Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit
Abstract
:1. Introduction
2. Patients and Methods
3. Results
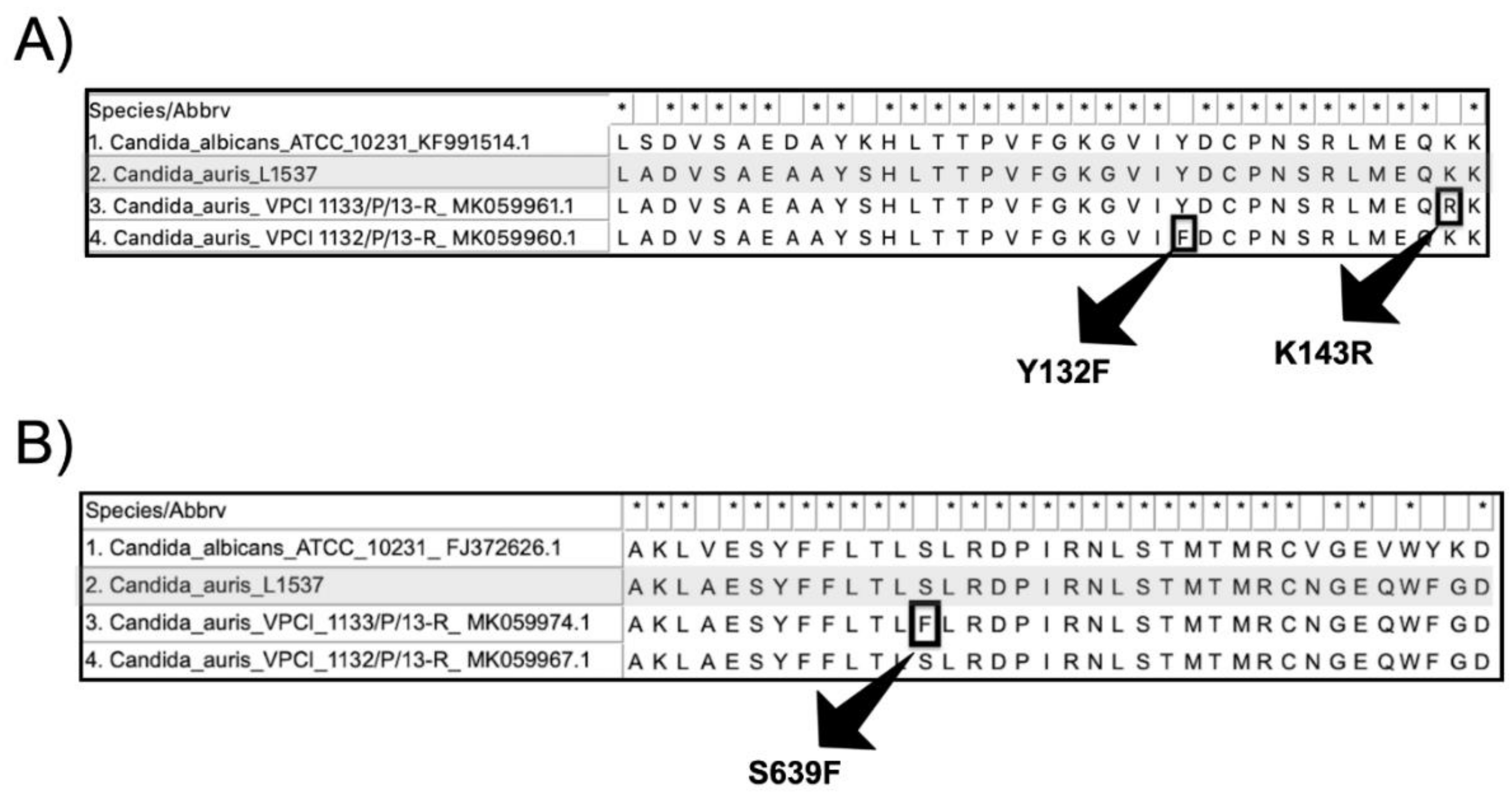
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvo, B.; Melo, A.S.A.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First Report of Candida auris in America: Clinical and Microbiological Aspects of 18 Episodes of Candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef] [PubMed]
- OPAS/OMS|3 October 2016: “Candida auris” Outbreaks in Health Care Services—Epidemiological Alert. Available online: https://www.paho.org/en/documents/epidemiological-alert-candida-auris-outbreaks-health-care-services-context-covid-19 (accessed on 11 February 2021).
- Gerência de Vigilância e Monitoramento em Serviços de Saúde; Gerência Geral de Tecnologia em Serviços de Saúde; Agência Nacional de Vigilância Sanitária. Comunicado de Riscono 01/2017–Gvims/Ggtes/Anvisa; Relatos de Surtos de Candida auris em Serviços de Saúde Da América Latina: Brasília, Brazil, 2017. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groot, T.; Puts, Y.; Berrio, I.; Chowdhary, A.; Meis, J.F. Development of Candida auris Short Tandem Repeat Typing and Its Application to a Global Collection of Isolates. mBio 2020, 11, e02971-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A Multicentre Study of Antifungal Susceptibility Patterns among 350 Candida auris Isolates (2009–17) in India: Role of the ERG11 and FKS1 Genes in Azole and Echinocandin Resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob. Agents Chemother. 2018, 62, e00238-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bing, J.; Zheng, Q.; Zhang, F.; Liu, J.; Yue, H.; Tao, L.; Du, H.; Wang, Y.; Wang, H.; et al. The First Isolate of Candida auris in China: Clinical and Biological Aspects. Emerg. Microbes Infect. 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva-Lozano, H.; de J Treviño-Rangel, R.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris Infection in a COVID-19 Hospital in Mexico. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A Latent Threat to Critically Ill Patients with COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.R.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris during the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. mBio 2020, 11, e03364-19. [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April-July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, J.N., Jr.; Francisco, E.C.; Hagen, F.; Brandão, I.B.; Pereira, F.M.; Presta Dias, P.H.; de Miranda Costa, M.M.; de Souza Jordão, R.T.; de Groot, T.; Colombo, A.L. Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. J. Fungi 2021, 7, 220. https://doi.org/10.3390/jof7030220
de Almeida JN Jr., Francisco EC, Hagen F, Brandão IB, Pereira FM, Presta Dias PH, de Miranda Costa MM, de Souza Jordão RT, de Groot T, Colombo AL. Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. Journal of Fungi. 2021; 7(3):220. https://doi.org/10.3390/jof7030220
Chicago/Turabian Stylede Almeida, João N., Jr., Elaine C. Francisco, Ferry Hagen, Igor B. Brandão, Felicidade M. Pereira, Pedro H. Presta Dias, Magda M. de Miranda Costa, Regiane T. de Souza Jordão, Theun de Groot, and Arnaldo L. Colombo. 2021. "Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit" Journal of Fungi 7, no. 3: 220. https://doi.org/10.3390/jof7030220
APA Stylede Almeida, J. N., Jr., Francisco, E. C., Hagen, F., Brandão, I. B., Pereira, F. M., Presta Dias, P. H., de Miranda Costa, M. M., de Souza Jordão, R. T., de Groot, T., & Colombo, A. L. (2021). Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. Journal of Fungi, 7(3), 220. https://doi.org/10.3390/jof7030220






