Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications
Abstract
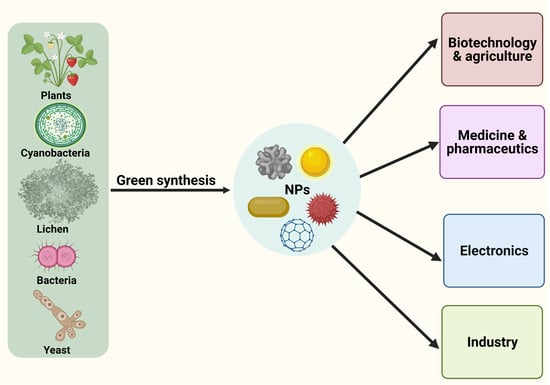
:1. Introduction
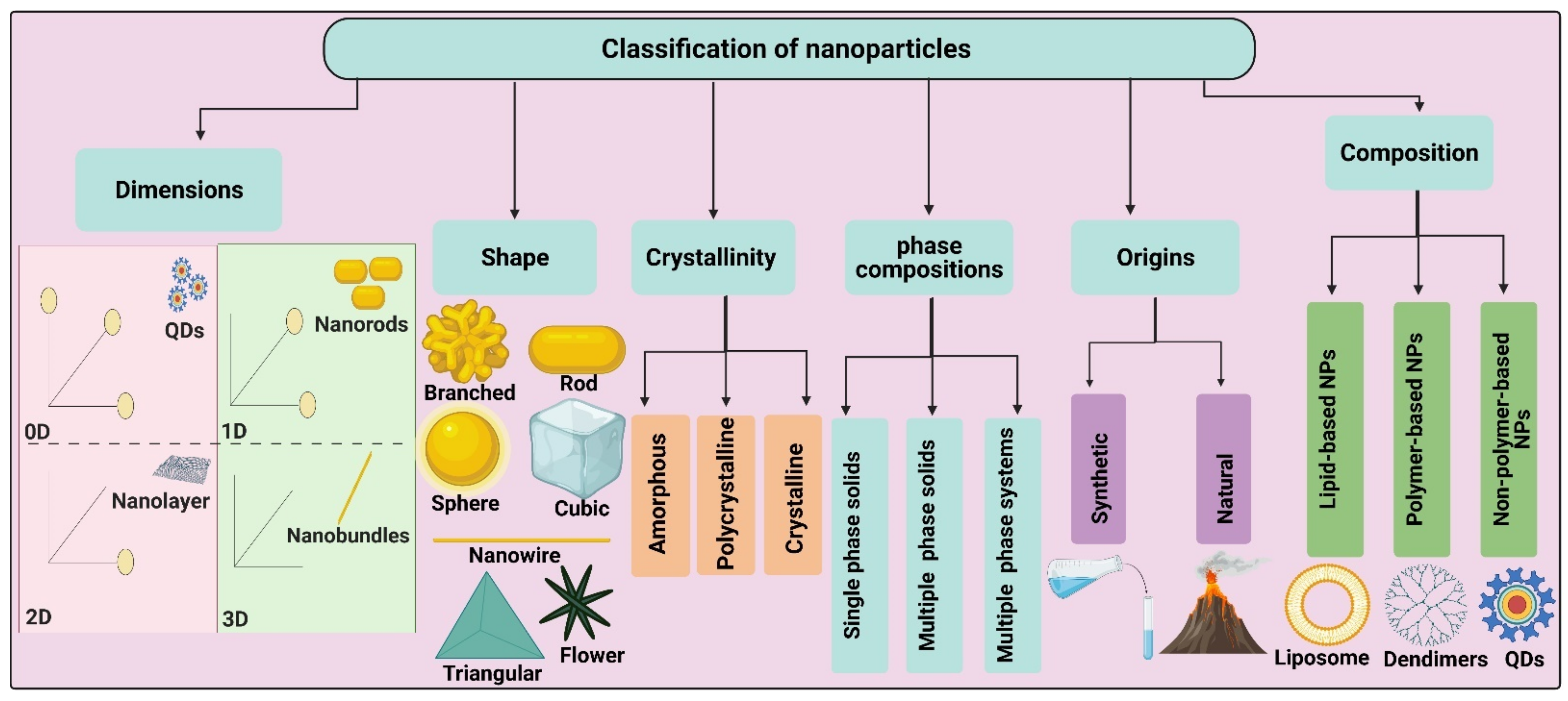
2. Classification of Nanoparticles
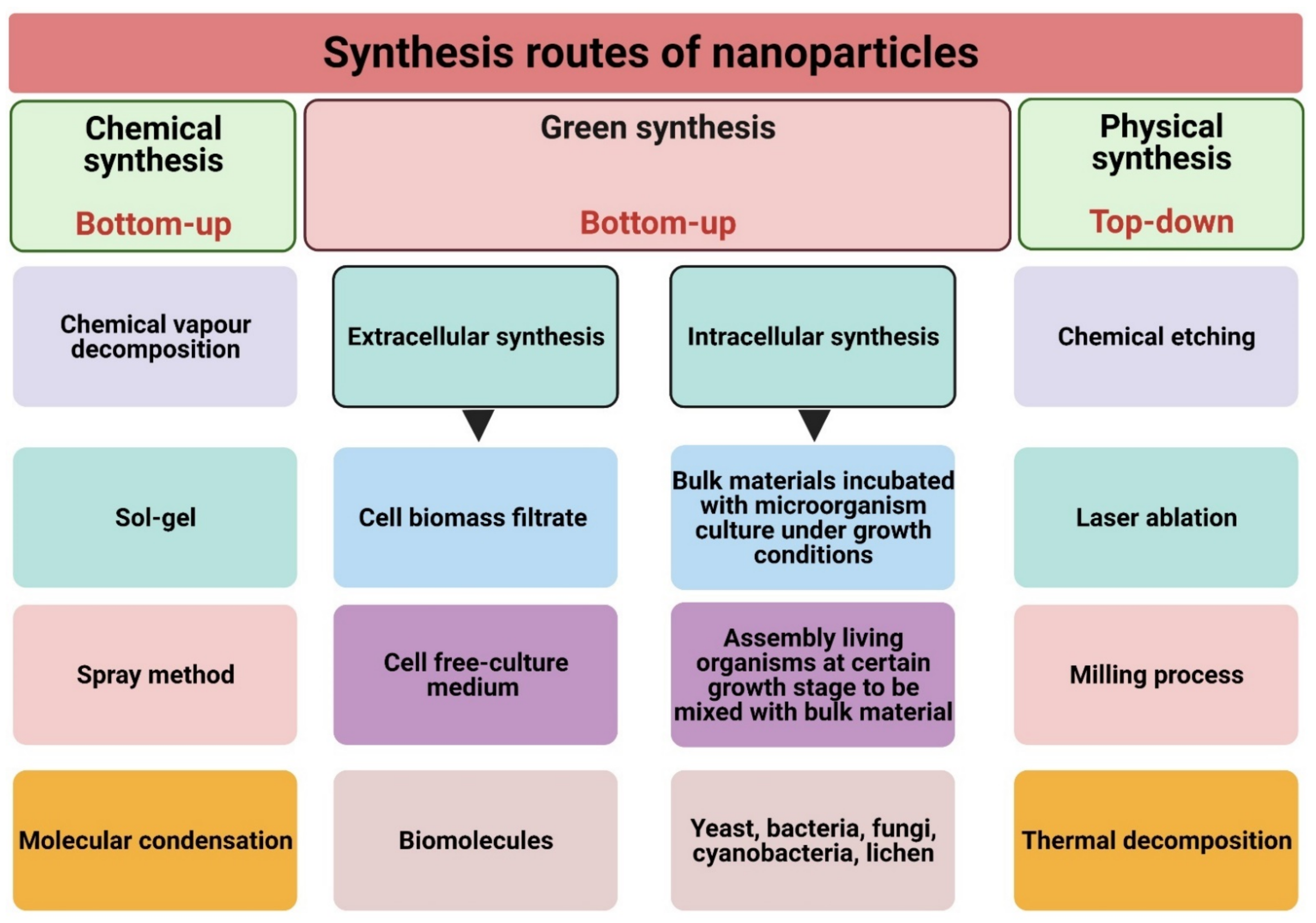
3. Synthesis Routes of Nanoparticles
3.1. Physical Synthesis
3.2. Chemical Synthesis
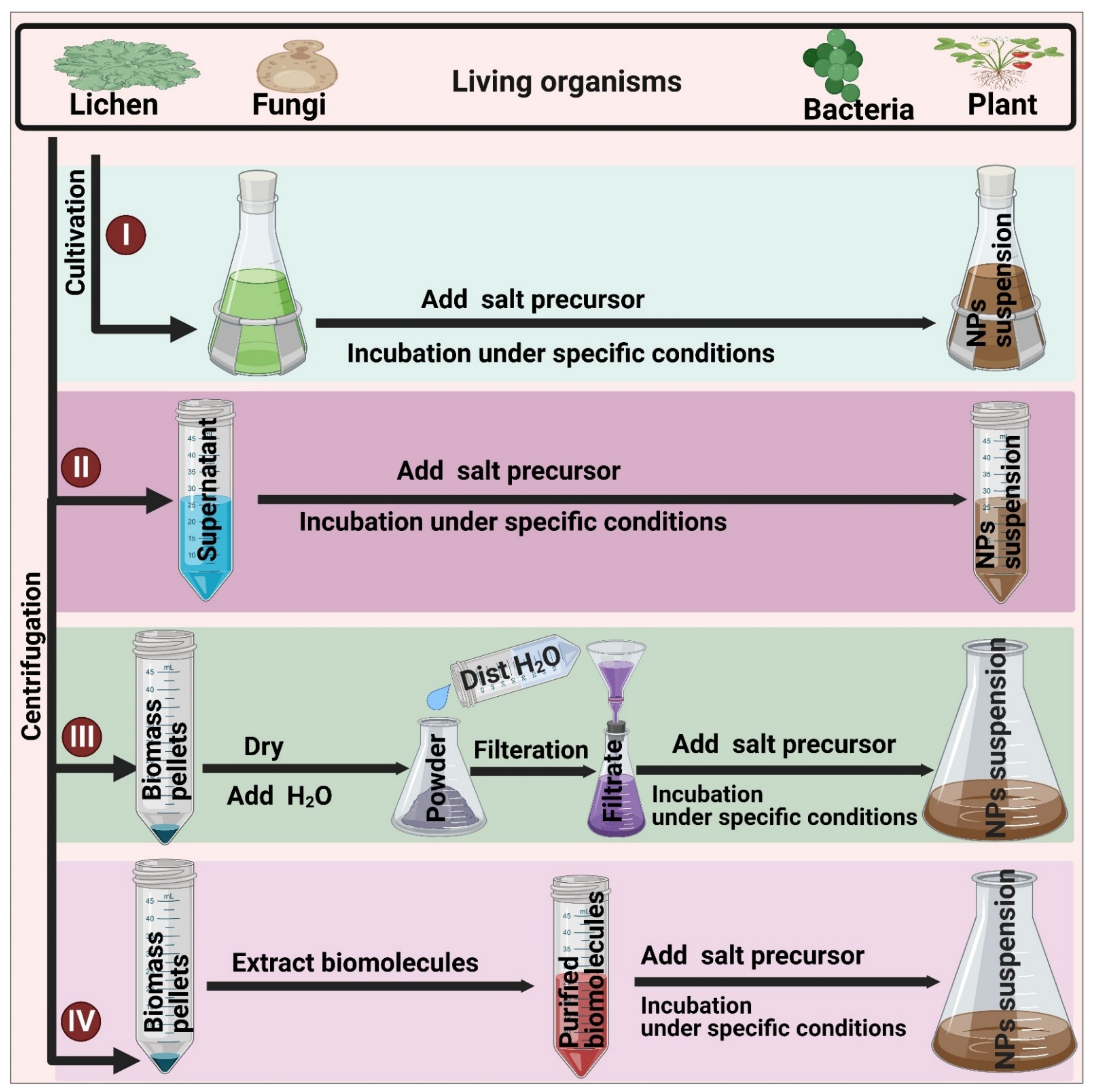
3.3. Biological (Green) Synthesis
3.3.1. Extracellular Synthesis
3.3.2. Intracellular Synthesis
4. Green Synthesis-Based Systems
4.1. Biomolecule-Mediated Fabrication of NPs
4.1.1. Pigments
4.1.2. Carbohydrates
4.1.3. Enzymes
4.1.4. Proteins
4.1.5. Lipids
4.1.6. Vitamins
4.1.7. Secondary Metabolites
4.2. Living Organisms-Mediated Fabrication of NPs
4.2.1. Plants
4.2.2. Algae, Microalgae, Cyanobacteria, and Diatoms
4.2.3. Actinomycetes
4.2.4. Bacteria
4.2.5. Fungi
4.2.6. Lichens
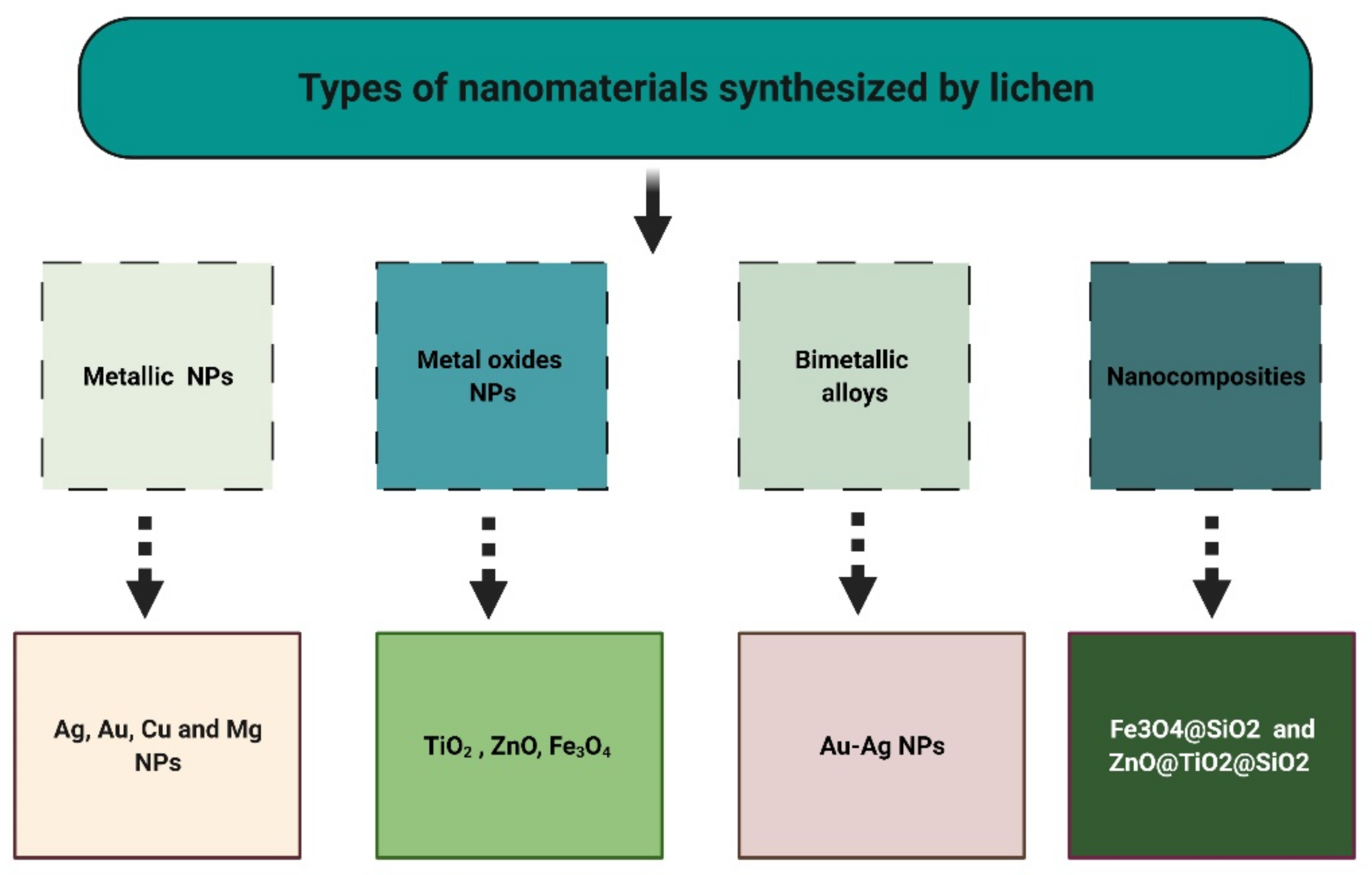
5. Lichens as Biosynthesizers for Nanoparticles
5.1. Metallic Nanoparticles (MNPs)
5.2. Metal Oxide Nanoparticles (MONPs)
5.3. Other Nanomaterials
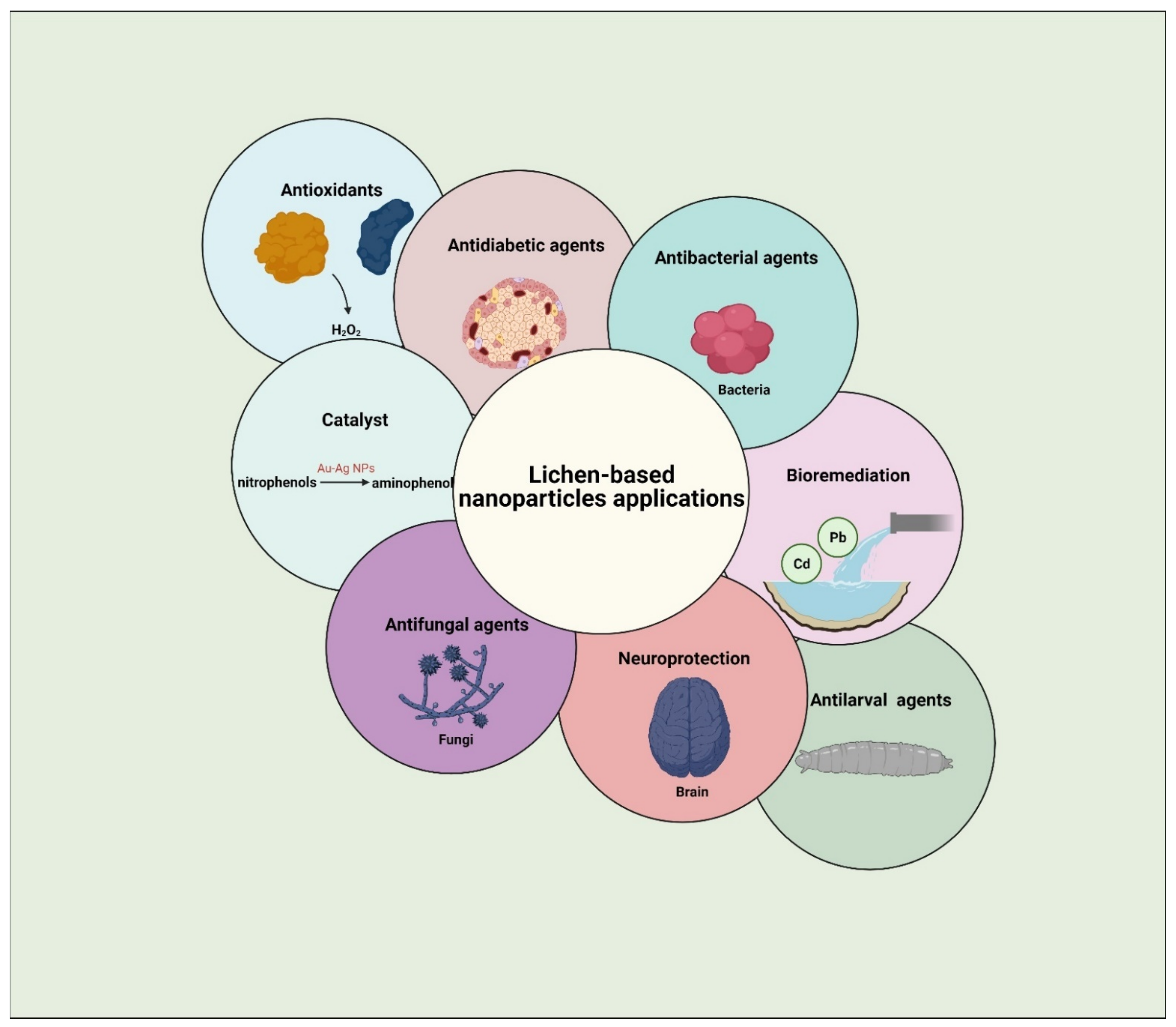
6. Prospective Applications of Lichen-Based Nanoparticles
6.1. Antimicrobial Activity
6.2. Antioxidants
6.3. Other Applications
7. Analysis and Characterization of Nanoparticles
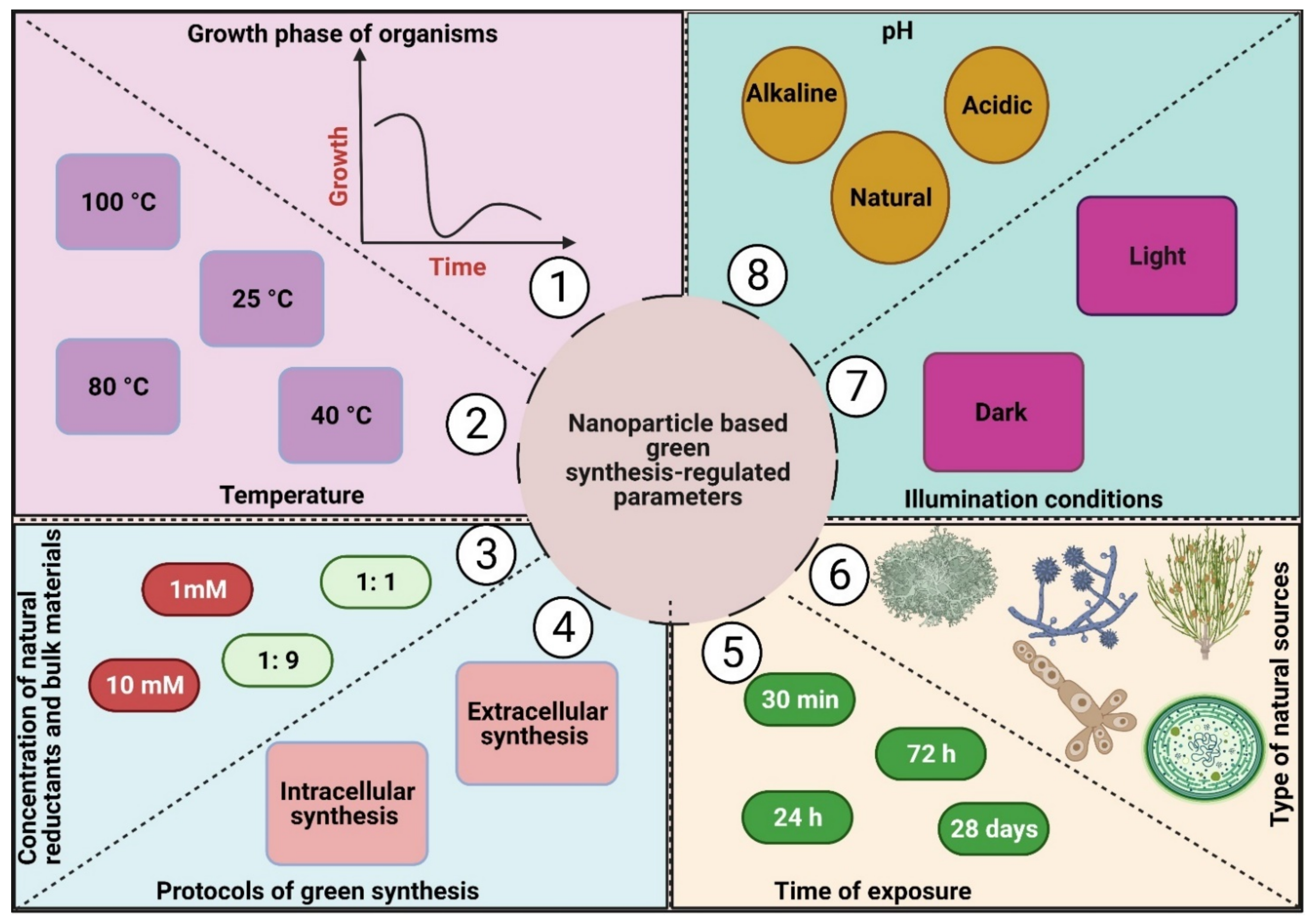
8. Nanoparticle-Based Green Synthesis-Regulated Parameters: Clues to Enhance Their Activity
8.1. Temperature
8.2. pH
8.3. Time of Exposure
8.4. Concentration of Natural Reductants, Stabilizing Agents, and Bulk Materials
8.5. Illumination
8.6. Protocol of Green Synthesis Method
8.7. Type of Natural Sources
8.8. Growth Phase of Organisms Used for NP Fabrication
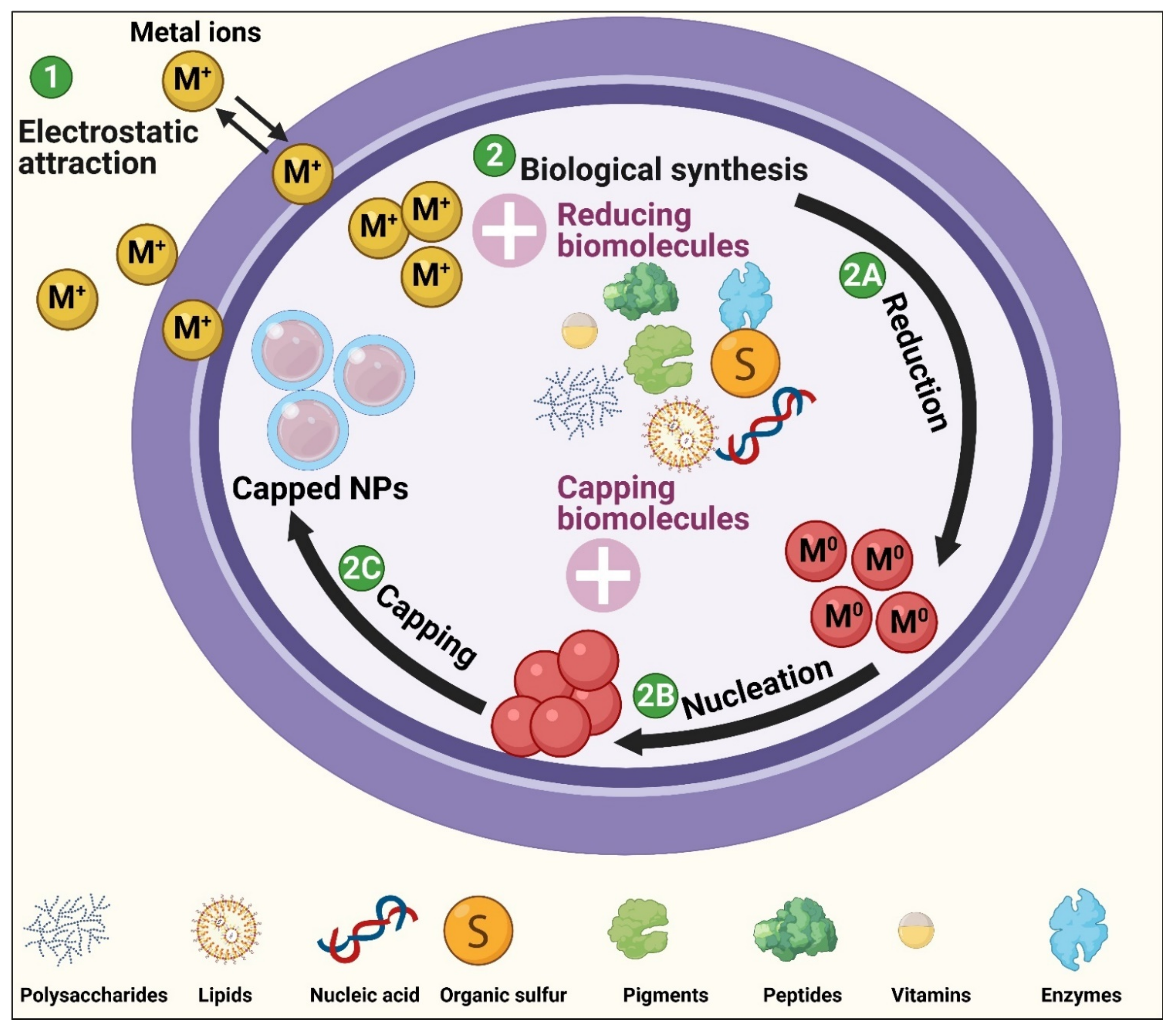
9. The Mechanism of Biological Synthesis of NPs
10. Toxicity of Nanoparticles
11. Future Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [Green Version]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.; Bin-Meferij, M.M. Cyanobacteria—A Promising Platform in Green Nanotechnology: A Review on Nanoparticles Fabrication and Their Prospective Applications. Int. J. Nanomed. 2020, 15, 6033–6066. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 1–24. [Google Scholar]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal nanoparticles as green catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, V.K.; Shukla, S.; Kang, S.-M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.-K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef] [Green Version]
- Hamida, R.S.; Albasher, G.; Bin-Meferij, M.M. Oxidative Stress and Apoptotic Responses Elicited by Nostoc-Synthesized Silver Nanoparticles against Different Cancer Cell Lines. Cancers 2020, 12, 2099. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, C.; Yang, Y.; Zhang, J.; Gao, H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm. Sin. B 2019, 9, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Agasti, S.S.; Rana, S.; Park, M.-H.; Kim, C.K.; You, C.-C.; Rotello, V.M. Nanoparticles for detection and diagnosis. Adv. Drug Deliv. Rev. 2010, 62, 316–328. [Google Scholar] [CrossRef] [Green Version]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Pawlak, A.; Satti, M.; Lee, H.; Wharton, T.; Gali, H.; Sarna, T.; Hamblin, M.R. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radic. Biol. Med. 2007, 43, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegos, G.P.; Demidova, T.N.; Arcila-Lopez, D.; Lee, H.; Wharton, T.; Gali, H.; Hamblin, M.R. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 2005, 12, 1127–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Wu, M.; Huang, S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment. Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Sun, J.; Li, S.; Shi, J.; Gao, H.; Leong, W.S.; Wu, Y.; Li, M.; Liu, C.; Li, P. Blood-triggered generation of platinum nanoparticle functions as an anti-cancer agent. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sonkusre, P. Specificity of biogenic selenium nanoparticles for prostate cancer therapy with reduced risk of toxicity: An in vitro and in vivo study. Front. Oncol. 2020, 9, 1541. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.T.V.; Huynh, T.-C.; Manivasagan, P.; Mondal, S.; Oh, J. An up-to-date review on biomedical applications of palladium nanoparticles. Nanomaterials 2020, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.S.; Shah, N.; Tiwari, M. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Escobar, A.; Reyes-López, S.Y. Antifungal susceptibility of Candida species to copper oxide nanoparticles on polycaprolactone fibers (PCL-CuONPs). PLoS ONE 2020, 15, e0228864. [Google Scholar] [CrossRef]
- Akhtar, S.; Shahzad, K.; Mushtaq, S.; Ali, I.; Rafe, M.H.; Fazal-ul-Karim, S.M. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express 2019, 6, 105409. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Kumbhakar, P.; Ray, S.S.; Stepanov, A.L. Optical properties of nanoparticles and nanocomposites. J. Nanomater. 2014, 2014, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Matsui, I. Nanoparticles for electronic device applications: A brief review. J. Chem. Eng. Jpn. 2005, 38, 535–546. [Google Scholar] [CrossRef]
- Wei, W.; Wang, H.; Wang, C.; Luo, H. Advanced Nanomaterials and Nanotechnologies for Solar Energy. Int. J. Photoenergy 2019. [Google Scholar] [CrossRef]
- Kiani, M.; Ansari, M.; Arshadi, A.A.; Houshfar, E.; Ashjaee, M. Hybrid thermal management of lithium-ion batteries using nanofluid, metal foam, and phase change material: An integrated numerical-experimental approach. J. Therm. Anal. Calorim. 2020, 141, 1–13. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.M.; Khalil, M.I. Synthesis of silver nanoparticles using a novel cyanobacteria Desertifilum sp. extract: Their antibacterial and cytotoxicity effects. Int. J. Nanomed. 2020, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Asmathunisha, N.; Kathiresan, K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf. B Biointerfaces 2013, 103, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Saratale, G.D.; Ghodake, G.; Cho, S.-K.; Kadam, A.; Kumar, G.; Jeon, B.-H.; Pant, D.; Bhatnagar, A.; Shin, H.S. Wheat straw extracted lignin in silver nanoparticles synthesis: Expanding its prophecy towards antineoplastic potency and hydrogen peroxide sensing ability. Int. J. Biol. Macromol. 2019, 128, 391–400. [Google Scholar] [CrossRef]
- Noruzi, M. Biosynthesis of gold nanoparticles using plant extracts. Bioprocess Biosyst. Eng. 2015, 38, 1–14. [Google Scholar] [CrossRef]
- Yuan, X.; Xiao, S.; Taylor, T.N. Lichen-like symbiosis 600 million years ago. Science 2005, 308, 1017–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, K. Pharmaceutically relevant metabolites from lichens. Appl. Microbiol. Biotechnol. 2001, 56, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.; Shukla, S.; Sharma, B.; Bhat, M. A Mini-Review on Lichen-Based Nanoparticles and Their Applications as Antimicrobial Agents. Front. Microbiol. 2021, 12, 336. [Google Scholar]
- Alqahtani, M.A.; Al Othman, M.R.; Mohammed, A.E. Bio fabrication of silver nanoparticles with antibacterial and cytotoxic abilities using lichens. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087. [Google Scholar] [CrossRef] [Green Version]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.; Harini, B.; Rakshith, D.; Satish, S. Marine microbes: Invisible nanofactories. J. Pharm. Res. 2013, 6, 383–388. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Murr, L.E. Classifications and Structures of Nanomaterials. In Handbook of Materials Structures, Properties, Processing and Performance; Springer International Publishing: Cham, Switzerland, 2015; pp. 719–746. [Google Scholar]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. In Supramolecular Chemistry: From Molecules to Nanomaterials; Steed, J.W., Gale, P.A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2012; Volume 8, pp. 3589–3602. [Google Scholar]
- Nakata, Y.; Mukai, K.; Sugawara, M.; Ohtsubo, K.; Ishikawa, H.; Yokoyama, N. Molecular beam epitaxial growth of InAs self-assembled quantum dots with light-emission at 1.3 μm. J. Cryst. Growth 2000, 208, 93–99. [Google Scholar] [CrossRef]
- Bertino, M.F.; Gadipalli, R.R.; Martin, L.A.; Rich, L.E.; Yamilov, A.; Heckman, B.R.; Leventis, N.; Guha, S.; Katsoudas, J.; Divan, R. Quantum dots by ultraviolet and x-ray lithography. Nanotechnology 2007, 18, 315603. [Google Scholar] [CrossRef] [Green Version]
- Patiño-Carachure, C.; Martínez-Vargas, S.; Flores-Chan, J.; Rosas, G. Synthesis of carbon nanostructures by graphite deformation during mechanical milling in air. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 869–876. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Javidi, J.; Dadkhah, M. Ball milling synthesis of silica nanoparticle from rice husk ash for drug delivery application. Comb. Chem. High Throughput Screen. 2013, 16, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Başoğlu, A.; Ocak, Ü.; Gümrükçüoğlu, A. Synthesis of Microwave-Assisted Fluorescence Carbon Quantum Dots Using Roasted–Chickpeas and its Applications for Sensitive and Selective Detection of Fe3+ Ions. J. Fluoresc. 2020, 30, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prosective biotechnological applications: An overview. Biol. Trace Elem. Res. 2020, 6, 1–27. [Google Scholar]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Patel, K.; Bharatiya, B.; Mukherjee, T.; Soni, T.; Shukla, A.; Suhagia, B. Role of stabilizing agents in the formation of stable silver nanoparticles in aqueous solution: Characterization and stability study. J. Dispers. Sci. Technol. 2017, 38, 626–631. [Google Scholar] [CrossRef]
- Mushtaq, K.; Saeed, M.; Gul, W.; Munir, M.; Firdous, A.; Yousaf, T.; Khan, K.; Sarwar, H.M.R.; Riaz, M.A.; Zahid, S. Synthesis and characterization of TiO2 via sol-gel method for efficient photocatalytic degradation of antibiotic ofloxacin. Inorg. Nano-Met. Chem. 2020, 50, 580–586. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, P.; Hu, S.; Hui, J.; Zhuang, J.; Wang, X. Hydrothermal synthesis of hollow silica spheres under acidic conditions. Langmuir 2011, 27, 7185–7191. [Google Scholar] [CrossRef]
- Moraes, D.A.; Junior, J.B.S.; Ferreira, F.F.; Mogili, N.V.V.; Varanda, L.C. Gold nanowire growth through stacking fault mechanism by oleylamine-mediated synthesis. Nanoscale 2020, 12, 13316–13329. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Bin-Meferij, M.M.; Hamida, R.S. Biofabrication and antitumor activity of silver nanoparticles utilizing novel nostoc sp. Bahar M. Int. J. Nanomed. 2019, 14, 9019. [Google Scholar] [CrossRef] [Green Version]
- Mata, Y.; Torres, E.; Blazquez, M.; Ballester, A.; González, F.; Munoz, J. Gold (III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J. Hazard. Mater. 2009, 166, 612–618. [Google Scholar] [CrossRef]
- Husain, S.; Sardar, M.; Fatma, T. Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J. Microbiol. Biotechnol. 2015, 31, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.-D.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. JBIC J. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef]
- Singaravelu, G.; Arockiamary, J.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Y.; Tang, Z.; Hu, J.; Su, R.; Lin, J.; Zhou, T.; Guo, H.; Wang, N.; Xu, R. A size-controlled green synthesis of silver nanoparticles by using the berry extract of Sea Buckthorn and their biological activities. New J. Chem. 2020, 44, 9304–9312. [Google Scholar] [CrossRef]
- Abdel-Gawad, E.I.; Hassan, A.I.; Awwad, S.A. Efficiency of calcium phosphate composite nanoparticles in targeting Ehrlich carcinoma cells transplanted in mice. J. Adv. Res. 2016, 7, 143–154. [Google Scholar] [CrossRef]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Hussein, M.H.; El-Sawah, A.A. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci. Rep. 2017, 7, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Naggar, N.E.-A.; Hussein, M.H.; El-Sawah, A.A. Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Sci. Rep. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Sharma, K.; Chetri, S.P.; Vashishtha, A.; Singh, P.; Kumar, R.; Rathi, B.; Agrawal, V. Algae as crucial organisms in advancing nanotechnology: A systematic review. J. Appl. Phycol. 2016, 28, 1759–1774. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir 2007, 23, 2694–2699. [Google Scholar] [CrossRef]
- Merin, D.D.; Prakash, S.; Bhimba, B.V. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pac. J. Trop. Med. 2010, 3, 797–799. [Google Scholar] [CrossRef] [Green Version]
- Jena, J.; Pradhan, N.; Dash, B.P.; Sukla, L.B.; Panda, P.K. Biosynthesis and characterization of silver nanoparticles using microalga Chlorococcum humicola and its antibacterial activity. Int. J. Nanomater. Biostruct. 2013, 3, 1–8. [Google Scholar]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B Biointerfaces 2011, 83, 42–48. [Google Scholar] [CrossRef]
- Solárová, Z.; Liskova, A.; Samec, M.; Kubatka, P.; Büsselberg, D.; Solár, P. Anticancer potential of lichens’ secondary metabolites. Biomolecules 2020, 10, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, C.; Capper, E.; Roshak, A.; Marquez, B.; Grace, K.; Gerwick, W.; Jacobs, R.; Marshall, L. Scytonemin-a marine natural product inhibitor of kinases key in hyperproliferative inflammatory diseases. Inflamm. Res. 2002, 51, 112. [Google Scholar] [CrossRef]
- Bernardo, P.H.; Chai, C.L.; Heath, G.A.; Mahon, P.J.; Smith, G.D.; Waring, P.; Wilkes, B.A. Synthesis, electrochemistry, and bioactivity of the cyanobacterial calothrixins and related quinones. J. Med. Chem. 2004, 47, 4958–4963. [Google Scholar] [CrossRef] [PubMed]
- Colin, J.A.; Pech-Pech, I.; Oviedo, M.; Águila, S.A.; Romo-Herrera, J.M.; Contreras, O.E. Gold nanoparticles synthesis assisted by marine algae extract: Biomolecules shells from a green chemistry approach. Chem. Phys. Lett. 2018, 708, 210–215. [Google Scholar] [CrossRef]
- Madhumitha, G.; Roopan, S.M. Devastated crops: Multifunctional efficacy for the production of nanoparticles. J. Nanomater. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Mutairi, A.; Al-Shamri, B.; Aabed, K. Antibacterial and cytotoxic potential of biosynthesized silver nanoparticles by some plant extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesharwani, J.; Yoon, K.Y.; Hwang, J.; Rai, M. Phytofabrication of silver nanoparticles by leaf extract of Datura metel: Hypothetical mechanism involved in synthesis. J. Bionanosci. 2009, 3, 39–44. [Google Scholar] [CrossRef]
- Adelere, I.A.; Lateef, A. A novel approach to the green synthesis of metallic nanoparticles: The use of agro-wastes, enzymes, and pigments. Nanotechnol. Rev. 2016, 5, 567–587. [Google Scholar] [CrossRef]
- Ali, J.; Ali, N.; Wang, L.; Waseem, H.; Pan, G. Revisiting the mechanistic pathways for bacterial mediated synthesis of noble metal nanoparticles. J. Microbiol. Methods 2019, 159, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Baraka, A.; Dickson, S.; Gobara, M.; El-Sayyad, G.S.; Zorainy, M.; Awaad, M.I.; Hatem, H.; Kotb, M.M.; Tawfic, A. Synthesis of silver nanoparticles using natural pigments extracted from Alfalfa leaves and its use for antimicrobial activity. Chem. Pap. 2017, 71, 2271–2281. [Google Scholar] [CrossRef]
- Manikprabhu, D.; Lingappa, K. Microwave assisted rapid and green synthesis of silver nanoparticles using a pigment produced by Streptomyces coelicolor klmp33. Bioinorg. Chem. Appl. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Manikprabhu, D.; Lingappa, K. Synthesis of silver nanoparticles using the Streptomyces coelicolor klmp33 pigment: An antimicrobial agent against extended-spectrum beta-lactamase (ESBL) producing Escherichia coli. Mater. Sci. Eng. C 2014, 45, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Kobori, T.; Ganesh, D.; Ogawa, K.; Aoyagi, H. Biosynthesis of silver nanoparticles mediated by extracellular pigment from talaromyces purpurogenus and their biomedical applications. Nanomaterials 2019, 9, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; Halder, U.; Bandopadhyay, R. Preparations and applications of polysaccharide based green synthesized metal nanoparticles: A state-of-the-art. J. Clust. Sci. 2017, 28, 1803–1813. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Bagheri, M.; Taghizadeh, S.-M.; Berenjian, A.; Ghasemi, Y. Biomimetic synthesis of silver nanoparticles using microalgal secretory carbohydrates as a novel anticancer and antimicrobial. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015018. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Wang, Z.; Liu, H. Green synthesis of palladium nanoparticles with carboxymethyl cellulose for degradation of azo-dyes. Mater. Chem. Phys. 2017, 187, 133–140. [Google Scholar] [CrossRef]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Adamu, S.M.; Koki, A.Y.; Adamu, S.; Musa, A.M.; Abdullahi, A.S. Biotechnology as a Cradle of Scientific Development: A Review on Historical Perspective. J. Adv. Biol. Biotechnol. 2016, 10, 1–12. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Tan, H.K.; Lau, S.Y.; Yap, P.-S.; Danquah, M.K. Potential and challenges of enzyme incorporated nanotechnology in dye wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103261. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Kang, K.-H.; Sivakumar, K.; Park, S.-J.; Kim, S.-K. Production of α-amylase for the biosynthesis of gold nanoparticles using Streptomyces sp. MBRC-82. Int. J. Biol. Macromol. 2015, 72, 71–78. [Google Scholar] [CrossRef]
- Gholami-Shabani, M.; Shams-Ghahfarokhi, M.; Gholami-Shabani, Z.; Akbarzadeh, A.; Riazi, G.; Ajdari, S.; Amani, A.; Razzaghi-Abyaneh, M. Enzymatic synthesis of gold nanoparticles using sulfite reductase purified from Escherichia coli: A green eco-friendly approach. Process. Biochem. 2015, 50, 1076–1085. [Google Scholar] [CrossRef]
- Subbaiya, R.; Saravanan, M.; Priya, A.R.; Shankar, K.R.; Selvam, M.; Ovais, M.; Balajee, R.; Barabadi, H. Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol. 2017, 11, 965–972. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef]
- Tareq, F.K.; Fayzunnesa, M.; Kabir, M.S.; Parvin, R.; Zahid, M.A. Biomolecule stabilized/functionalized nanomaterials: Advanced synthesis strategies, characterization and unique properties as antimicroorganism agent. Import Appl. Nanotechnol. 2020, 2, 1–7. [Google Scholar]
- Kim, M.; Jee, S.-C.; Shinde, S.K.; Mistry, B.M.; Saratale, R.G.; Saratale, G.D.; Ghodake, G.S.; Kim, D.-Y.; Sung, J.-S.; Kadam, A.A. Green-synthesis of anisotropic peptone-silver nanoparticles and its potential application as anti-bacterial agent. Polymers 2019, 11, 271. [Google Scholar] [CrossRef] [Green Version]
- Tugarova, A.V.; Kamnev, A.A. Proteins in microbial synthesis of selenium nanoparticles. Talanta 2017, 174, 539–547. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P. pH dependant fungal proteins in the ‘green’synthesis of gold nanoparticles. Adv. Mater. Lett. 2010, 1, 193–199. [Google Scholar] [CrossRef]
- Ga’al, H.; Yang, G.; Fouad, H.; Guo, M.; Mo, J. Mannosylerythritol lipids mediated biosynthesis of silver nanoparticles: An eco-friendly and operative approach against chikungunya vector Aedes albopictus. J. Clust. Sci. 2020, 32, 1–9. [Google Scholar] [CrossRef]
- Kikuchi, F.; Kato, Y.; Furihata, K.; Kogure, T.; Imura, Y.; Yoshimura, E.; Suzuki, M. Formation of gold nanoparticles by glycolipids of Lactobacillus casei. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nadagouda, M.N.; Varma, R.S. Green and controlled synthesis of gold and platinum nanomaterials using vitamin B2: Density-assisted self-assembly of nanospheres, wires and rods. Green Chem. 2006, 8, 516–518. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Yuan, C.; Chen, S.; Ding, T.; Ye, X.; Hu, Y. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int. J. Biol. Macromol. 2018, 111, 1281–1292. [Google Scholar] [CrossRef]
- Malassis, L.; Dreyfus, R.; Murphy, R.J.; Hough, L.A.; Donnio, B.; Murray, C.B. One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv. 2016, 6, 33092–33100. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S.; Singh, V.; Shamsi, S.; Fatma, A.; Mehta, B. Biosynthesis of silver nanoparticles from Desmodium triflorum: A novel approach towards weed utilization. Biotechnol. Res. Int. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Korany, M.; Mahmoud, B.; Ayoub, S.M.; Sakr, T.M.; Ahmed, S.A. Synthesis and radiolabeling of vitamin C-stabilized selenium nanoparticles as a promising approach in diagnosis of solid tumors. J. Radioanal. Nucl. Chem. 2020, 325, 237–244. [Google Scholar] [CrossRef]
- Han, C.; Nagendra, V.; Baig, R.; Varma, R.S.; Nadagouda, M.N. Expeditious synthesis of noble metal nanoparticles using vitamin B12 under microwave irradiation. Appl. Sci. 2015, 5, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Suman, T.; Rajasree, S.R.; Kanchana, A.; Elizabeth, S.B. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf. B Biointerfaces 2013, 106, 74–78. [Google Scholar] [CrossRef]
- Bagherzade, G.; Tavakoli, M.M.; Namaei, M.H. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 227–233. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, X.; Vigila, A.V.G.; Parimelazhagan, T.; Muralidhara-Rao, D.; Zhang, S. Biosynthesis, characterization, and evaluation of bioactivities of leaf extract-mediated biocompatible silver nanoparticles from an early tracheophyte, Pteris tripartita Sw. Int. J. Nanomed. 2016, 11, 5789. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Kim, D.-S.; Kim, D.-Y.; Shin, H.-S. Exploiting fruit waste grape pomace for silver nanoparticles synthesis, assessing their antioxidant, antidiabetic potential and antibacterial activity against human pathogens: A novel approach. Nanomaterials 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Yang, D.C. A strategic approach for rapid synthesis of gold and silver nanoparticles by Panax ginseng leaves. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1949–1957. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Saratale, G.D.; Cho, S.-K.; Ghodake, G.; Kadam, A.; Kumar, S.; Mulla, S.I.; Kim, D.-S.; Jeon, B.-H.; Chang, J.S. Phyto-fabrication of silver nanoparticles by Acacia nilotica leaves: Investigating their antineoplastic, free radical scavenging potential and application in H2O2 sensing. J. Taiwan Inst. Chem. Eng. 2019, 99, 239–249. [Google Scholar] [CrossRef]
- Krishnan, V.; Bupesh, G.; Manikandan, E.; Thanigai, A.; Magesh, S.; Kalyanaraman, R.; Maaza, M. Green synthesis of silver nanoparticles using Piper nigrum concoction and its anticancer activity against MCF-7 and Hep-2 cell lines. J. Antimicro 2016, 2. ISSN 2472-1212. [Google Scholar]
- Gómez-Graña, S.; Perez-Ameneiro, M.; Vecino, X.; Pastoriza-Santos, I.; Perez-Juste, J.; Cruz, J.M.; Moldes, A.B. Biogenic synthesis of metal nanoparticles using a biosurfactant extracted from corn and their antimicrobial properties. Nanomaterials 2017, 7, 139. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.B.A.; Subramanian, S.; Sivasubramanian, A.; Veerappan, G.; Veerappan, A. Preparation of gold nanoparticles using Salicornia brachiata plant extract and evaluation of catalytic and antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 54–58. [Google Scholar] [CrossRef]
- Omomowo, I.; Adenigba, V.; Ogunsona, S.; Adeyinka, G.; Oluyide, O.; Adedayo, A.; Fatukasi, B. Antimicrobial and antioxidant activities of algal-mediated silver and gold nanoparticles. In Proceedings of IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2020; p. 012010. [Google Scholar]
- El-Kassas, H.Y.; El-Sheekh, M.M. Cytotoxic activity of biosynthesized gold nanoparticles with an extract of the red seaweed Corallina officinalis on the MCF-7 human breast cancer cell line. Asian Pac. J. Cancer Prev. 2014, 15, 4311–4317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkinson, J.; Gordon, R. Beyond micromachining: The potential of diatoms. Trends Biotechnol. 1999, 17, 190–196. [Google Scholar] [CrossRef]
- Atazadeh, I.; Sharifi, M. Algae as bioindicators. In The Effects of Heavy Metals on Algae and Development of an Algal Index System for Assessing Water Quality; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2010. [Google Scholar]
- Graham, J.M.; Graham, L.E.; Zulkifly, S.B.; Pfleger, B.F.; Hoover, S.W.; Yoshitani, J. Freshwater diatoms as a source of lipids for biofuels. J. Ind. Microbiol. Biotechnol. 2012, 39, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Gutu, T.; Gale, D.K.; Jeffryes, C.; Wang, W.; Chang, C.-H.; Rorrer, G.L.; Jiao, J. Electron microscopy and optical characterization of cadmium sulphide nanocrystals deposited on the patterned surface of diatom biosilica. J. Nanomater. 2009, 2009, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jeffryes, C.; Gutu, T.; Jiao, J.; Rorrer, G.L. Metabolic insertion of nanostructured TiO2 into the patterned biosilica of the diatom Pinnularia sp. by a two-stage bioreactor cultivation process. Acs Nano 2008, 2, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Jena, J.; Pradhan, N.; Dash, B.P.; Panda, P.K.; Mishra, B.K. Pigment mediated biogenic synthesis of silver nanoparticles using diatom Amphora sp. and its antimicrobial activity. J. Saudi Chem. Soc. 2015, 19, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Ranjitha, V.; Rai, V.R. Actinomycetes mediated synthesis of gold nanoparticles from the culture supernatant of Streptomyces griseoruber with special reference to catalytic activity. 3 Biotech 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Avilala, J.; Golla, N. Antibacterial and antiviral properties of silver nanoparticles synthesized by marine actinomycetes. Int. J. Pharm. Sci. Res. 2019, 10, 1223–1228. [Google Scholar]
- Menon, S.; Rajeshkumar, S.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour. Effic. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Patil, M.P.; Kang, M.-J.; Niyonizigiye, I.; Singh, A.; Kim, J.-O.; Seo, Y.B.; Kim, G.-D. Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf. B Biointerfaces 2019, 183, 110455. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilger-Casagrande, M.; Lima, R.D. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [Green Version]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.-C.; Al Khulaifi, M.M.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Rashid, M.; Rahman, A.; Husen, A.; Rehman, S. Biogenic fabrication and characterization of silver nanoparticles using aqueous-ethanolic extract of lichen (Usnea longissima) and their antimicrobial activity. Biomater. Res. 2018, 22, 23. [Google Scholar] [CrossRef]
- Ingólfsdóttir, K.; Gudmundsdóttir, G.; Ögmundsdóttir, H.; Paulus, K.; Haraldsdóttir, S.; Kristinsson, H.; Bauer, R. Effects of tenuiorin and methyl orsellinate from the lichen Peltigera leucophlebia on 5-/15-lipoxygenases and proliferation of malignant cell lines in vitro. Phytomedicine 2002, 9, 654–658. [Google Scholar] [CrossRef]
- Reddy, V.M.; O’Sullivan, J.F.; Gangadharam, P.R. Antimycobacterial activities of riminophenazines. J. Antimicrob. Chemother. 1999, 43, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, N.; Ateş, Ç.; Yılmaz, M.; Demir, D.; Yıldız, A.; Çalımlı, A. Investigation of lichen based green synthesis of silver nanoparticles with response surface methodology. Green Process. Synth. 2014, 3, 259–270. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.; Crespo, A.; Gómez-Serranillos, M. Evaluation of the antioxidant capacities and cytotoxic effects of ten parmeliaceae lichen species. Evid. Based Complement. Altern. Med. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Felczykowska, A.; Pastuszak-Skrzypczak, A.; Pawlik, A.; Bogucka, K.; Herman-Antosiewicz, A.; Guzow-Krzemińska, B. Antibacterial and anticancer activities of acetone extracts from in vitro cultured lichen-forming fungi. Bmc Complementary Altern. Med. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Tapia, A.; Lima, B.; Pertino, M.; Sortino, M.; Zacchino, S.; Arias, A.R.D.; Feresin, G.E. A new antifungal and antiprotozoal depside from the Andean lichen Protousnea poeppigii. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 349–355. [Google Scholar]
- Abdolmaleki, H.; Sohrabi, M. Biosynthesis of silver nanoparticles by two lichens of “Usnea articulate” and “Ramalina sinensis” and investigation of their antibacterial activity against some pathogenic bacteria. Ebnesina 2016, 17, 33–42. [Google Scholar]
- Çıplak, Z.; Gökalp, C.; Getiren, B.; Yıldız, A.; Yıldız, N. Catalytic performance of Ag, Au and Ag-Au nanoparticles synthesized by lichen extract. Green Process. Synth. 2018, 7, 433–440. [Google Scholar] [CrossRef]
- Rai, H.; Gupta, R.K. Biogenic fabrication, characterization, and assessment of antibacterial activity of silver nanoparticles of a high altitude Himalayan lichen-Cladonia rangiferina (L.) Weber ex FH Wigg. Trop. Plant Res. 2019, 6, 293–298. [Google Scholar] [CrossRef]
- Esmaeili, A.; Rajaee, S. The preparation of hyaluronic acid nanoparticles from Aspicilia lichens using Bifido Bacteria for help in the treatment of diabetes in rats in vivo. Phytother. Res. 2017, 31, 1590–1599. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.-L.; Gu, Y.; Huang, H.; Zhang, G.-W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold nanoparticles-enabled efficient dual delivery of anticancer therapeutics to HeLa cells. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Khandel, P.; Kumar Shahi, S.; Kanwar, L.; Kumar Yadaw, R.; Kumar Soni, D. Biochemical profiling of microbes inhibiting Silver nanoparticles using symbiotic organisms. Int. J. Nano Dimens. 2018, 9, 273–285. [Google Scholar]
- Leela, K.; Devi, C. A Study on The Applications of Silver Nanoparticle Synthesized Using The Aqueous Extract And The Purified Secondary Metabolites of Lichen Parmelia perlata. Int. J. Pharm. Sci. Invent. 2017, 6, 42–59. [Google Scholar]
- Mie, R.; Samsudin, M.W.; Din, L.B.; Ahmad, A. Green synthesis of silver nanoparticles using two lichens species: Parmotrema praesorediosum and Ramalina dumeticola. Proc. Appl. Mech. Mater. 2012, 229–231, 256–259. [Google Scholar] [CrossRef]
- Mie, R.; Samsudin, M.W.; Din, L.B.; Ahmad, A.; Ibrahim, N.; Adnan, S.N.A. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 2014, 9, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, S.; Suresh, K.; Rajesh, M.; Reddy, S.; Samba, C.; Hemalatha, C.; Wudayagiri, R.; Valluru, L. Biosynthesis, characterization, antibacterial and antioxidant activity of silver nanoparticles produced by lichens. J. Bionanosci. 2013, 7, 237–244. [Google Scholar] [CrossRef]
- Din, L.B.; Mie, R.; Samsudin, M.W.; Ahmad, A.; Ibrahim, N. Biomimetic synthesis of silver nanoparticles using the lichen Ramalina dumeticola and the antibacterial activity. Malays. J. Anal. Sci. 2015, 19, 369–376. [Google Scholar]
- Baláž, M.; Goga, M.; Hegedus, M.; Daneu, N.; Kováčová, M.; Tkáčiková, L.; Balážová, L.; Bakčor, M. Biomechanochemical solid-state synthesis of silver nanoparticles with antibacterial activity using lichens. ACS Sustain. Chem. Eng. 2020, 8, 13945–13955. [Google Scholar]
- Goga, M.; Baláž, M.; Daneu, N.; Elečko, J.; Tkáčiková, L.; Marcinčinová, M.; Bakčor, M. Biological activity of selected lichens and lichen-based Ag nanoparticles prepared by a green solid-state mechanochemical approach. Mater. Sci. Eng. C 2021, 119, 111640. [Google Scholar]
- Senthil Prabhu, S.; Ramanujam, J.R.; Sudha, S. Antibacterial Activity of Silver Nanoparticles Synthesized by using Lichens Heterodermia boryi and Parmotrema stuppeum. Int. J. Pharm. Biol. Sci. 2019, 9, 1397–1402. [Google Scholar]
- Alqahtani, M.A.; Mohammed, A.E.; Daoud, S.I.; Alkhalifah, D.H.M.; Albrahim, J.S. Lichens (Parmotrema clavuliferum) extracts: Bio-mediator in silver nanoparticles formation and antibacterial potential. J. Bionanosci. 2017, 11, 410–415. [Google Scholar] [CrossRef]
- Debnath, R.; Purkayastha, D.D.; Hazra, S.; Ghosh, N.N.; Bhattacharjee, C.R.; Rout, J. Biogenic synthesis of antioxidant, shape selective gold nanomaterials mediated by high altitude lichens. Mater. Lett. 2016, 169, 58–61. [Google Scholar] [CrossRef]
- Gandhi, A.D.; Murugan, K.; Umamahesh, K.; Babujanarthanam, R.; Kavitha, P.; Selvi, A. Lichen Parmelia sulcata mediated synthesis of gold nanoparticles: An eco-friendly tool against Anopheles stephensi and Aedes aegypti. Environ. Sci. Pollut. Res. 2019, 26, 23886–23898. [Google Scholar] [CrossRef]
- Devasena, T.; Ashok, V.; Dey, N.; Francis, A. Phytosynthesis of magnesium nanoparticles using lichens. World J. Pharm. Res. 2014, 3, 4625–4632. [Google Scholar]
- Alavi, M.; Karimi, N.; Valadbeigi, T. Antibacterial, antibiofilm, antiquorum sensing, antimotility, and antioxidant activities of green fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via protoparmeliopsis muralis lichen aqueous extract against multi-drug-resistant bacteria. ACS Biomater. Sci. Eng. 2019, 5, 4228–4243. [Google Scholar] [CrossRef]
- Arjaghi, S.K.; Alasl, M.K.; Sajjadi, N.; Fataei, E.; Rajaei, G.E. Green Synthesis of Iron Oxide Nanoparticles by RS Lichen Extract and its Application in Removing Heavy Metals of Lead and Cadmium. Biol. Trace Elem. Res. 2020, 199, 1–6. [Google Scholar] [CrossRef]
- Abdullah, S.M.; Kolo, K.; Sajadi, S.M. Greener pathway toward the synthesis of lichen-based ZnO@ TiO2@ SiO2 and Fe3O4@ SiO2 nanocomposites and investigation of their biological activities. Food Sci. Nutr. 2020, 8, 4044–4054. [Google Scholar] [CrossRef]
- Safarkar, R.; Ebrahimzadeh Rajaei, G.; Khalili-Arjagi, S. The study of antibacterial properties of iron oxide nanoparticles synthesized using the extract of lichen Ramalina sinensis. Asian J. Nanosci. Mater. 2020, 3, 157–166. [Google Scholar]
- Reveathy, M.; Mathiazhagan, A.; Annadurai, G. Biosynthesis, characterization, antibacterial activity of silver nanoparticles using the lichen Parmotrema perlatum. Eur. J. Biomed. Pharm. Sci. 2015, 2, 348–361. [Google Scholar]
- Uthreshwaranath, K.; Sasidharan, J.; Yuvraj, P.; Aruna, V.; Johnson, A.W.; Karthik, S. Green synthesis of silver nanoparticles using Parmelia perlata. Asian J. Microbiol. Biotechnol. Environ. Sci. 2015, 17, 145–152. [Google Scholar]
- Shakouri, M.; Amani, A.; Mollaei, S.; Jeddi, M. Biosynthesis of Silver Nanoparticles by Umbilicaria Americana. In Proceedings of the 24th Iranian Seminar of Organic Chemistry, Azarbaijan Shahid Madani University, Tabriz, Iran, 24–26 August 2016. [Google Scholar]
- Koca, F.D.; Ünal, G.; Halici, M.G. Lichen Based Synthesis of Zinc Oxide Nanoparticles and Evaluation of its Neurotoxic Effects on Human Neuroblastoma Cells. Proc. J. Nano Res. 2019, 59, 15–24. [Google Scholar] [CrossRef]
- Sanchez-Moreno, P.; Ortega-Vinuesa, J.L.; Peula-Garcia, J.M.; Marchal, J.A.; Boulaiz, H. Smart drug-delivery systems for cancer nanotherapy. Curr. Drug Targets 2018, 19, 339–359. [Google Scholar] [CrossRef]
- Kumar, S.P.; Kekuda, T.P.; Vinayaka, K.; Yogesh, M. Synergistic efficacy of lichen extracts and silver nanoparticles against bacteria causing food poisoning. Asian J. Res. Chem. 2010, 3, 67–70. [Google Scholar]
- Rai, A.; Singh, A.; Ahmad, A.; Sastry, M. Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir 2006, 22, 736–741. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, J.; Wei, J. Effect of temperature on the size of biosynthesized silver nanoparticle: Deep insight into microscopic kinetics analysis. Arab. J. Chem. 2020, 13, 1011–1019. [Google Scholar] [CrossRef]
- Pandey, B. Synthesis of zinc-based nanomaterials: A biological perspective. IET Nanobiotechnol. 2012, 6, 144–148. [Google Scholar]
- Mohammadi, F.M.; Ghasemi, N. Influence of temperature and concentration on biosynthesis and characterization of zinc oxide nanoparticles using cherry extract. J. Nanostruct. Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Hamouda, R.A.; Hussein, M.H.; Abo-elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Černík, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889. [Google Scholar]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015, 5, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Chutrakulwong, F.; Thamaphat, K.; Limsuwan, P. Photo-irradiation induced green synthesis of highly stable silver nanoparticles using durian rind biomass: Effects of light intensity, exposure time and pH on silver nanoparticles formation. J. Phys. Commun. 2020, 4, 095015. [Google Scholar] [CrossRef]
- Mankad, M.; Patil, G.; Patel, D.; Patel, P.; Patel, A. Comparative studies of sunlight mediated green synthesis of silver nanoparaticles from Azadirachta indica leaf extract and its antibacterial effect on Xanthomonas oryzae pv. oryzae. Arab. J. Chem. 2020, 13, 2865–2872. [Google Scholar] [CrossRef]
- Sonker, A.S.; Pathak, J.; Kannaujiya, V.K.; Sinha, R.P. Characterization and in vitro antitumor, antibacterial and antifungal activities of green synthesized silver nanoparticles using cell extract of Nostoc sp. strain HKAR-2. Can. J. Biotechnol. 2017, 1, 26. [Google Scholar] [CrossRef]
- Sweeney, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Yang, X.; Hu, L.; Tan, Z.; Zhao, L.; Zhang, Z.; Liu, J.; Jiang, G. Superoxide-mediated extracellular biosynthesis of silver nanoparticles by the fungus Fusarium oxysporum. Environ. Sci. Technol. Lett. 2016, 3, 160–165. [Google Scholar] [CrossRef]
- Sneha, K.; Sathishkumar, M.; Mao, J.; Kwak, I.; Yun, Y.-S. Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem. Eng. J. 2010, 162, 989–996. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Deepak, V.; Ramkumarpandian, S.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413. [Google Scholar] [CrossRef]
- Husseiny, M.; Abd El-Aziz, M.; Badr, Y.; Mahmoud, M. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef]
- Divya, M.; Kiran, G.S.; Hassan, S.; Selvin, J. Biogenic synthesis and effect of silver nanoparticles (AgNPs) to combat catheter-related urinary tract infections. Biocatal. Agric. Biotechnol. 2019, 18, 101037. [Google Scholar] [CrossRef]
- Hamedi, S.; Ghaseminezhad, M.; Shokrollahzadeh, S.; Shojaosadati, S.A. Controlled biosynthesis of silver nanoparticles using nitrate reductase enzyme induction of filamentous fungus and their antibacterial evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1588–1596. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Gutierrez, T.; Morris, G.; Green, D.H. Yield and physicochemical properties of EPS from Halomonas sp. strain TG39 identifies a role for protein and anionic residues (sulfate and phosphate) in emulsification of n-hexadecane. Biotechnol. Bioeng. 2009, 103, 207–216. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahmed, H.B. Polysaccharides templates for assembly of nanosilver. Carbohydr. Polym. 2016, 135, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Alvarez, P.J.; Zhu, D. Microbial extracellular polymeric substances reduce Ag+ to silver nanoparticles and antagonize bactericidal activity. Environ. Sci. Technol. 2014, 48, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Reith, F.; Lengke, M.F.; Falconer, D.; Craw, D.; Southam, G. The geomicrobiology of gold. ISME J. 2007, 1, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M.; Monchy, S.; Vallaeys, T.; Auquier, V.; Benotmane, A.; Bertin, P.; Taghavi, S.; Dunn, J.; Van Der Lelie, D.; Wattiez, R. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: Towards a catalogue of metal-responsive genes. FEMS Microbiol. Rev. 2003, 27, 385–410. [Google Scholar] [CrossRef]
- Perez-Gonzalez, T.; Jimenez-Lopez, C.; Neal, A.L.; Rull-Perez, F.; Rodriguez-Navarro, A.; Fernandez-Vivas, A.; Iañez-Pareja, E. Magnetite biomineralization induced by Shewanella oneidensis. Geochim. Cosmochim. Acta 2010, 74, 967–979. [Google Scholar] [CrossRef]
- Senut, M.C.; Zhang, Y.; Liu, F.; Sen, A.; Ruden, D.M.; Mao, G. Size-dependent toxicity of gold nanoparticles on human embryonic stem cells and their neural derivatives. Small 2016, 12, 631–646. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef]
- Wan, R.; Mo, Y.; Zhang, Z.; Jiang, M.; Tang, S.; Zhang, Q. Cobalt nanoparticles induce lung injury, DNA damage and mutations in mice. Part. Fibre Toxicol. 2017, 14, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapied, E.; Nahmani, J.Y.; Moudilou, E.; Chaurand, P.; Labille, J.; Rose, J.; Exbrayat, J.-M.; Oughton, D.H.; Joner, E.J. Ecotoxicological effects of an aged TiO2 nanocomposite measured as apoptosis in the anecic earthworm Lumbricus terrestris after exposure through water, food and soil. Environ. Int. 2011, 37, 1105–1110. [Google Scholar] [CrossRef]
- Rahman, Q.; Lohani, M.; Dopp, E.; Pemsel, H.; Jonas, L.; Weiss, D.G.; Schiffmann, D. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ. Health Perspect. 2002, 110, 797–800. [Google Scholar] [CrossRef] [Green Version]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chokkalingam, M.; Singh, P.; Huo, Y.; Soshnikova, V.; Ahn, S.; Kang, J.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.C. Facile synthesis of Au and Ag nanoparticles using fruit extract of Lycium chinense and their anticancer activity. J. Drug Deliv. Sci. Technol. 2019, 49, 308–315. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Harper, S.L.; Yun, S.-I. In Vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). J. Hazard. Mater. 2016, 301, 480–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Strains | Type of NPs | Size (nm) | Shape | Illumination | Time of Exposure | pH | Temperature (°C) | Mode of Synthesis | Application | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Usnea longissima | Ag-NPs | 9.40–11.23 | Spherical | Dark | 72 h | 7 | RT | - | Antibacterial agent | [136] |
| Parmotrema praesorediosum | Ag-NPs | 19 | Cubic structure | NM | 24 h | NM | RT | - | Antibacterial agent | [152] |
| Cetraria islandica (L) Ach | Ag-NPs | 5-29 | Spherical | NM | 19.09, 60 120, 180 and 220.91 min | NM | 16.48, 25, 37.5, 50, 58.52 | - | NA | [139] |
| Parmotrema praesorediosum | Ag-NPs | 42 | Spherical | NM | 72 h | Alkaline | RT | - | NA | [151] |
| Ramalina dumeticola | Ag-NPs | 20 | Spherical | NM | 72 h | Alkaline | RT | - | NA | [151] |
| Ramalina dumeticola | Ag-NPs | 13 | Spherical | NM | 24 h | NM | RT | - | NA | [154] |
| Cetraria islandica (L.) Ach | Ag-NPs | 6 | Spherical | NM | 30 min | NM | 80 | - | Catalytic activity | [144] |
| Au-NPs | 19 | Spherical | NM | 30 min | NM | 80 | - | |||
| Ag-Au NPs | 6 and 21 | Polygonal and Spherical | NM | 30 min | NM | 80 | - | |||
| Parmelia perlata | Ag-NPs | NA | Spherical | NM | 30 min | NM | 60 | - | Antimicrobial, antioxidant and antidiabetic agents | [150] |
| Ramalina sinensis | Fe3O4 NPs | 20-40 | Uniform Spherical | NM | 1 h | NM | 70 | - | Removing heavy metals such as Pb and Cd | [163] |
| Lecanora muralis | ZnO@TiO2@SiO2 nanocomposites | 55–90 | Spherical | NM | 5 h | NM | 80 | - | Antimicrobial agent | [164] |
| Fe3O4@SiO2 nanocomposites | 55–85 | Spherical | NM | 5 h | NM | 80 | - | |||
| Ramalina sinensis | Iron oxide nanoparticles | 31.74-53.91 | Uniform spherical | NM | 1 h | 7 | 70 | - | Antibacterial agent | [165] |
| Protoparmeliopsis muralis | Ag-NPs | 33.49 ± 22.91 | Spherical | NM | 24 h | 8 | RT | - | Antibacterial, antibiofilm, antiquorum sensing, antimotility, and antioxidant activities | [162] |
| Cu-NPs | 253.97 ± 57.2 | Triangular | NM | 24 h | 8 | RT | - | |||
| Fe3O4 NPs | 307 ± 154 | Spherical | NM | 24 h | 8 | RT | - | |||
| TiO2 NPs | 133.32 ± 35.33 | Polyhedral | NM | 24 h | 8 | RT | - | |||
| ZnO NPs | 178.06 ± 49.97 | Cubic | NM | 24 h | 8 | RT | - | |||
| Parmeliopsis ambigua | Ag-NPs | 150–250 | NM | Light | 24 h | NM | RT | +, - | Antibacterial and antioxidant agents | [153] |
| Punctelia subrudecta | Ag-NPs | 150–250 | NM | Light | 24 h | NM | RT | +, - | ||
| Evernia mesomorpha | Ag-NPs | 150–250 | NM | Light | 24 h | NM | RT | +, - | ||
| Xanthoparmelia plitti | Ag-NPs | 150–250 | NM | Light | 24 h | NM | RT | +, - | ||
| Cladonia rangiferina | Mg-NPs | 23 | NM | NM | 24 h | NM | NM | NM | NA | [161] |
| Parmotrema tinctorum | Ag-NPs | 15 ± 5.1 | Spherical | Dark | 24 h | NA | RT | - | Antibacterial agent | [149] |
| Acroscyphus sphaerophoroides | Ag-NPs | 5–35 | Twinned quasi-spherical and prismatic shapes | NM | 12 h | NM | RT | - | Antioxidant agent | [159] |
| Sticta nylanderiana | Ag-NPs | 20–50 | Multiply twinned | NM | 12 h | NM | RT | - | ||
| Parmotrema clavuliferum | Ag-NPs | 106 | Spherical | Dark | 48 h | NM | 80 °C | - | Antibacterial agent | [158] |
| Parmotrema perlatum | Ag-NPs | NM | NM | NM | NM | NM | NM | NM | Antibacterial agent | [166] |
| Xanthoria parietina | Ag-NPs | 1–40 | Spherical | Dark | 72 h | NM | 40 °C | - | Anticancer and antibacterial agents | [37] |
| Flavopunctelia flaventior | Ag-NPs | 1–40 | Spherical | Dark | 72 h | NM | 40 °C | - | ||
| Parmelia perlata | Ag-NPs | NM | NM | NM | NM | NM | NM | NM | Antibacterial agent | [167] |
| Umbilicaria Americana | Ag-NPs | NM | NM | NM | NM | NM | NM | NM | NM | [168] |
| Cladonia rangiferina | Ag-NPs | 20 | Spherical and rods | NM | 72 h | Alkaline | RT | - | Antibacterial agent | [145] |
| Usnea articulata | Ag-NPs | 10–50 | Spherical | NM | 72 h | Alkaline | 27 °C | - | Antibacterial agent | [143] |
| Ramalina sinensis | Ag-NPs | 50–80 | Spherical | NM | 72 h | Alkaline | 27 °C | - | ||
| Parmelia sulcate | Au-NP | 54 | Spherical | NM | 20 min | NM | 60 °C | - | Antioxidant and mosquitocidal agents | [160] |
| Ramalina fraxinea | ZnO-NPs | 21 | Spherical | NM | Up to 2 h | NM | 60 °C | - | Neuroprotection activity | [169] |
| Aspicilia lichens | Nanohyaluronic acid | 29–89 | Spherical | NM | 48 h | Alkaline then neutralize by acid | 50 °C | - | Antidiabetic agent | [146] |
| Xanthoria elegans | Ag-NPs | Bimodal | 5-100 | NM | 2 h | NM | NM | - | Antibacterial agent | [155] |
| Usnea antarctica | Bimodal | 5-100 | NM | 6 h | NM | NM | - | Antibacterial agent | ||
| Leptogium puberulum | Bimodal | 5-100 | NM | 6 h | NM | NM | - | Antibacterial agent | ||
| Cetraria islandica | Bimodal | 5-100 | NM | 2 h | NM | NM | - | Antibacterial agent | ||
| Pseudevernia furfuracea | Ag-NPs | Bimodal | ˂10-100 | NM | 2 h | NM | NM | - | Antibacterial and antioxidant agents | [156] |
| Lobaria pulmonaria | Bimodal | ˂10-100 | NM | 2 h | NM | NM | - | Antibacterial and antioxidant agents | ||
| Heterodermia boryi | Ag-NPs | Cubic | 27.91–37.21 | NM | NM | NM | NM | - | Antibacterial agent | [157] |
| Parmotrema stuppeum | Cubic | 27.69–36.00 | NM | NM | NM | NM | - | Antibacterial agent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamida, R.S.; Ali, M.A.; Abdelmeguid, N.E.; Al-Zaban, M.I.; Baz, L.; Bin-Meferij, M.M. Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications. J. Fungi 2021, 7, 291. https://doi.org/10.3390/jof7040291
Hamida RS, Ali MA, Abdelmeguid NE, Al-Zaban MI, Baz L, Bin-Meferij MM. Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications. Journal of Fungi. 2021; 7(4):291. https://doi.org/10.3390/jof7040291
Chicago/Turabian StyleHamida, Reham Samir, Mohamed Abdelaal Ali, Nabila Elsayed Abdelmeguid, Mayasar Ibrahim Al-Zaban, Lina Baz, and Mashael Mohammed Bin-Meferij. 2021. "Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications" Journal of Fungi 7, no. 4: 291. https://doi.org/10.3390/jof7040291
APA StyleHamida, R. S., Ali, M. A., Abdelmeguid, N. E., Al-Zaban, M. I., Baz, L., & Bin-Meferij, M. M. (2021). Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications. Journal of Fungi, 7(4), 291. https://doi.org/10.3390/jof7040291