Breakdown of Symbiosis in Radiation-Induced Oral Mucositis
Abstract
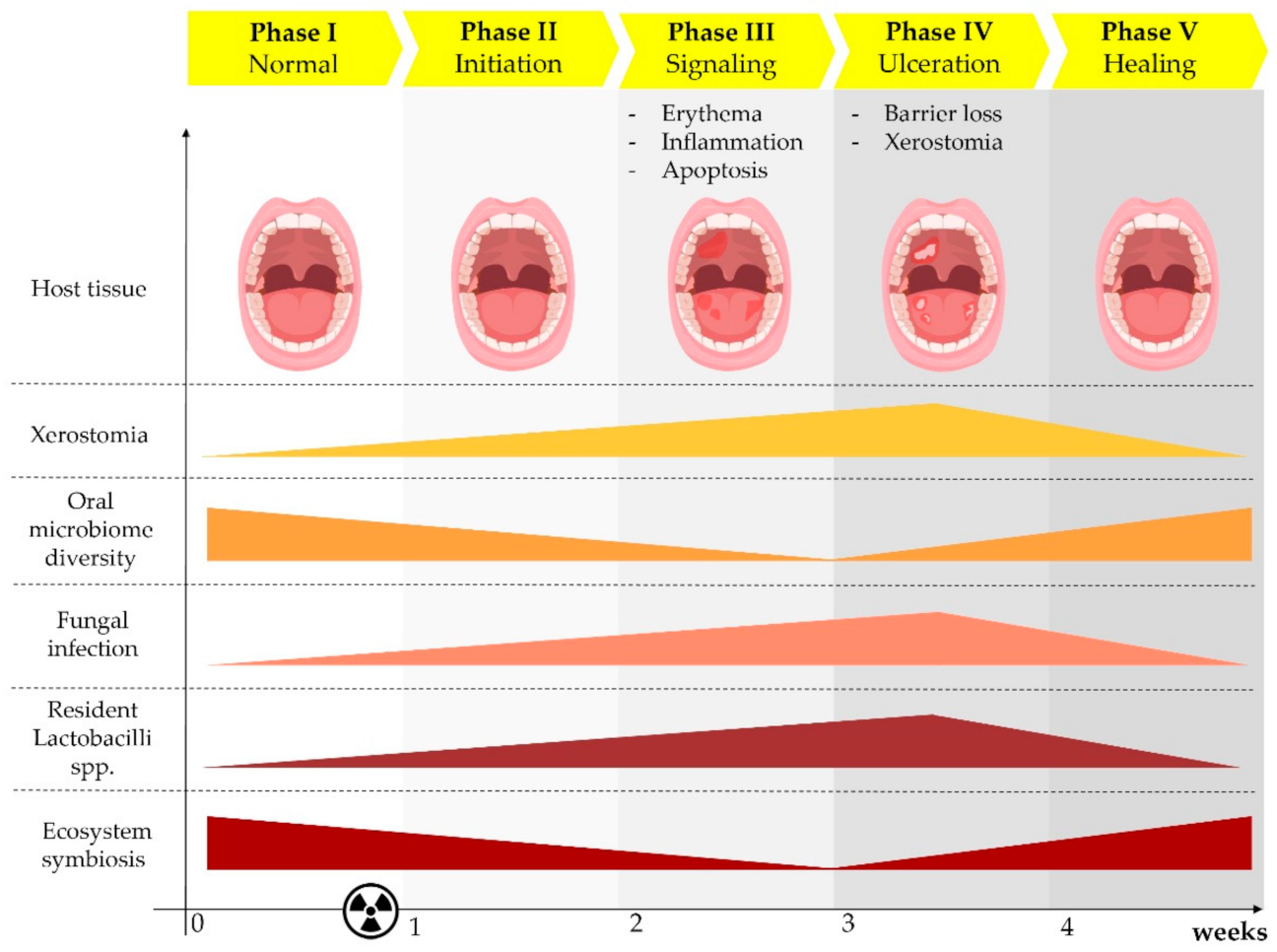
:1. Radiation-Induced Oral Mucositis
2. Grading of OM
3. Risk Factors for OM
4. Opportunistic Fungal Infections in Patients Treated with RT
5. RT-Dependent Oral Infections: What We Know about Oral Anti-Fungal Immunity
6. Prevention and Treatment of OM and Related Infections
7. Can We Harness the Microbiota to Counteract RT-Dependent Fungal Infections?
7.1. The Oral Microbiota
7.2. Novel Insights into OM Therapy Based on Microbiome Modulation
8. Future Directions
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1. 2018. J. Natl. Compr. Canc. Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Gupta, T.; Kannan, S.; Ghosh-Laskar, S.; Agarwal, J.P. Systematic review and meta-analyses of intensity-modulated radiation therapy versus conventional two-dimensional and/or or three-dimensional radiotherapy in curative-intent management of head and neck squamous cell carcinoma. PLoS ONE 2018, 13, 0200137. [Google Scholar] [CrossRef]
- Zhang, B.; Mo, Z.; Du, W.; Wang, Y.; Liu, L.; Wei, Y. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol. 2015, 51, 1041–1046. [Google Scholar] [CrossRef]
- Marta, G.N.; Silva, V.; de Andrade Carvalho, H.; de Arruda, F.F.; Hanna, S.A.; Gadia, R.; da Silva, J.L.; Correa, S.F.; Vita Abreu, C.E.; Riera, R. Intensity-modulated radiation therapy for head and neck cancer: Systematic review and meta-analysis. Radiother. Oncol. 2014, 110, 9–15. [Google Scholar] [CrossRef]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Sonis, S.T. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009, 45, 1015–1020. [Google Scholar] [CrossRef]
- Gruber, S.; Dorr, W. Tissue reactions to ionizing radiation-Oral mucosa. Mutat. Res. 2016, 770, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Ferreira, J.A.; Jaen Olasolo, J.; Azinovic, I.; Jeremic, B. Effect of radiotherapy delay in overall treatment time on local control and survival in head and neck cancer: Review of the literature. Rep. Pract. Oncol. Radiother. 2015, 20, 328–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.J.; Shao, Z.Y.; Wang, Q.; Jiang, Y.T.; Ma, R.; Tang, Z.S.; Liu, Z.; Liang, J.P.; Huang, Z.W. Exploring the dynamic core microbiome of plaque microbiota during head-and-neck radiotherapy using pyrosequencing. PLoS ONE 2013, 8, 56343. [Google Scholar]
- Hou, J.; Zheng, H.; Li, P.; Liu, H.; Zhou, H.; Yang, X. Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother. Oncol. 2018, 129, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Guobis, Z.; Kareiviene, V.; Baseviciene, N.; Paipaliene, P.; Niedzelskiene, I.; Sabalys, G.; Kubilius, R.; Gervickas, A. Microflora of the oral cavity in patients with xerostomia. Medicina (Kaunas) 2011, 47, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Vanhoecke, B.; De Ryck, T.; Stringer, A.; Van de Wiele, T.; Keefe, D. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis. 2015, 21, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Bensadoun, R.J.; Patton, L.L.; Lalla, R.V.; Epstein, J.B. Oropharyngeal candidiasis in head and neck cancer patients treated with radiation: Update 2011. Support Care Cancer 2011, 19, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Riesenbeck, D.; Dorr, W. Documentation of radiation-induced oral mucositis. Scoring systems. Strahlenther. Onkol. 1998, 174, 44–46. [Google Scholar] [PubMed]
- Assessing stomatitis: Refinement of the Western Consortium for Cancer Nursing Research (WCCNR) stomatitis staging system. Can. Oncol. Nurs. J. 1998, 8, 160–165. [CrossRef] [Green Version]
- Sonis, S.T.; Eilers, J.P.; Epstein, J.B.; LeVeque, F.G.; Liggett, W.H., Jr.; Mulagha, M.T.; Peterson, D.E.; Rose, A.H.; Schubert, M.M.; Spijkervet, F.K.; et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer 1999, 85, 2103–2113. [Google Scholar] [CrossRef]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef]
- Bonar-Alvarez, P.; Padin-Iruegas, E.; Chamorro-Petronacci, C.; Gandara-Vila, P.; Lorenzo-Pouso, A.I.; Somoza-Martin, M.; Blanco-Carrion, A.; Garcia-Garcia, A.; Perez-Sayans, M. Assessment of saliva and oral candidiasis levels 12, 24 and 36 months after radiotherapy in patients with head and neck cancer. J. Stomatol. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Tarapan, S.; Matangkasombut, O.; Trachootham, D.; Sattabanasuk, V.; Talungchit, S.; Paemuang, W.; Phonyiam, T.; Chokchaitam, O.; Mungkung, O.O.; Lam-Ubol, A. Oral Candida colonization in xerostomic postradiotherapy head and neck cancer patients. Oral Dis. 2019, 25, 1798–1808. [Google Scholar] [CrossRef]
- Zhu, X.X.; Yang, X.J.; Chao, Y.L.; Zheng, H.M.; Sheng, H.F.; Liu, H.Y.; He, Y.; Zhou, H.W. The Potential Effect of Oral Microbiota in the Prediction of Mucositis During Radiotherapy for Nasopharyngeal Carcinoma. EBioMedicine 2017, 18, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.H.; Hong, M.H.; Guo, L.; Cao, K.J.; Deng, M.Q.; Mo, H.Y. Analysis of oral mucositis risk factors during radiotherapy for nasopharyngeal carcinoma patients and establishment of a discriminant model. Ai Zheng 2005, 24, 850–854. [Google Scholar]
- Chen, S.C.; Lai, Y.H.; Huang, B.S.; Lin, C.Y.; Fan, K.H.; Chang, J.T. Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur. J. Oncol Nurs. 2015, 19, 214–219. [Google Scholar] [CrossRef]
- Khan, S.A.; Wingard, J.R. Infection and mucosal injury in cancer treatment. J. Natl. Cancer Inst. Monogr. 2001, 29, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Da Silva, E.M.; Mansano, E.S.B.; Miazima, E.S.; Rodrigues, F.A.V.; Hernandes, L.; Svidzinski, T.I.E. Radiation used for head and neck cancer increases virulence in Candida tropicalis isolated from a cancer patient. BMC Infect. Dis. 2017, 17, 783. [Google Scholar] [CrossRef] [Green Version]
- Lalla, R.V.; Latortue, M.C.; Hong, C.H.; Ariyawardana, A.; D’Amato-Palumbo, S.; Fischer, D.J.; Martof, A.; Nicolatou-Galitis, O.; Patton, L.L.; Elting, L.S.; et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Support. Care Cancer 2010, 18, 985–992. [Google Scholar] [CrossRef] [Green Version]
- Mirabile, A.; Vismara, C.; Crippa, F.; Bossi, P.; Locati, L.; Bergamini, C.; Granata, R.; Resteghini, C.; Conte, E.; Morelli, D.; et al. Health care-associated infections in patients with head and neck cancer treated with chemotherapy and/or radiotherapy. Head Neck. 2016, 38, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, B.R. Prevention and treatment of the orofacial complications of radiotherapy. J. Am. Dent. Assoc. 1987, 114, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, O.; Puri, S.; Tati, S.; Edgerton, M. Innate Immunity and Saliva in Candida albicans-mediated Oral Diseases. J. Dent Res. 2016, 95, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millet, N.; Solis, N.V.; Swidergall, M. Mucosal IgA Prevents Commensal Candida albicans Dysbiosis in the Oral Cavity. Front. Immunol. 2020, 11, 555363. [Google Scholar] [CrossRef]
- Bossi, P.; Bergamini, C.; Miceli, R.; Cova, A.; Orlandi, E.; Resteghini, C.; Locati, L.; Alfieri, S.; Imbimbo, M.; Granata, R.; et al. Salivary Cytokine Levels and Oral Mucositis in Head and Neck Cancer Patients Treated With Chemotherapy and Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 959–966. [Google Scholar] [CrossRef]
- Epstein, J.B.; Gorsky, M.; Guglietta, A.; Le, N.; Sonis, S.T. The correlation between epidermal growth factor levels in saliva and the severity of oral mucositis during oropharyngeal radiation therapy. Cancer 2000, 89, 2258–2265. [Google Scholar] [CrossRef]
- Tong, H.C.; Gao, X.J.; Dong, X.Z. Non-mutans streptococci in patients receiving radiotherapy in the head and neck area. Caries Res. 2003, 37, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The Chicken or the Egg? Changes in Oral Microbiota as Cause or Consequence of Mucositis During Radiation Therapy. EBioMedicine 2017, 18, 7–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchner, F.R.; Littringer, K.; Altmeier, S.; Tran, V.D.T.; Schonherr, F.; Lemberg, C.; Pagni, M.; Sanglard, D.; Joller, N.; Leibund, S. Gut-Landmann Persistence of Candida albicans in the Oral Mucosa Induces a Curbed Inflammatory Host Response That Is Independent of Immunosuppression. Front. Immunol. 2019, 10, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Aggor, F.E.Y.; Break, T.J.; Trevejo-Nunez, G.; Whibley, N.; Coleman, B.M.; Bailey, R.D.; Kaplan, D.H.; Naglik, J.R.; Shan, W.; Shetty, A.C.; et al. Oral epithelial IL-22/STAT3 signaling licenses IL-17-mediated immunity to oral mucosal candidiasis. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Kaleviste, E.; Ruhlemann, M.; Karner, J.; Haljasmagi, L.; Tserel, L.; Org, E.; Trebusak Podkrajsek, K.; Battelino, T.; Bang, C.; Franke, A.; et al. IL-22 Paucity in APECED Is Associated With Mucosal and Microbial Alterations in Oral Cavity. Front. Immunol. 2020, 11, 838. [Google Scholar] [CrossRef]
- Ps, S.K.; Balan, A.; Sankar, A.; Bose, T. Radiation induced oral mucositis. Indian J. Palliat. Care 2009, 15, 95–102. [Google Scholar] [PubMed]
- Hong, B.Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Beaven, A.W.; Shea, T.C. Recombinant human keratinocyte growth factor palifermin reduces oral mucositis and improves patient outcomes after stem cell transplant. Drugs Today 2007, 43, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Kanuga, S. Cryotherapy and keratinocyte growth factor may be beneficial in preventing oral mucositis in patients with cancer, and sucralfate is effective in reducing its severity. J. Am. Dent Assoc. 2013, 144, 928–929. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Bardet, E.; Martin, L.; Calais, G.; Alfonsi, M.; Feham, N.E.; Tuchais, C.; Boisselier, P.; Dessard-Diana, B.; Seng, S.H.; Garaud, P.; et al. Subcutaneous compared with intravenous administration of amifostine in patients with head and neck cancer receiving radiotherapy: Final results of the GORTEC2000-02 phase III randomized trial. J. Clin. Oncol 2011, 29, 127–133. [Google Scholar] [CrossRef]
- Karacetin, D.; Yucel, B.; Leblebicioglu, B.; Aksakal, O.; Maral, O.; Incekara, O. A randomized trial of amifostine as radioprotector in the radiotherapy of head and neck cancer. J. BUON 2004, 9, 23–26. [Google Scholar]
- Wasserman, T.H.; Brizel, D.M.; Henke, M.; Monnier, A.; Eschwege, F.; Sauer, R.; Strnad, V. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and- neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 985–990. [Google Scholar] [CrossRef]
- Komaki, R.; Lee, J.S.; Milas, L.; Lee, H.K.; Fossella, F.V.; Herbst, R.S.; Allen, P.K.; Liao, Z.; Stevens, C.W.; Lu, C.; et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small-cell lung cancer: Report of a randomized comparative trial. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Bensadoun, R.J.; Franquin, J.C.; Ciais, G.; Darcourt, V.; Schubert, M.M.; Viot, M.; Dejou, J.; Tardieu, C.; Benezery, K.; Nguyen, T.D.; et al. Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Support. Care Cancer 1999, 7, 244–252. [Google Scholar] [CrossRef]
- Lalla, R.V.; Ashbury, F.D. The MASCC/ISOO mucositis guidelines: Dissemination and clinical impact. Support. Care Cancer 2013, 21, 3161–3163. [Google Scholar] [CrossRef] [Green Version]
- Barber, C.; Powell, R.; Ellis, A.; Hewett, J. Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: A preliminary, double centre, randomised controlled trial on patients with radiotherapy-induced oral mucositis. Support. Care Cancer 2007, 15, 427–440. [Google Scholar] [CrossRef]
- Huang, E.Y.; Leung, S.W.; Wang, C.J.; Chen, H.C.; Sun, L.M.; Fang, F.M.; Yeh, S.A.; Hsu, H.C.; Hsiung, C.Y. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 535–539. [Google Scholar] [CrossRef]
- Chan, A.; Ignoffo, R.J. Survey of topical oral solutions for the treatment of chemo-induced oral mucositis. J. Oncol. Pharm. Pract. 2005, 11, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.N.; Singer, J. A comparison of artificial saliva and pilocarpine in radiation-induced xerostomia. J. Laryngol. Otol. 1994, 108, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.R.; Fleck, J.F.; Diehl, A.; Barletta, D.; Braga-Filho, A.; Barletta, A.; Ilha, L. Protective effect of alpha-tocopherol in head and neck cancer radiation-induced mucositis: A double-blind randomized trial. Head Neck 2004, 26, 313–321. [Google Scholar] [CrossRef] [PubMed]
- High, K.P.; Legault, C.; Sinclair, J.A.; Cruz, J.; Hill, K.; Hurd, D.D. Low plasma concentrations of retinol and alpha-tocopherol in hematopoietic stem cell transplant recipients: The effect of mucositis and the risk of infection. Am. J. Clin. Nutr. 2002, 76, 1358–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshitani, T.; Okada, K.; Kushima, T.; Suematsu, T.; Obayashi, K.; Hirata, Y.; Takada, Y.; Ishida, T.; Yoshida, M.; Narabayashi, I.; et al. Clinical evaluation of sodium alginate on oral mucositis associated with radiotherapy. Nihon Gan Chiryo Gakkai Shi 1990, 25, 1129–1137. [Google Scholar]
- Saldi, S.; Perrucci, E.; Fulcheri, C.P.L.; Mariucci, C.; Chierchini, S.; Ingrosso, G.; Falcinelli, L.; Podlesko, A.M.; Merluzzi, M.; Bini, V.; et al. Zinc-L-carnosine prevented dysphagia in breast cancer patients undergoing adjuvant radiotherapy: Results of a phase III randomized trial. Breast J. 2020, 26, 1882–1884. [Google Scholar] [CrossRef] [PubMed]
- Podlesko, A.M.; Ramacciati, N.; Panzolini, S.; Saldi, S.; Fiorucci, S.; Pierini, D.; Mancini, M.; Merolla, M.S.; Lancellotta, V.; Aristei, C. Effects of topical polydeoxyribonucleotide on radiation-induced oral mucositis. Tech. Innov. Patient Support Radiat. Oncol. 2018, 7, 17–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elad, S.; Yarom, N. The Search for an Effective Therapy and Pain Relief for Oral Mucositis. JAMA 2019, 321, 1459–1461. [Google Scholar] [CrossRef]
- Sonis, S.T.; O’Donnell, K.E.; Popat, R.; Bragdon, C.; Phelan, S.; Cocks, D.; Epstein, J.B. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol. 2004, 40, 170–176. [Google Scholar] [CrossRef]
- Pillsbury, H.C., 3rd; Webster, W.P.; Rosenman, J. Prostaglandin inhibitor and radiotherapy in advanced head and neck cancers. Arch. Otolaryngol. Head Neck Surg. 1986, 112, 552–553. [Google Scholar] [CrossRef]
- He, D.; Behar, S.; Roberts, J.E.; Lim, H.W. The effect of L-cysteine and N-acetylcysteine on porphyrin/heme biosynthetic pathway in cells treated with 5-aminolevulinic acid and exposed to radiation. Photodermatol. Photoimmunol. Photomed. 1996, 12, 194–199. [Google Scholar] [CrossRef]
- Alfieri, S.; Ripamonti, C.I.; Marceglia, S.; Orlandi, E.; Iacovelli, N.A.; Granata, R.; Cavallo, A.; Pozzi, P.; Boffi, R.; Bergamini, C.; et al. Temporal course and predictive factors of analgesic opioid requirement for chemoradiation-induced oral mucositis in oropharyngeal cancer. Head Neck 2016, 38, 1521–1527. [Google Scholar] [CrossRef]
- Osaki, T.; Ueta, E.; Yoneda, K.; Hirota, J.; Yamamoto, T. Prophylaxis of oral mucositis associated with chemoradiotherapy for oral carcinoma by Azelastine hydrochloride (Azelastine) with other antioxidants. Head Neck 1994, 16, 331–339. [Google Scholar] [CrossRef]
- Leborgne, J.H.; Leborgne, F.; Zubizarreta, E.; Ortega, B.; Mezzera, J. Corticosteroids and radiation mucositis in head and neck cancer. A double-blind placebo-controlled randomized trial. Radiother. Oncol. 1998, 47, 145–148. [Google Scholar] [CrossRef]
- Matthews, R.H.; Ercal, N. Prevention of mucositis in irradiated head and neck cancer patients. J. Exp. Ther. Oncol. 1996, 1, 135–138. [Google Scholar]
- Spijkervet, F.K.; Van Saene, H.K.; Van Saene, J.J.; Panders, A.K.; Vermey, A.; Mehta, D.M.; Fidler, V. Effect of selective elimination of the oral flora on mucositis in irradiated head and neck cancer patients. J. Surg. Oncol. 1991, 46, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Shenep, J.L. Combination and single-agent empirical antibacterial therapy for febrile cancer patients with neutropenia and mucositis. NCI Monogr. 1990, 9, 117–122. [Google Scholar]
- Nicolatou-Galitis, O.; Velegraki, A.; Sotiropoulou-Lontou, A.; Dardoufas, K.; Kouloulias, V.; Kyprianou, K.; Kolitsi, G.; Skarleas, C.; Pissakas, G.; Papanicolaou, V.S.; et al. Effect of fluconazole antifungal prophylaxis on oral mucositis in head and neck cancer patients receiving radiotherapy. Support. Care Cancer 2006, 14, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.; Whiteson, K.; Huse, S.; Hernandez, D.; Farinelli, L.; Osteras, M.; Schrenzel, J.; Francois, P. Metagenomic study of the oral microbiota by Illumina high-throughput sequencing. J. Microbiol. Methods 2009, 79, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, W.G.; Prosdocimi, E.M. Profiling of Oral Bacterial Communities. J. Dent Res. 2020, 99, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Daglia, M.; Arguelles Castilla, S.; Sanadgol, N.; Fazel Nabavi, S.; Khan, H.; Belwal, T.; Jeandet, P.; Marchese, A.; Pistollato, F.; et al. Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol. Res. 2020, 151, 104582. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.M.; Joshi, V.; Fellows, M.; Dabdoub, S.M.; Nagaraja, H.N.; O’Donnell, B.; Deshpande, N.R.; Kumar, P.S. A tale of two risks: Smoking, diabetes and the subgingival microbiome. ISME J. 2017, 11, 2075–2089. [Google Scholar] [CrossRef] [Green Version]
- Mason, M.R.; Preshaw, P.M.; Nagaraja, H.N.; Dabdoub, S.M.; Rahman, A.; Kumar, P.S. The subgingival microbiome of clinically healthy current and never smokers. ISME J. 2015, 9, 268–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belda-Ferre, P.; Alcaraz, L.D.; Cabrera-Rubio, R.; Romero, H.; Simon-Soro, A.; Pignatelli, M.; Mira, A. The oral metagenome in health and disease. ISME J. 2012, 6, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.J.; Wang, Q.; Jiang, Y.T.; Ma, R.; Xia, W.W.; Tang, Z.S.; Liu, Z.; Liang, J.P.; Huang, Z.W. Characterization of oral bacterial diversity of irradiated patients by high-throughput sequencing. Int. J. Oral Sci. 2013, 5, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, H.; Liang, X.; Zhang, M.; Wang, R.; Peng, G.; Li, J. Investigation of salivary function and oral microbiota of radiation caries-free people with nasopharyngeal carcinoma. PLoS ONE 2015, 10, 0123137. [Google Scholar]
- Vanhoecke, B.W.; De Ryck, T.R.; De boel, K.; Wiles, S.; Boterberg, T.; Van de Wiele, T.; Swift, S. Low-dose irradiation affects the functional behavior of oral microbiota in the context of mucositis. Exp. Biol. Med. (Maywood) 2016, 241, 60–70. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Biswas, K.; Mackenzie, B.W.; Taylor, M.W.; Douglas, R.G. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support. Care Cancer 2020, 28, 2683–2691. [Google Scholar] [CrossRef]
- Laheij, A.M.; de Soet, J.J.; von dem Borne, P.A.; Kuijper, E.J.; Kraneveld, E.A.; van Loveren, C.; Raber-Durlacher, J.E. Oral bacteria and yeasts in relationship to oral ulcerations in hematopoietic stem cell transplant recipients. Support. Care Cancer 2012, 20, 3231–3240. [Google Scholar] [CrossRef] [Green Version]
- De Sanctis, V.; Belgioia, L.; Cante, D.; La Porta, M.R.; Caspiani, O.; Guarnaccia, R.; Argenone, A.; Muto, P.; Musio, D.; De Felice, F.; et al. Lactobacillus brevis CD2 for Prevention of Oral Mucositis in Patients With Head and Neck Tumors: A Multicentric Randomized Study. Anticancer Res. 2019, 39, 1935–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossoni, R.D.; de Barros, P.P.; Mendonca, I.D.C.; Medina, R.P.; Silva, D.H.S.; Fuchs, B.B.; Junqueira, J.C.; Mylonakis, E. The Postbiotic Activity of Lactobacillus paracasei 28.4 Against Candida auris. Front. Cell Infect. Microbiol. 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Granados, M.J.; Franco-Robles, E. Postbiotics in human health: Possible new functional ingredients? Food Res. Int. 2020, 137, 109660. [Google Scholar] [CrossRef]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Wieland Brown, L.C.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell 2014, 158, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Ferreira, J.; Hong, C.H.L.; Tan, K.S. Lactobacillus reuteri DSM 17938 and ATCC PTA 5289 ameliorates chemotherapy-induced oral mucositis. Sci. Rep. 2020, 10, 16189. [Google Scholar] [CrossRef]
- Hisha, H.; Tanaka, T.; Ueno, H. Lingual Epithelial Stem Cells and Organoid Culture of Them. Int. J. Mol. Sci. 2016, 17, 168. [Google Scholar] [CrossRef] [Green Version]
- Vincent-Chong, V.K.; Seshadri, M. Development and Radiation Response Assessment in A Novel Syngeneic Mouse Model. of Tongue Cancer: 2D Culture, 3D Organoids and Orthotopic Allografts. Cancers 2020, 12, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Agent | Effect | Reference |
|---|---|---|
| Glycyrrhetinic acid/povidone/sodium hyaluronate gel | Adherence to the mouth mucosal surface, soothing oral lesions | [51] |
| L-glutamine | Counteraction of RT-induced metabolic deficiencies | [52] |
| Local anesthetics | Short-term relief of OM-associated pain (e.g., diphenhydramine, viscous xylocaine, lidocaine, and dyclonine hydrochloride) | [53] |
| Artificial saliva sprays | Alleviate mucosal dryness in mild cases of OM | [54] |
| Vitamin E (tocopherol) | Reduction of oral mucosa oxidative damage and, consequently, incidence of symptomatic OM | [55,56] |
| Sodium alginate | Reduction of OM-linked discomfort and OM severity | [57] |
| Zinc-L-carnosine | Physical barrier protection and repair of damaged areas | [58] |
| Polideoxyribonucleotide (PDRN) | Regenerative and anti-inflammatory device | [53,59,60] |
| Agent | Effect | Reference |
|---|---|---|
| Cyclooxygenase-2 inhibitors | Suppression of NF-κB, reduction of pro-inflammatory cytokine production, inhibition of angiogenesis | [61,62] |
| N-acetylcysteine | Antioxidant agent that suppresses NF-κB activation | [63] |
| Minor Analgesics and Opioids | Mitigation of OM-related pain | [44,64] |
| Azelastine | Potent second-generation selective histamine antagonist used as an anti-inflammatory and antioxidant agent | [65] |
| Systemic corticosteroids | Anti-inflammatory and antioxidant agent | [66] |
| Antibacterial agents | Prophylaxis of aerobic (e.g., Pseudomonas spp. and Staphylococcus epidermidis) and anaerobic (e.g., Bacteroides spp. and Veillonella spp.) bacterial infections | [67,68,69] |
| Systemic administration of Fluconazole | Prophylaxis of fungal infections, which can complicate the clinical scenario, especially in immunocompromised patients. Fluconazole significantly reduced the severity of OM and the risk of RT interruption | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingrosso, G.; Saldi, S.; Marani, S.; Wong, A.Y.W.; Bertelli, M.; Aristei, C.; Zelante, T. Breakdown of Symbiosis in Radiation-Induced Oral Mucositis. J. Fungi 2021, 7, 290. https://doi.org/10.3390/jof7040290
Ingrosso G, Saldi S, Marani S, Wong AYW, Bertelli M, Aristei C, Zelante T. Breakdown of Symbiosis in Radiation-Induced Oral Mucositis. Journal of Fungi. 2021; 7(4):290. https://doi.org/10.3390/jof7040290
Chicago/Turabian StyleIngrosso, Gianluca, Simonetta Saldi, Simona Marani, Alicia Y. W. Wong, Matteo Bertelli, Cynthia Aristei, and Teresa Zelante. 2021. "Breakdown of Symbiosis in Radiation-Induced Oral Mucositis" Journal of Fungi 7, no. 4: 290. https://doi.org/10.3390/jof7040290
APA StyleIngrosso, G., Saldi, S., Marani, S., Wong, A. Y. W., Bertelli, M., Aristei, C., & Zelante, T. (2021). Breakdown of Symbiosis in Radiation-Induced Oral Mucositis. Journal of Fungi, 7(4), 290. https://doi.org/10.3390/jof7040290








