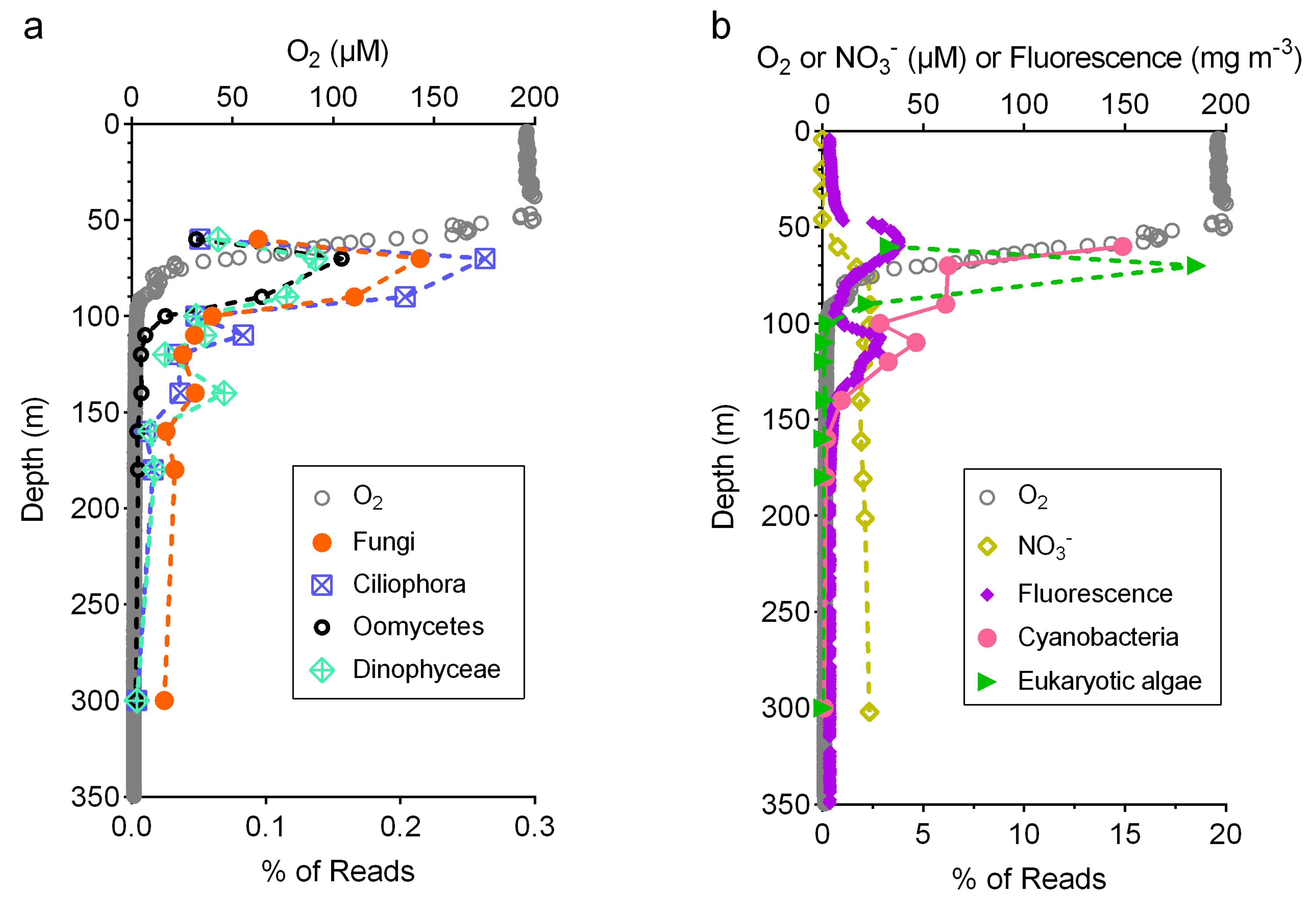
3.3. Relative Abundance of Fungi Compared to Other Taxa
3.4. Potential Contribution of Fungal N2O Production
3.5. Search for Fungal Denitrifiers and Functional Genes
4. Discussion
4.1. Fungal Diversity in Different Size Fractions
4.2. Ecology of Marine Fungi in the Oxygen Minimum Zone
4.3. Fungal N2O Production in the Oxygen Minimum Zone
4.4. Molecular Evidence for Fungal N2O Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Extrapolation of N2O Production by a Putative Fungal Denitrifier
References
- Paulmier, A.; Ruiz-Pino, D. Oxygen minimum zones (OMZs) in the modern ocean. Prog. Oceanogr. 2009, 80, 113–128. [Google Scholar] [CrossRef]
- Thamdrup, B.; Steinsdóttir, H.G.R.; Bertagnolli, A.D.; Padilla, C.C.; Patin, N.V.; Garcia-Robledo, E.; Bristow, L.A.; Stewart, F.J. Anaerobic methane oxidation is an important sink for methane in the ocean’s largest oxygen minimum zone. Limnol. Oceanogr. 2019, 64, 2569–2585. [Google Scholar] [CrossRef]
- Callbeck, C.M.; Lavik, G.; Ferdelman, T.G.; Fuchs, B.; Gruber-Vodicka, H.R.; Hach, P.F.; Littmann, S.; Schoffelen, N.J.; Kalvelage, T.; Thomsen, S.; et al. Oxygen minimum zone cryptic sulfur cycling sustained by offshore transport of key sulfur oxidizing bacteria. Nat. Commun. 2018, 9, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulloa, O.; Canfield, D.E.; DeLong, E.F.; Letelier, R.M.; Stewart, F.J. Microbial oceanography of anoxic oxygen minimum zones. Proc. Natl. Acad. Sci. USA 2012, 109, 15996–16003. [Google Scholar] [CrossRef] [Green Version]
- Beman, J.M.; Vargas, S.M.; Vazquez, S.; Wilson, J.M.; Yu, A.; Cairo, A.; Perez-Coronel, E. Biogeochemistry and hydrography shape microbial community assembly and activity in the eastern tropical North Pacific Ocean oxygen minimum zone. Environ. Microbiol. 2020. [Google Scholar] [CrossRef]
- Glass, J.B.; Kretz, C.B.; Ganesh, S.; Ranjan, P.; Seston, S.L.; Buck, K.N.; Landing, W.M.; Morton, P.L.; Moffett, J.W.; Giovannoni, S.J.; et al. Meta-omic signatures of microbial metal and nitrogen cycling in marine oxygen minimum zones. Front. Microbiol. 2015, 6, 998. [Google Scholar] [CrossRef] [Green Version]
- Duret, M.T.; Pachiadaki, M.G.; Stewart, F.J.; Sarode, N.; Christaki, U.; Monchy, S.; Srivasava, A.; Edgcomb, V.P. Size-fractionated diversity of eukaryotic microbial communities in the Eastern Tropical North Pacific oxygen minimum zone. FEMS Microbiol. Ecol. 2015, 91, fiv037. [Google Scholar] [CrossRef]
- Parris, D.J.; Ganesh, S.; Edgcomb, V.P.; DeLong, E.F.; Stewart, F.J. Microbial eukaryote diversity in the marine oxygen minimum zone off northern Chile. Front. Microbiol. 2014, 5, 543. [Google Scholar] [CrossRef] [Green Version]
- Fell, J.W. Distribution of Yeasts in the Indian Ocean. Bull. Mar. Sci. 1967, 17, 454–470. [Google Scholar]
- Gutiérrez, M.H.; Pantoja, S.; Quiñones, R.A.; González, R.R. First record of filamentous fungi in the coastal upwelling ecosystem off central Chile. Gayana 2010, 74, 66–73. [Google Scholar]
- Gutiérrez, M.H.; Jara, A.M.; Pantoja, S. Fungal parasites infect marine diatoms in the upwelling ecosystem of the Humboldt current system off central Chile. Environ. Microbiol. 2016, 18, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.A.; Jones, M.D.M.; Leonard, G.; Bass, D. Marine Fungi: Their Ecology and Molecular Diversity. Annu. Rev. Mar. Science 2012, 4, 495–522. [Google Scholar] [CrossRef] [PubMed]
- Grossart, H.-P.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Heitman, J.; Hom, E.F.Y.; Ianiri, G.; Jones, A.C.; et al. Fungi in the Marine Environment: Open Questions and Unsolved Problems. mBio 2019, 10, e01189-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, M.H.; Pantoja, S.; Tejos, E.; Quiñones, R.A. The role of fungi in processing marine organic matter in the upwelling ecosystem off Chile. Mar. Biol. 2011, 158, 205–219. [Google Scholar] [CrossRef]
- Shoun, H.; Tanimoto, T. Denitrification by the fungus Fusarium oxysporum and involvement of cytochrome P-450 in the respiratory nitrite reduction. J. Biol. Chem. 1991, 266, 11078–11082. [Google Scholar] [CrossRef]
- Shoun, H.; Kim, D.-H.; Uchiyama, H.; Sugiyama, J. Denitrification by fungi. FEMS Microbiol. Lett. 1992, 94, 277–281. [Google Scholar] [CrossRef]
- Stein, L.Y.; Yung, Y.L. Production, Isotopic Composition, and Atmospheric Fate of Biologically Produced Nitrous Oxide. Annu. Rev. Earth Planet. Sci. 2003, 31, 329–356. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Spor, A.; Edel-Hermann, V.; Heraud, C.; Breuil, M.-C.; Bizouard, F.; Toyoda, S.; Yoshida, N.; Steinberg, C.; Philippot, L. N2O production, a widespread trait in fungi. Sci. Rep. 2015, 5, srep09697. [Google Scholar] [CrossRef] [Green Version]
- Stief, P.; Fuchs-Ocklenburg, S.; Kamp, A.; Manohar, C.-S.; Houbraken, J.; Boekhout, T.; de Beer, D.; Stoeck, T. Dissimilatory nitrate reduction by Aspergillus terreus isolated from the seasonal oxygen minimum zone in the Arabian Sea. BMC Microbiol. 2014, 14, 35. [Google Scholar] [CrossRef] [Green Version]
- Wankel, S.D.; Ziebis, W.; Buchwald, C.; Charoenpong, C.; de Beer, D.; Dentinger, J.; Xu, Z.; Zengler, K. Evidence for fungal and chemodenitrification based N2O flux from nitrogen impacted coastal sediments. Nat. Commun. 2017, 8, 15595. [Google Scholar] [CrossRef]
- Cathrine, S.J.; Raghukumar, C. Anaerobic denitrification in fungi from the coastal marine sediments off Goa, India. Mycol. Res. 2009, 113, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.E.; Locarnini, R.A.; Boyer, T.P.; Antonov, J.I.; Baranova, O.K.; Zweng, M.M.; Reagan, J.R.; Johnson, D.R. World Ocean Atlas 2013, Volume 4: Dissolved Inorganic Nutrients (phosphate, nitrate, silicate). In NOAA Atlas NESDIS; Levitus, S., Ed.; U.S. Department of Commerce: Silver Spring, MD, USA, 2013; pp. 1–25. [Google Scholar]
- QIAGEN. DNeasy Plant Handbook. 2015. Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/dna-purification/genomic-dna/dneasy-plant-mini-kit/#resources (accessed on 8 March 2019).
- Illumina. 16S Metagenomic Sequencing Library Preparation. Available online: https://support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation.html (accessed on 8 March 2019).
- Tedersoo, L.; Anslan, S.; Bahram, M.; Põlme, S.; Riit, T.; Liiv Kõljalg, U.; Kisand, V.; Nilsson, H.; Hildebrand, F.; Bork, P.; et al. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 2015, 10, 1–43. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suiji, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivers, A.R.; Weber, K.C.; Gardner, T.G.; Liu, S.; Armstrong, S.D. ITSxpress: Software to rapidly trim internally transcribed spacer sequences with quality scores for marker gene analysis. F1000Research 2018, 7, 1418. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 2016, 081257. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- UNITE Community. UNITE QIIME Release for Fungi. Version 02.02.2019. Available online: https://unite.ut.ee/repository.php (accessed on 8 March 2019).
- Kearns, P.J.; Bulseco-McKim, A.N.; Hoyt, H.; Angell, J.H.; Bowen, J.L. Nutrient Enrichment Alters Salt Marsh Fungal Communities and Promotes Putative Fungal Denitrifiers. Microb. Ecol. 2018, 77, 358–369. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchsman, C.A.; Devol, A.H.; Saunders, J.K.; McKay, C.; Rocap, G. Niche Partitioning of the N Cycling Microbial Community of an Offshore Oxygen Deficient Zone. Front. Microbiol 2017, 8, 2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushnell, B. BBMap Short-Read Aligner, and Other Bioinformatics Tools. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 8 March 2019).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Takaya, N.; Shoun, H. Nitric oxide reduction, the last step in denitrification by Fusarium oxysporum, is obligatorily mediated by cytochrome P450nor. Mol. Gen. Genet. 2000, 263, 342–348. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [Green Version]
- Higgins, S.A.; Schadt, C.W.; Matheny, P.B.; Löffler, F.E. Phylogenomics Reveal the Dynamic Evolution of Fungal Nitric Oxide Reductases and Their Relationship to Secondary Metabolism. Genome Biol. Evol. 2018, 10, 2474–2489. [Google Scholar] [CrossRef] [Green Version]
- Holmes, R.M.; Aminot, A.; Kérouel, R.; Hooker, B.A.; Peterson, B.J. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci 1999, 56, 1801–1808. [Google Scholar] [CrossRef]
- Hales, B.; van Geen, A.; Takahashi, T. High-frequency measurement of seawater chemistry: Flow-injection analysis of macronutrients. Limnol. Oceanogr. Methods 2004, 2, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Ji, Q.; Grundle, D.S. An automated, laser-based measurement system for nitrous oxide isotope and isotopomer ratios at nanomolar levels. Rapid Commun. Mass Spectrom. 2019, 33, 1553–1564. [Google Scholar] [CrossRef]
- Peng, X.; Fuchsman, C.A.; Jayakumar, A.; Oleynik, S.; Martens-Habbena, W.; Devol, A.H.; Ward, B.B. Ammonia and nitrite oxidation in the Eastern Tropical North Pacific. Glob. Biogeochem. Cycles 2015, 29, 2034–2049. [Google Scholar] [CrossRef] [Green Version]
- Balabanova, L.A.; Gafurov, Y.M.; Pivkin, M.V.; Terentyeva, N.A.; Likhatskaya, G.N.; Rasskazov, V.A. An Extracellular S1-Type Nuclease of Marine Fungus Penicillium melinii. Mar. Biotechnol. 2012, 14, 87–95. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 678–692. [Google Scholar]
- Boekhout, T.; Theelen, B.; Houbraken, J.; Robert, V.; Scorzetti, G.; Gafni, A.; Gerson, U.; Sztejnberg, A. Novel anamorphic mite-associated fungi belonging to the Ustilaginomycetes: Meira geulakonigii gen. nov., sp. nov., Meira argovae sp. nov. and Acaromyces ingoldii gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 1655–1664. [Google Scholar] [CrossRef] [Green Version]
- Zalar, P.; Gostinčar, C.; de Hoog, G.S.; Uršič, V.; Sudhadham, M.; Gunde-Cimerman, N. Redefinition of Aureobasidium pullulans and its varieties. Stud. Mycol. 2008, 61, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.D.; Jones, E.B.G.; Leaño, E.; Pointing, S.B.; Poonyth, A.D.; Vrijmoed, L.L.P. Role of fungi in marine ecosystems. Biodivers. Conserv. 1998, 7, 1147–1161. [Google Scholar] [CrossRef]
- Hassett, B.T.; Vonnahme, T.R.; Peng, X.; Jones, E.B.G.; Heuzé, C. Global diversity and geography of planktonic marine fungi. Bot. Mar. 2020, 63, 121–139. [Google Scholar] [CrossRef] [Green Version]
- Worden, A.Z.; Follows, M.J.; Giovannoni, S.J.; Wilken, S.; Zimmerman, A.E.; Keeling, P.J. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 2015, 347, 1257594. [Google Scholar] [CrossRef] [Green Version]
- Jeffries, T.C.; Curlevski, N.J.; Brown, M.V.; Harrison, D.P.; Doblin, M.A.; Petrou, K.; Ralph, P.J.; Seymour, J.R. Partitioning of fungal assemblages across different marine habitats. Environ. Microbiol. Rep. 2016, 8, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Singh, P.; Gao, Z.; Zhang, X.; Johnson, Z.I.; Wang, G. Distribution and Diversity of Planktonic Fungi in the West Pacific Warm Pool. PLoS ONE 2014, 9, e101523. [Google Scholar]
- Berbee, M.L.; James, T.Y.; Strullu-Derrien, C. Early Diverging Fungi: Diversity and Impact at the Dawn of Terrestrial Life. Annu. Rev. Microbiol. 2017, 71, 41–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassett, B.T.; Gradinger, R. Chytrids dominate arctic marine fungal communities. Environ. Microbiol. 2016, 18, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Robledo, E.; Padilla, C.C.; Aldunate, M.; Stewart, F.J.; Ulloa, O.; Paulmier, A.; Gregori, G.; Revsbech, N.P. Cryptic oxygen cycling in anoxic marine zones. Proc. Natl. Acad. Sci. USA 2017, 114, 8319–8324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauchop, T.; Mountfort, D.O. Cellulose Fermentation by a Rumen Anaerobic Fungus in Both the Absence and the Presence of Rumen Methanogens. Appl. Environ. Microbiol. 1981, 42, 1103–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Stewart, F.J.; Thamdrup, B.; Brabandere, L.D.; Revsbech, N.P.; Ulloa, O.; Candield, D.E.; DeLong, E.F. Oxygen at Nanomolar Levels Reversibly Suppresses Process Rates and Gene Expression in Anammox and Denitrification in the Oxygen Minimum Zone off Northern Chile. mBio 2014, 5, e01966. [Google Scholar] [CrossRef] [Green Version]
- Hallin, S.; Philippot, L.; Löffler, F.E.; Sanford, R.A.; Jones, C.M. Genomics and Ecology of Novel N2O-Reducing Microorganisms. Trends Microbiol. 2018, 26, 43–55. [Google Scholar] [CrossRef]
- Watson, S.W.; Novitsky, T.J.; Quinby, H.L.; Valois, F.W. Determination of bacterial number and biomass in the marine environment. Appl. Environ. Microbiol. 1977, 33, 940–946. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Valentine, D.L. Diversity and N2O Production Potential of Fungi in an Oceanic Oxygen Minimum Zone. J. Fungi 2021, 7, 218. https://doi.org/10.3390/jof7030218
Peng X, Valentine DL. Diversity and N2O Production Potential of Fungi in an Oceanic Oxygen Minimum Zone. Journal of Fungi. 2021; 7(3):218. https://doi.org/10.3390/jof7030218
Chicago/Turabian StylePeng, Xuefeng, and David L. Valentine. 2021. "Diversity and N2O Production Potential of Fungi in an Oceanic Oxygen Minimum Zone" Journal of Fungi 7, no. 3: 218. https://doi.org/10.3390/jof7030218
APA StylePeng, X., & Valentine, D. L. (2021). Diversity and N2O Production Potential of Fungi in an Oceanic Oxygen Minimum Zone. Journal of Fungi, 7(3), 218. https://doi.org/10.3390/jof7030218





