Fungal Melanin and the Mammalian Immune System
Abstract
:1. Introduction
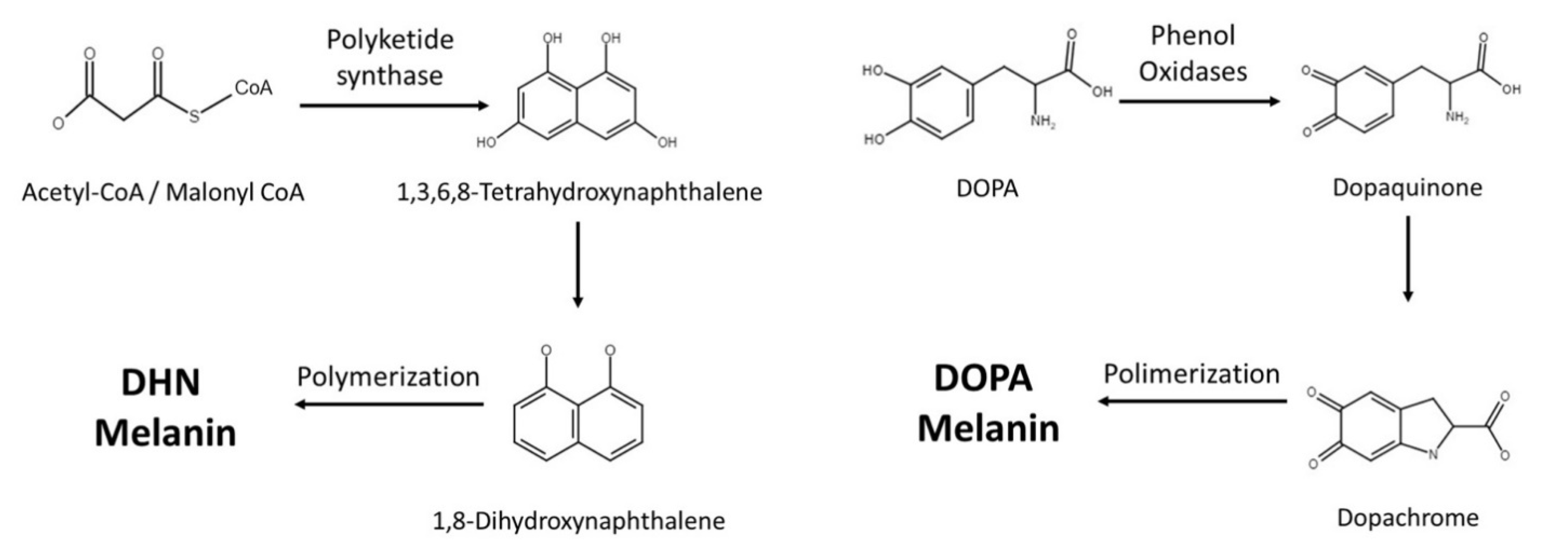
2. Melanin Synthesis
3. Cryptococcus neoformans
3.1. Melanosomes and Melanin Location
3.2. Melanin and Cell Wall
3.3. Survival Advantage
3.4. Melanin and Host Effector Cells
3.5. Antibody
3.6. Complement System
4. Aspergillus fumigatus
5. Other Melanotic Fungi and Their Interactions with the Immune System
6. Open Questions in Melanin Biology
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suwannarach, N.; Kumla, J.; Watanabe, B.; Matsui, K.; Lumyong, S. Characterization of melanin and optimal conditions for pigment production by an endophytic fungus, Spissiomyces endophytica SDBR-CMU319. PLoS ONE 2019, 14, e0222187. [Google Scholar] [CrossRef] [Green Version]
- Riley, P.A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239. [Google Scholar] [CrossRef]
- Eisen, T.G. The control of gene expression in melanocytes and melanomas. Melanoma Res. 1996, 6, 277–284. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, Á.; Neptuno Rodríguez-López, J.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Wheeler, M.H.; Bell, A.A. Melanins and their importance in pathogenic fungi. In Current Topics in Medical Mycology; Springer: Berlin/Heidelberg, Germany, 1988; pp. 338–387. [Google Scholar]
- Staunton, J.; Weissman, K.J. Polyketide biosynthesis: A millennium review. Nat. Prod. Rep. 2001, 18, 380–416. [Google Scholar] [CrossRef]
- Butler, M.; Day, A. Fungal melanins: A review. J. Can. J. Microbiol. 1998, 44, 1115–1136. [Google Scholar] [CrossRef]
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158. [Google Scholar] [CrossRef]
- Katz, L. Manipulation of Modular Polyketide Synthases. Chem. Rev. 1997, 97, 2557–2576. [Google Scholar] [CrossRef]
- Huffman, J.; Gerber, R.; Du, L. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers 2010, 93, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D.A. Complex enzymes in microbial natural product biosynthesis, part B: Polyketides, aminocoumarins and carbohydrates. Preface. Methods Enzymol. 2009, 459, xvii–xix. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, H.C.; Greer, E.M.; McGrail, C.W. The role of melanins in melanotic fungi for pathogenesis and environmental survival. Appl. Microbiol. Biotechnol. 2020, 104, 4247–4257. [Google Scholar] [CrossRef]
- Morris-Jones, R.; Youngchim, S.; Gomez, B.L.; Aisen, P.; Hay, R.J.; Nosanchuk, J.D.; Casadevall, A.; Hamilton, A.J. Synthesis of Melanin-Like Pigments by Sporothrix schenckiix In Vitro and during Mammalian Infection. Infect. Immun. 2003, 71, 4026–4033. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.A.; Gómez, B.L.; Mora-Montes, H.M.; Mackenzie, K.S.; Munro, C.A.; Brown, A.J.P.; Gow, N.A.R.; Kibbler, C.C.; Odds, F.C. Melanin externalization in Candida albicans depends on cell wall chitin structures. Eukaryot Cell 2010, 9, 1329–1342. [Google Scholar] [CrossRef] [Green Version]
- Nosanchuk, J.D.; Van Duin, D.; Mandal, P.; Aisen, P.; Legendre, A.M.; Casadevall, A. Blastomyces dermatitidis produces melanin in vitro and during infection. FEMS Microbiol. Lett. 2004, 239, 187–193. [Google Scholar] [CrossRef]
- McClelland, E.E.; Bernhardt, P.; Casadevall, A. Estimating the relative contributions of virulence factors for pathogenic microbes. Infect. Immun. 2006, 74, 1500–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Prados-Rosales, R.; Frases, S.; Itin, B.; Casadevall, A.; Stark, R.E. Using solid-state NMR to monitor the molecular consequences of Cryptococcus neoformans melanization with different catecholamine precursors. Biochemistry 2012, 51, 6080–6088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida-Paes, R.; Frases, S.; Fialho Monteiro, P.C.; Gutierrez-Galhardo, M.C.; Zancope-Oliveira, R.M.; Nosanchuk, J.D. Growth conditions influence melanization of Brazilian clinical Sporothrix schenckii isolates. Microbes Infect. 2009, 11, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas, A.L.; Nosanchuk, J.D.; Feldmesser, M.; Cox, G.M.; McDade, H.C.; Casadevall, A. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect. Immun. 2000, 68, 2845–2853. [Google Scholar] [CrossRef] [Green Version]
- Kwon-Chung, K.J.; Polacheck, I.; Popkin, T.J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 1982, 150, 1414–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, J.C.; Polacheck, I.; Kwon-Chung, K.J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect. Immun. 1982, 36, 1175–1184. [Google Scholar] [CrossRef] [Green Version]
- Williamson, P.R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: Identification as a laccase. J. Bacteriol. 1994, 176, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Salas, S.D.; Bennett, J.E.; Kwon-Chung, K.J.; Perfect, J.R.; Williamson, P.R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef]
- Pukkila-Worley, R.; Gerrald, Q.D.; Kraus, P.R.; Boily, M.-J.; Davis, M.J.; Giles, S.S.; Cox, G.M.; Heitman, J.; Alspaugh, J.A. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell 2005, 4, 190–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Frases, S.; Wang, H.; Casadevall, A.; Stark, R.E. Following fungal melanin biosynthesis with solid-state NMR: Biopolymer molecular structures and possible connections to cell-wall polysaccharides. Biochemistry 2008, 47, 4701–4710. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Frases, S.; Nicola, A.M.; Rodrigues, M.L.; Casadevall, A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 2009, 155, 3860–3867. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.L.; Nakayasu, E.S.; Oliveira, D.L.; Nimrichter, L.; Nosanchuk, J.D.; Almeida, I.C.; Casadevall, A. Extracellular Vesicles Produced by Cryptococcus neoformans Contain Protein Components Associated with Virulence. J. Eukaryotic. Cell 2008, 7, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Panepinto, J.; Komperda, K.; Frases, S.; Park, Y.-D.; Djordjevic, J.T.; Casadevall, A.; Williamson, P.R. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol. Microbiol. 2009, 71, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- San-Blas, G.; Guanipa, O.; Moreno, B.; Pekerar, S.; San-Blas, F. Cladosporium carrionii and Hormoconis resinae (C. resinae): Cell wall and melanin studies. Curr. Microbiol. 1996, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Xu, X.; Lowry, D.; Jackson, J.C.; Roberson, R.W.; Lin, X. Subcellular Compartmentalization and Trafficking of the Biosynthetic Machinery for Fungal Melanin. Cell Rep. 2016, 14, 2511–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosanchuk, J.D.; Stark, R.E.; Casadevall, A. Fungal Melanin: What do We Know About Structure? Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Nosanchuk, J.D.; Casadevall, A. Budding of melanized Cryptococcus neoformans in the presence or absence of L-dopa. Microbiology 2003, 149, 1945–1951. [Google Scholar] [CrossRef] [Green Version]
- Morris-Jones, R.; Gomez, B.L.; Diez, S.; Uran, M.; Morris-Jones, S.D.; Casadevall, A.; Nosanchuk, J.D.; Hamilton, A.J. Synthesis of Melanin Pigment by Candida albicans In Vitro and during Infection. Infect. Immun. 2005, 73, 6147–6150. [Google Scholar] [CrossRef] [Green Version]
- Rosas, Á.L.; Nosanchuk, J.D.; Gómez, B.L.; Edens, W.A.; Henson, J.M.; Casadevall, A. Isolation and serological analyses of fungal melanins. J. Immunol. Methods 2000, 244, 69–80. [Google Scholar] [CrossRef]
- Gómez, B.L.; Nosanchuk, J.D.; Díez, S.; Youngchim, S.; Aisen, P.; Cano, L.E.; Restrepo, A.; Casadevall, A.; Hamilton, A.J. Detection of Melanin-Like Pigments in the Dimorphic Fungal Pathogen Paracoccidioides brasiliensis In Vitro and during Infection. J. Infect. Immun. 2001, 69, 5760–5767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosanchuk, J.D.; Yu, J.-J.; Hung, C.-Y.; Casadevall, A.; Cole, G.T. Coccidioides posadasii produces melanin in vitro and during infection. Fungal Genet. Biol. 2007, 44, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Gómez, B.L.; Youngchim, S.; Díez, S.; Aisen, P.; Zancopé-Oliveira, R.M.; Restrepo, A.; Casadevall, A.; Hamilton, A.J. Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect. Immun. 2002, 70, 5124–5131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenman, H.C.; Nosanchuk, J.D.; Webber, J.B.; Emerson, R.J.; Camesano, T.A.; Casadevall, A. Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry 2005, 44, 3683–3693. [Google Scholar] [CrossRef] [PubMed]
- Pihet, M.; Vandeputte, P.; Tronchin, G.; Renier, G.; Saulnier, P.; Georgeault, S.; Mallet, R.; Chabasse, D.; Symoens, F.; Bouchara, J.P. Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol. 2009, 9, 177. [Google Scholar] [CrossRef]
- Jacobson, E.S.; Ikeda, R. Effect of melanization upon porosity of the cryptococcal cell wall. Med. Mycol. 2005, 43, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Franzen, A.J.; Cunha, M.M.; Batista, E.J.; Seabra, S.H.; De Souza, W.; Rozental, S. Effects of tricyclazole (5-methyl-1,2,4-triazol [3,4] benzothiazole), a specific DHN-melanin inhibitor, on the morphology of Fonsecaea pedrosoi conidia and sclerotic cells. Microsc. Res. Tech. 2006, 69, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Hegnauer, H.; Nyhle´n, L.E.; Rast, D.M. Ultrastructure of native and synthetic Agaricus bisporus melanins—Implications as to the compartmentation of melanogenesis in fungi. Exp. Mycol. 1985, 9, 1–29. [Google Scholar] [CrossRef]
- Kogej, T.; Stein, M.; Volkmann, M.; Gorbushina, A.A.; Galinski, E.A.; Gunde-Cimerman, N. Osmotic adaptation of the halophilic fungus Hortaea werneckii: Role of osmolytes and melanization. Microbiology 2007, 153, 4261–4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, C.A.; Gow, N.A. Chitin synthesis in human pathogenic fungi. Med. Mycol. 2001, 39 (Suppl. 1), 41–53. [Google Scholar] [CrossRef]
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot Cell 2007, 6, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Banks, I.R.; Specht, C.A.; Donlin, M.J.; Gerik, K.J.; Levitz, S.M.; Lodge, J.K. A Chitin Synthase and Its Regulator Protein Are Critical for Chitosan Production and Growth of the Fungal Pathogen Cryptococcus neoformans. J. Eukaryot. Cell 2005, 4, 1902–1912. [Google Scholar] [CrossRef] [Green Version]
- Walton, F.J.; Idnurm, A.; Heitman, J. Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol. Microbiol. 2005, 57, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Lam, W.C.; Maybruck, B.; Specht, C.A.; Levitz, S.M.; Lodge, J.K. Induction of Protective Immunity to Cryptococcal Infection in Mice by a Heat-Killed, Chitosan-Deficient Strain of Cryptococcus neoformans. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosanchuk, J.D.; Rudolph, J.; Rosas, A.L.; Casadevall, A. Evidence That Cryptococcus neoformans Is Melanized in Pigeon Excreta: Implications for Pathogenesis. Infect. Immun. 1999, 67, 5477–5479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas, Á.L.; Casadevall, A. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol. Lett. 1997, 153, 265–272. [Google Scholar] [CrossRef]
- Zhdanova, N.; Gavriushina, A.; Vasilevskaia, A. Effect of gamma and UV irradiation on the survival of Cladosporium sp. and Oidiodendron cerealis. Mikrobiolohichnyi Zhurnal 1973, 35, 449–452. [Google Scholar] [PubMed]
- Mironenko, N.V.; Alekhina, I.A.; Zhdanova, N.N.; Bulat, S.A. Intraspecific variation in gamma-radiation resistance and genomic structure in the filamentous fungus Alternaria alternata: A case study of strains inhabiting Chernobyl reactor no. 4. Ecotoxicol. Environ. Saf. 2000, 45, 177–187. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Casadevall, A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 2006, 50, 3519–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadachova, E.; Bryan, R.A.; Huang, X.; Moadel, T.; Schweitzer, A.D.; Aisen, P.; Nosanchuk, J.D.; Casadevall, A. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2007, 2, e457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, K.L.; Mostaghim, A.; Cuomo, C.A.; Soto, C.M.; Lebedev, N.; Bailey, R.F.; Wang, Z. Adaptation of the black yeast Wangiella dermatitidis to ionizing radiation: Molecular and cellular mechanisms. PLoS ONE 2012, 7, e48674. [Google Scholar] [CrossRef] [Green Version]
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef]
- Rosas, A.L.; Casadevall, A. Melanization decreases the susceptibility of Cryptococcus neoformans to enzymatic degradation. Mycopathologia 2001, 151, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, R.V.; Tobin, J.M. Fungal melanins and their interactions with metals. Enzym. Microb. Technol. 1996, 19, 311–317. [Google Scholar] [CrossRef]
- Garcia-Rivera, J.; Casadevall, A. Melanization of Cryptococcus neoformans reduces its susceptibility to the antimicrobial effects of silver nitrate. Sabouraudia 2001, 39, 353–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, R.; Sugita, T.; Jacobson, E.S.; Shinoda, T. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol. Immunol. 2003, 47, 271–277. [Google Scholar] [CrossRef]
- Barza, M.; Baum, J.; Kane, A. Inhibition of antibiotic activity in vitro by synthetic melanin. Antimicrob. Agents Chemother. 1976, 10, 569. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, M.; Sasaki, K. Changes in the antibacterial activity of melanin-bound drugs. Ophthalmic Res. 1990, 22, 123–127. [Google Scholar] [CrossRef]
- White, L.P. Melanin: A Naturally Occurring Cation Exchange Material. Nature 1958, 182, 1427–1428. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Casadevall, A. Cellular charge of Cryptococcus neoformans: Contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect. Immun. 1997, 65, 1836–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozel, T.R. Dissociation of a hydrophobic surface from phagocytosis of encapsulated and non-encapsulated Cryptococcus neoformans. Infect. Immun. 1983, 39, 1214–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, H.; Graham, L.L.; Krob, E.J.; Hill, M. Correlation between phagocytic and membrane surface properties reflected by partitioning of human peripheral blood monocytes in two-polymer aqueous phases. Biochim. Biophys. Acta. 1980, 602, 309–322. [Google Scholar] [CrossRef]
- Eggleton, P.; Crawford, N.; Fisher, D. Fractionation of human neutrophils into subpopulations by countercurrent distribution: Surface charge and functional heterogeneity. Eur. J. Cell Biol. 1992, 57, 265–272. [Google Scholar]
- Mednick, A.J.; Nosanchuk, J.D.; Casadevall, A. Melanization of Cryptococcus neoformans affects lung inflammatory responses during cryptococcal infection. Infect. Immun. 2005, 73, 2012–2019. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Aisen, P.; Casadevall, A. Cryptococcus neoformans melanin and virulence: Mechanism of action. Infect. Immun. 1995, 63, 3131–3136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doering, T.L.; Nosanchuk, J.D.; Roberts, W.K.; Casadevall, A. Melanin as a potential cryptococcal defence against microbicidal proteins. Med. Mycol. 1999, 37, 175–181. [Google Scholar] [CrossRef]
- Alvarez, M.; Casadevall, A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 2006, 16, 2161–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazão, S.d.O.; Sousa, H.R.d.; Silva, L.G.d.; Folha, J.d.S.; Gorgonha, K.C.d.M.; Oliveira, G.P.d.; Felipe, M.S.S.; Silva-Pereira, I.; Casadevall, A.; Nicola, A.M.; et al. Laccase Affects the Rate of Cryptococcus neoformans Nonlytic Exocytosis from Macrophages. mBio 2020, 11, e02085-20. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Sabiiti, W.; Robertson, E.; Beale, M.A.; Johnston, S.A.; Brouwer, A.E.; Loyse, A.; Jarvis, J.N.; Gilbert, A.S.; Fisher, M.C.; Harrison, T.S.; et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J. Clin. Investig. 2014, 124, 2000–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas, A.L.; MacGill, R.S.; Nosanchuk, J.D.; Kozel, T.R.; Casadevall, A. Activation of the alternative complement pathway by fungal melanins. Clin. Diagn Lab. Immunol. 2002, 9, 144–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, D.L.; Lee, S.C.; Mednick, A.J.; Montella, L.; Casadevall, A. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect. Immun. 2000, 68, 832–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Tewari, R.P.; Williamson, P.R. Laccase Protects Cryptococcus neoformans from Antifungal Activity of Alveolar Macrophages. Infect. Immun. 1999, 67, 6034–6039. [Google Scholar] [CrossRef] [Green Version]
- Minton, K. Granuloma macrophage differentiation. Nat. Rev. Immunol. 2016, 16, 719. [Google Scholar] [CrossRef]
- Tajima, K.; Yamanaka, D.; Ishibashi, K.I.; Adachi, Y.; Ohno, N. Solubilized melanin suppresses macrophage function. FEBS Open Bio 2019, 9, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, T.G.; Friedman, L. In vitro phagocytosis and intracellular fate of variously encapsulated strains of Cryptococcus neoformans. Infect. Immun. 1972, 5, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Huffnagle, G.B.; Chen, G.H.; Curtis, J.L.; McDonald, R.A.; Strieter, R.M.; Toews, G.B. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 1995, 155, 3507–3516. [Google Scholar] [PubMed]
- Barluzzi, R.; Brozzetti, A.; Mariucci, G.; Tantucci, M.; Neglia, R.G.; Bistoni, F.; Blasi, E. Establishment of protective immunity against cerebral cryptococcosis by means of an avirulent, non melanogenic Cryptococcus neoformans strain. J. Neuroimmunol. 2000, 109, 75–86. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Rosas, A.L.; Casadevall, A. The antibody response to fungal melanin in mice. J. Immunol. 1998, 160, 6026–6031. [Google Scholar]
- Nosanchuk, J.D.; Valadon, P.; Feldmesser, M.; Casadevall, A. Melanization of Cryptococcus neoformans in murine infection. Mol. Cell Biol. 1999, 19, 745–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas, A.L.; Nosanchuk, J.D.; Casadevall, A. Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect. Immun. 2001, 69, 3410–3412. [Google Scholar] [CrossRef] [Green Version]
- Kozel, T.R.; Highison, B.; Stratton, C.J. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect. Immun. 1984, 43, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Davies, S.F.; Clifford, D.P.; Hoidal, J.R.; Repine, J.E. Opsonic requirements for the uptake of Cryptococcus neoformans by human polymorphonuclear leukocytes and monocytes. J. Infect. Dis. 1982, 145, 870–874. [Google Scholar] [CrossRef]
- Tsai, H.F.; Chang, Y.C.; Washburn, R.G.; Wheeler, M.H.; Kwon-Chung, K.J. The developmentally regulated alb1 gene of Aspergillus fumigatus: Its role in modulation of conidial morphology and virulence. J. Bacteriol. 1998, 180, 3031–3038. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.F.; Wheeler, M.H.; Chang, Y.C.; Kwon-Chung, K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1999, 181, 6469–6477. [Google Scholar] [CrossRef] [Green Version]
- Abad, A.; Fernández-Molina, J.V.; Bikandi, J.; Ramírez, A.; Margareto, J.; Sendino, J.; Hernando, F.L.; Pontón, J.; Garaizar, J.; Rementeria, A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 2010, 27, 155–182. [Google Scholar] [CrossRef]
- Jahn, B.; Boukhallouk, F.; Lotz, J.; Langfelder, K.; Wanner, G.; Brakhage, A.A. Interaction of human phagocytes with pigmentless Aspergillus conidia. Infect. Immun. 2000, 68, 3736–3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, L.Y.; Netea, M.G.; Sugui, J.; Vonk, A.G.; Van de Sande, W.W.; Warris, A.; Kwon-Chung, K.J.; Kullberg, B.J. Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 2010, 215, 915–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luther, K.; Torosantucci, A.; Brakhage, A.A.; Heesemann, J.; Ebel, F. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell. Microbiol. 2007, 9, 368–381. [Google Scholar] [CrossRef]
- Jahn, B.; Langfelder, K.; Schneider, U.; Schindel, C.; Brakhage, A.A. PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell. Microbiol. 2002, 4, 793–803. [Google Scholar] [CrossRef]
- Thywißen, A.; Heinekamp, T.; Dahse, H.M.; Schmaler-Ripcke, J.; Nietzsche, S.; Zipfel, P.F.; Brakhage, A.A. Conidial Dihydroxynaphthalene Melanin of the Human Pathogenic Fungus Aspergillus fumigatus Interferes with the Host Endocytosis Pathway. Front. Microbiol. 2011, 2, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef]
- Kyrmizi, I.; Ferreira, H.; Carvalho, A.; Figueroa, J.A.L.; Zarmpas, P.; Cunha, C.; Akoumianaki, T.; Stylianou, K.; Deepe, G.S.; Samonis, G.; et al. Calcium sequestration by fungal melanin inhibits calcium–calmodulin signalling to prevent LC3-associated phagocytosis. Nat. Microbiol. 2018, 3, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Malireddi, R.S.; Lu, Q.; Cunha, L.D.; Pelletier, S.; Gingras, S.; Orchard, R.; Guan, J.-L.; Tan, H.; Peng, J. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 2015, 17, 893–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-S.; Lee, J.-S.; Rodgers, M.; Min, C.-K.; Lee, J.-Y.; Kim, H.J.; Lee, K.-H.; Kim, C.-J.; Oh, B.; Zandi, E. Autophagy protein Rubicon mediates phagocytic NADPH oxidase activation in response to microbial infection or TLR stimulation. Cell Host Microbe 2012, 11, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.-C.; Kontoyiannis, D.P. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Bayry, J.; Beaussart, A.; Dufrêne, Y.F.; Sharma, M.; Bansal, K.; Kniemeyer, O.; Aimanianda, V.; Brakhage, A.A.; Kaveri, S.V.; Kwon-Chung, K.J.; et al. Surface structure characterization of Aspergillus fumigatus conidia mutated in the melanin synthesis pathway and their human cellular immune response. Infect. Immun. 2014, 82, 3141–3153. [Google Scholar] [CrossRef] [Green Version]
- Stappers, M.H.T.; Clark, A.E.; Aimanianda, V.; Bidula, S.; Reid, D.M.; Asamaphan, P.; Hardison, S.E.; Dambuza, I.M.; Valsecchi, I.; Kerscher, B.; et al. Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus. Nature 2018, 555, 382–386. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Duarte-Oliveira, C.; Campos, C.F.; Aimanianda, V.; Ter Horst, R.; Leite, L.; Mercier, T.; Pereira, P.; Fernández-García, M.; Antunes, D.; et al. Phagosomal removal of fungal melanin reprograms macrophage metabolism to promote antifungal immunity. Nat. Commun. 2020, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.K.; Febbo, P.G.; Bikoff, R.; Berger, R.; Xue, Q.; McMahon, L.M.; Manola, J.; Brugarolas, J.; McDonnell, T.J.; Golub, T.R.; et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004, 10, 594–601. [Google Scholar] [CrossRef]
- Sturtevant, J.; Latgé, J.P. Participation of complement in the phagocytosis of the conidia of Aspergillus fumigatus by human polymorphonuclear cells. J. Infect. Dis. 1992, 166, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Behnsen, J.; Hartmann, A.; Schmaler, J.; Gehrke, A.; Brakhage, A.A.; Zipfel, P.F. The Opportunistic Human Pathogenic Fungus Aspergillus fumigatus Evades the Host Complement System. J. Infect. Immun. 2008, 76, 820–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.F.; Washburn, R.G.; Chang, Y.C.; Kwon-Chung, K.J. Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol. Microbiol. 1997, 26, 175–183. [Google Scholar] [CrossRef]
- Langfelder, K.; Jahn, B.; Gehringer, H.; Schmidt, A.; Wanner, G.; Brakhage, A.A. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med. Microbiol. Immunol. 1998, 187, 79–89. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xi, L.; Huang, H.; Hu, Y.; Li, X.; Huang, X.; Lu, S.; Sun, J. Melanin in a meristematic mutant of Fonsecaea monophora inhibits the production of nitric oxide and Th1 cytokines of murine macrophages. Mycopathologia 2013, 175, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sun, J.; Lu, S.; Qin, J.; Xi, L.; Zhang, J. Transcriptional profiling of macrophages infected with Fonsecaea monophora. Mycoses 2019, 62, 374–383. [Google Scholar] [CrossRef]
- Pinto, L.; Granja, L.F.Z.; Almeida, M.A.d.; Alviano, D.S.; Silva, M.H.d.; Ejzemberg, R.; Rozental, S.; Alviano, C.S. Melanin particles isolated from the fungus Fonsecaea pedrosoi activates the human complement system. Memórias do Instituto Oswaldo Cruz 2018, 113. [Google Scholar] [CrossRef] [PubMed]
- Youngchim, S.; Hay, R.J.; Hamilton, A.J. Melanization of Penicillium marneffei in vitro and in vivo. Microbiology 2005, 151, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uran, M.E.; Nosanchuk, J.D.; Restrepo, A.; Hamilton, A.J.; Gomez, B.L.; Cano, L.E. Detection of antibodies against Paracoccidioides brasiliensis melanin in in vitro and in vivo studies during infection. Clin. Vaccine Immunol. 2011, 18, 1680–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emidio, E.C.P.; Uran, M.E.; Silva, L.B.R.; Dias, L.S.; Doprado, M.; Nosanchuk, J.D.; Taborda, C.P. Melanin as a Virulence Factor in Different Species of Genus Paracoccidioides. J. Fungi. 2020, 6, 291. [Google Scholar] [CrossRef]
- Silva, M.B.; Thomaz, L.; Marques, A.F.; Svidzinski, A.E.; Nosanchuk, J.D.; Casadevall, A.; Travassos, L.R.; Taborda, C.P. Resistance of melanized yeast cells of Paracoccidioides brasiliensis to antimicrobial oxidants and inhibition of phagocytosis using carbohydrates and monoclonal antibody to CD18. Memórias do Instituto Oswaldo Cruz 2009, 104, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Paes, R.; Almeida, M.A.; Baeza, L.C.; Marmello, L.A.M.; Trugilho, M.R.O.; Nosanchuk, J.D.; Soares, C.M.A.; Valente, R.H.; Zancopé-Oliveira, R.M. Beyond Melanin: Proteomics Reveals Virulence-Related Proteins in Paracoccidioides brasiliensis and Paracoccidioides lutzii Yeast Cells Grown in the Presence of L-Dihydroxyphenylalanine. J. Fungi. 2020, 6, 328. [Google Scholar] [CrossRef]
- Tam, E.W.; Tsang, C.C.; Lau, S.K.; Woo, P.C. Polyketides, toxins and pigments in Penicillium marneffei. Toxins 2015, 7, 4421–4436. [Google Scholar] [CrossRef] [Green Version]
- Sapmak, A.; Kaewmalakul, J.; Nosanchuk, J.D.; Vanittanakom, N.; Andrianopoulos, A.; Pruksaphon, K.; Youngchim, S. Talaromyces marneffei laccase modifies THP-1 macrophage responses. Virulence 2016, 7, 702–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaewmalakul, J.; Nosanchuk, J.D.; Vanittanakom, N.; Youngchim, S. Melanization and morphological effects on antifungal susceptibility of Penicillium marneffei. Antonie Van Leeuwenhoek 2014, 106, 1011–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, K.J.; McLauchlan, A.; Schreider, L.; Andrianopoulos, A. Intracellular growth is dependent on tyrosine catabolism in the dimorphic fungal pathogen Penicillium marneffei. PLoS Pathog. 2015, 11, e1004790. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Paes, R.; Frases, S.; Araújo Gde, S.; De Oliveira, M.M.; Gerfen, G.J.; Nosanchuk, J.D.; Zancopé-Oliveira, R.M. Biosynthesis and functions of a melanoid pigment produced by species of the Sporothrix complex in the presence of L-tyrosine. Appl. Environ. Microbiol. 2012, 78, 8623–8630. [Google Scholar] [CrossRef] [Green Version]
- Cruz, I.L.R.; Figueiredo-Carvalho, M.H.G.; Zancopé-Oliveira, R.M.; Almeida-Paes, R. Evaluation of melanin production by Sporothrix luriei. Memórias do Instituto Oswaldo Cruz 2018, 113, 68–70. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Yao, L.; Zhen, Y.; Cui, Y.; Zhong, S.; Liu, Y.; Li, S. Sporothrix globosa melanin inhibits antigen presentation by macrophages and enhances deep organ dissemination. Braz J. Microbiol. 2020. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; de Oliveira, L.C.; Oliveira, M.M.; Gutierrez-Galhardo, M.C.; Nosanchuk, J.D.; Zancope-Oliveira, R.M. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. BioMed Res. Int. 2015, 2015, 212308. [Google Scholar] [CrossRef] [Green Version]
- Madrid, I.M.; Xavier, M.O.; Mattei, A.S.; Fernandes, C.G.; Guim, T.N.; Santin, R.; Schuch, L.F.; Nobre Mde, O.; Araújo Meireles, M.C. Role of melanin in the pathogenesis of cutaneous sporotrichosis. Microbes Infect. 2010, 12, 162–165. [Google Scholar] [CrossRef]
- Mario, D.A.; Santos, R.C.; Denardi, L.B.; Vaucher Rde, A.; Santurio, J.M.; Alves, S.H. Interference of melanin in the susceptibility profile of Sporothrix species to amphotericin B. Rev. Iberoam. Micol. 2016, 33, 21–25. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; Figueiredo-Carvalho, M.H.; Brito-Santos, F.; Almeida-Silva, F.; Oliveira, M.M.; Zancopé-Oliveira, R.M. Melanins Protect Sporothrix brasiliensis and Sporothrix schenckii from the Antifungal Effects of Terbinafine. PLoS ONE 2016, 11, e0152796. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Hamdy, R.; Elseginy, S.A.; Gebremariam, T.; Hamoda, A.M.; Madkour, M.; Venkatachalam, T.; Ershaid, M.N.; Mohammad, M.G.; Chamilos, G.; et al. Selective inhibition of Rhizopus eumelanin biosynthesis by novel natural product scaffold-based designs caused significant inhibition of fungal pathogenesis. Biochem. J. 2020, 477, 2489–2507. [Google Scholar] [CrossRef]
- Chatterjee, S.; Prados-Rosales, R.; Tan, S.; Phan, V.C.; Chrissian, C.; Itin, B.; Wang, H.; Khajo, A.; Magliozzo, R.S.; Casadevall, A.; et al. The melanization road more traveled by: Precursor substrate effects on melanin synthesis in cell-free and fungal cell systems. J. Biol. Chem. 2018, 293, 20157–20168. [Google Scholar] [CrossRef] [Green Version]
- Cordero, R.J.B.; Casadevall, A. Melanin. Curr. Biol. 2020, 30, R142–R143. [Google Scholar] [CrossRef]
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef] [PubMed]
- Galeb, H.A.; Wilkinson, E.L.; Stowell, A.F.; Lin, H.; Murphy, S.T.; Martin-Hirsch, P.L.; Mort, R.L.; Taylor, A.M.; Hardy, J.G. Melanins as Sustainable Resources for Advanced Biotechnological Applications. Glob. Chall. 2020, 5, 2000102. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B. Melanin for space travel radioprotection. Environ. Microbiol. 2017, 19, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
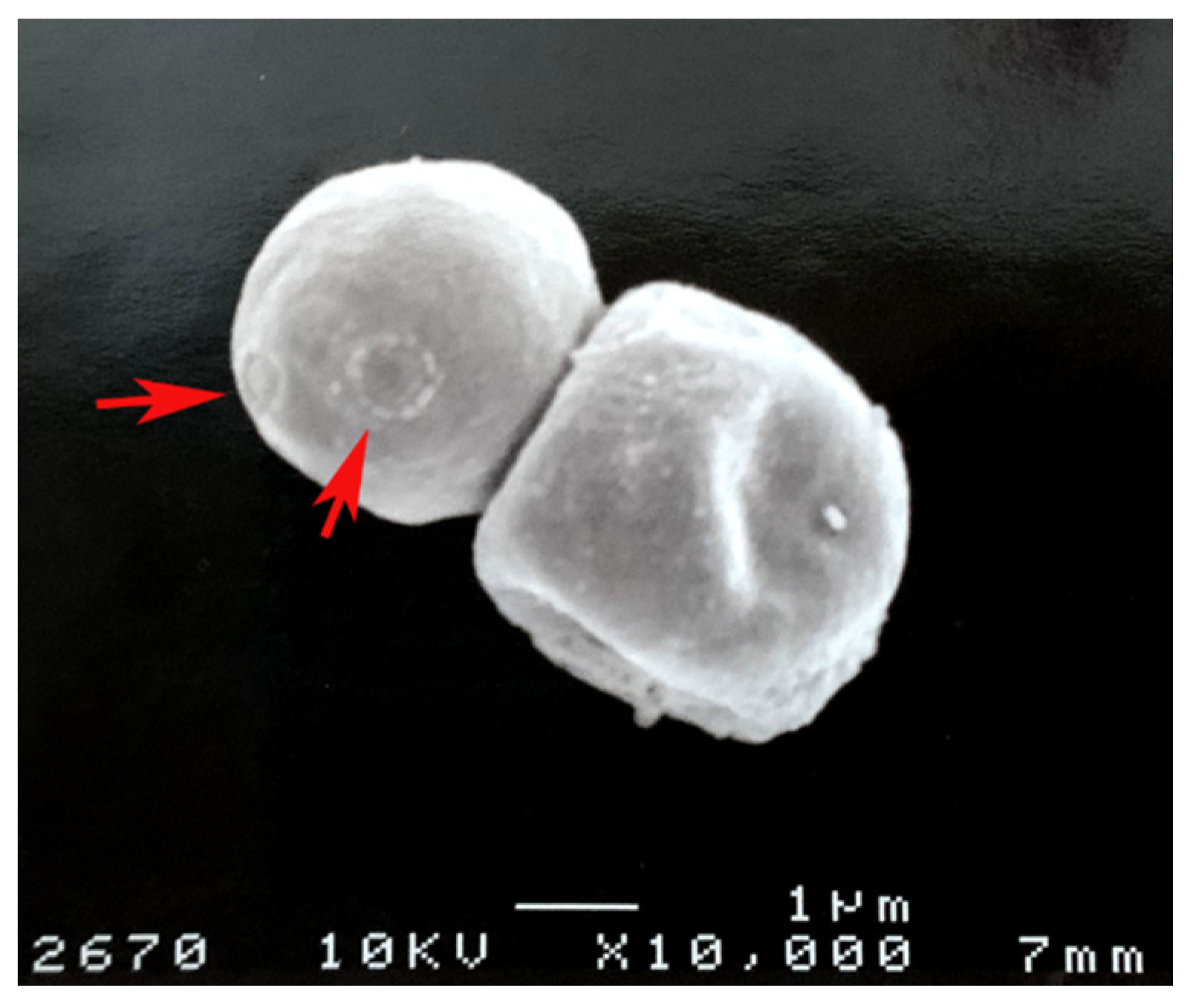
| Species | Isolate Environment | Melanin Types |
|---|---|---|
| Aspergillus fumigatus | Clinical | DHN and pyo-melanin |
| Aspergillus niger | Industrial fermentation | DHN and L-DOPA |
| Blastomyces dermatitidis | Clinical | DHN |
| Candida Albicans | Clinical | L-DOPA |
| Cryptococcus neoformans | Clinical | L-DOPA |
| Histoplasma capsulatum | Clinical | DHN and L-DOPA |
| Paracoccidioides brasiliensis | Clinical | DHN and L-DOPA |
| Fonsecaea monophora | Clinical | DHN and L-DOPA |
| Fonsecaea pedrosoi | Clinical | DHN |
| Sporothrix schenckii | Clinical | DHN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Youngchim, S.; Zamith-Miranda, D.; Nosanchuk, J.D. Fungal Melanin and the Mammalian Immune System. J. Fungi 2021, 7, 264. https://doi.org/10.3390/jof7040264
Liu S, Youngchim S, Zamith-Miranda D, Nosanchuk JD. Fungal Melanin and the Mammalian Immune System. Journal of Fungi. 2021; 7(4):264. https://doi.org/10.3390/jof7040264
Chicago/Turabian StyleLiu, Sichen, Sirida Youngchim, Daniel Zamith-Miranda, and Joshua D. Nosanchuk. 2021. "Fungal Melanin and the Mammalian Immune System" Journal of Fungi 7, no. 4: 264. https://doi.org/10.3390/jof7040264
APA StyleLiu, S., Youngchim, S., Zamith-Miranda, D., & Nosanchuk, J. D. (2021). Fungal Melanin and the Mammalian Immune System. Journal of Fungi, 7(4), 264. https://doi.org/10.3390/jof7040264








