Calcium Affects Polyphosphate and Lipid Accumulation in Mucoromycota Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Growth Media and Cultivation Conditions
2.3. Fourier-Transform Infrared Spectroscopy
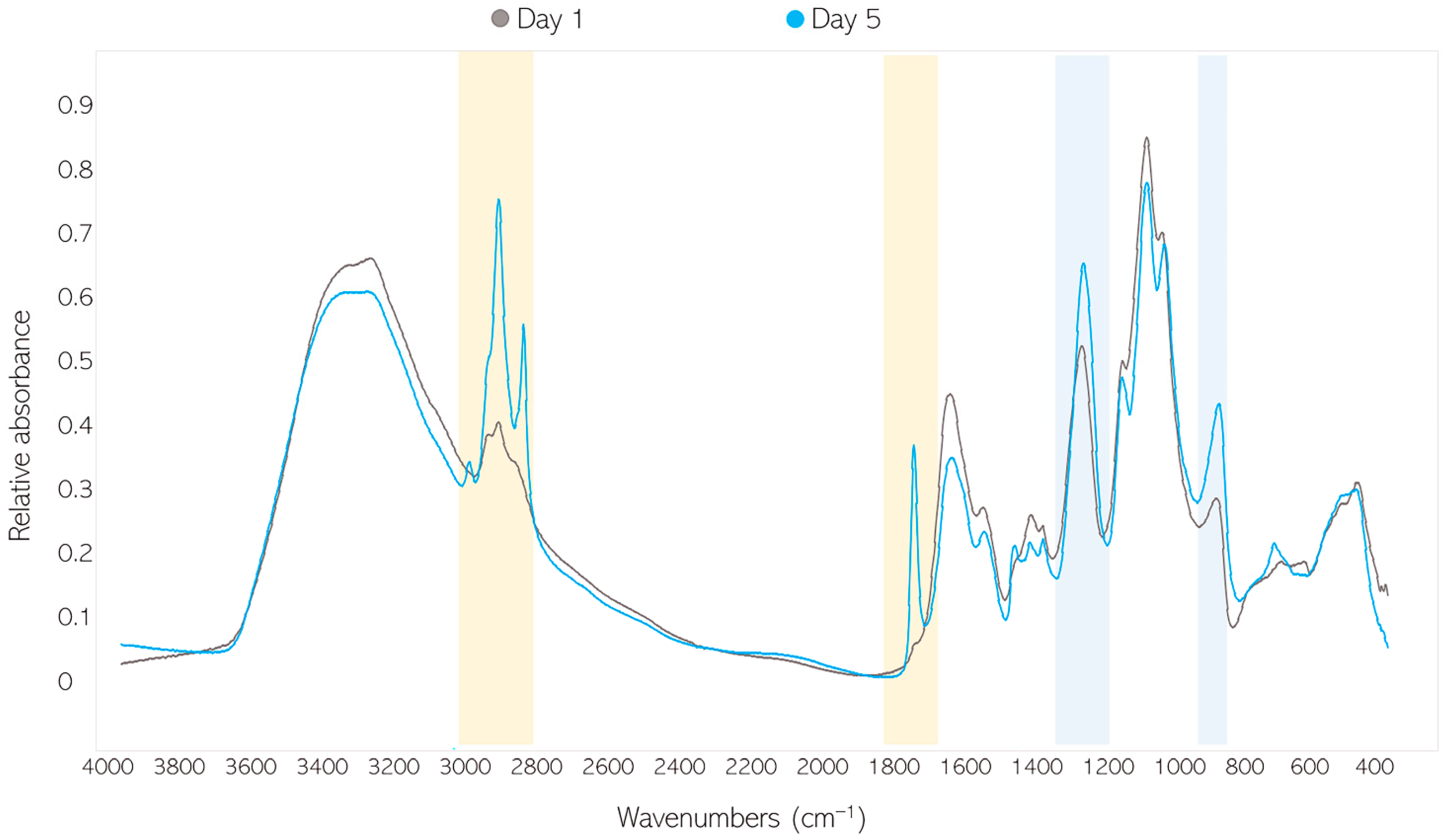
2.3.1. FTIR-HTS of Fungal Biomass
2.3.2. FTIR-ATR of Culture Supernatant
2.4. Analysis of Cellular Phosphorus
2.4.1. Analysis of Total P in Fungal Biomass
2.4.2. Solid-State NMR (SSNMR) Characterization of Phosphates in Fungal Biomass
2.5. Lipid Extraction and GC-FID Analysis of Fatty Acid Profile
2.6. Data Analysis
2.6.1. Analysis of FTIR Spectral Data of Fungal Biomass
2.6.2. FTIR-ATR Spectral Data of Culture Supernatants—Glucose Estimation
3. Results
3.1. Growth Characteristics of Mucoromycota Fungi
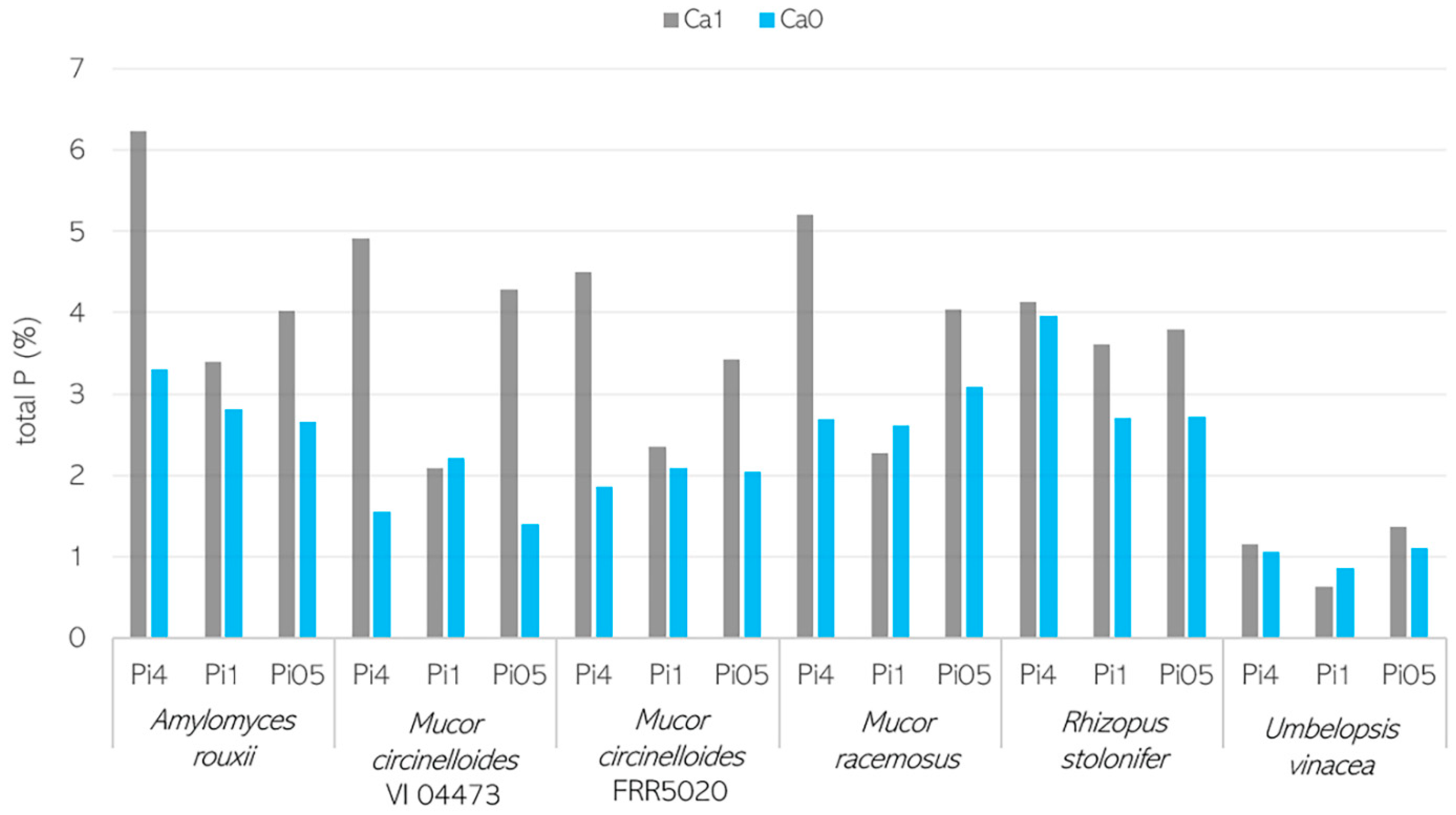
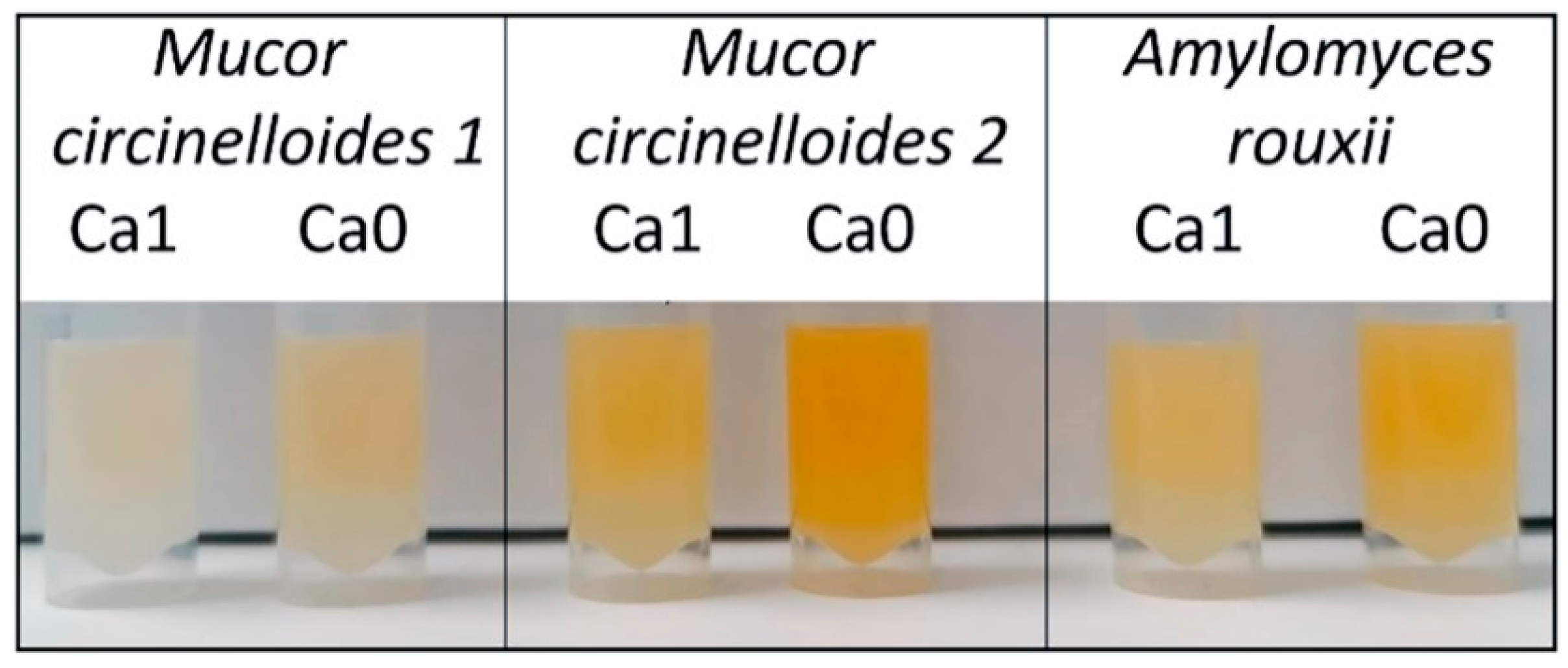
3.2. Importance of Ca Ions’ Availability for Polyphosphate Accumulation in Mucoromycota
3.3. Ca Ion Deficiency Can Trigger Lipid Accumulation in Mucoromycota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.K.; Treichel, H.; Shapaval, V.O.; de Oliveira, L.A.; Tuohy, M.G. Microbial Functional Foods and Nutraceuticals; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Rodrigues Reis, C.E.; Bento, H.B.; Carvalho, A.K.; Rajendran, A.; Hu, B.; De Castro, H.F. Critical applications of Mucor circinelloides within a biorefinery context. Crit. Rev. Biotechnol. 2019, 39, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Single cell oils for the 21st century. In Single Cell Oils; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–26. [Google Scholar]
- Dzurendova, S.; Zimmermann, B.; Kohler, A.; Tafintseva, V.; Slany, O.; Certik, M.; Shapaval, V. Microcultivation and FTIR spectroscopy-based screening revealed a nutrient-induced co-production of high-value metabolites in oleaginous Mucoromycota fungi. PLoS ONE 2020, 15, e0234870. [Google Scholar] [CrossRef] [PubMed]
- Dzurendova, S.; Zimmermann, B.; Tafintseva, V.; Kohler, A.; Ekeberg, D.; Shapaval, V. The influence of phosphorus source and the nature of nitrogen substrate on the biomass production and lipid accumulation in oleaginous Mucoromycota fungi. Appl. Microbiol. Biotechnol. 2020, 104, 8065–8076. [Google Scholar] [CrossRef] [PubMed]
- Dzurendova, S.; Zimmermann, B.; Tafintseva, V.; Kohler, A.; Horn, S.J.; Shapaval, V. Metal and Phosphate Ions Show Remarkable Influence on the Biomass Production and Lipid Accumulation in Oleaginous Mucor circinelloides. J. Fungi 2020, 6, 260. [Google Scholar] [CrossRef]
- Jackson, S.; Heath, I. Roles of calcium ions in hyphal tip growth. Microbiol. Mol. Biol. Rev. 1993, 57, 367–382. [Google Scholar] [CrossRef]
- Benčina, M.; Legiša, M.; Read, N.D. Cross-talk between cAMP and calcium signalling in Aspergillus niger. Mol. Microbiol. 2005, 56, 268–281. [Google Scholar] [CrossRef]
- Meyer, V.; Arentshorst, M.; Flitter, S.J.; Nitsche, B.M.; Kwon, M.J.; Reynaga-Pena, C.G.; Bartnicki-Garcia, S.; van den Hondel, C.A.; Ram, A.F. Reconstruction of signaling networks regulating fungal morphogenesis by transcriptomics. Eukaryot. Cell 2009, 8, 1677–1691. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.B.; Kadotani, N.; Kasahara, S.; Tosa, Y.; Mayama, S.; Nakayashiki, H. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol. Microbiol. 2008, 68, 1348–1365. [Google Scholar] [CrossRef]
- Benčina, M.; Bagar, T.; Lah, L.; Kraševec, N. A comparative genomic analysis of calcium and proton signaling/homeostasis in Aspergillus species. Fungal Genet. Biol. 2009, 46, S93–S104. [Google Scholar] [CrossRef]
- Roy, A.; Kumar, A.; Baruah, D.; Tamuli, R. Calcium signaling is involved in diverse cellular processes in fungi. Mycology 2021, 12, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Tisi, R.; Rigamonti, M.; Groppi, S.; Belotti, F. Calcium homeostasis and signaling in fungi and their relevance for pathogenicity of yeasts and filamentous fungi. AIMS Mol. Sci. 2016, 3, 505–549. [Google Scholar] [CrossRef]
- Puigpinós, J.; Casas, C.; Herrero, E. Altered intracellular calcium homeostasis and endoplasmic reticulum redox state in Saccharomyces cerevisiae cells lacking Grx6 glutaredoxin. Mol. Biol. Cell 2015, 26, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, Y.; Hijikata, N.; Yokoyama, K.; Ohtomo, R.; Handa, Y.; Kawaguchi, M.; Saito, K.; Ezawa, T. Polyphosphate accumulation is driven by transcriptome alterations that lead to near-synchronous and near-equivalent uptake of inorganic cations in an arbuscular mycorrhizal fungus. New Phytol. 2014, 204, 638–649. [Google Scholar] [CrossRef]
- Allen, N.S.; Schumm, J.H. Endoplasmic reticulum, calciosomes and their possible roles in signal transduction. Protoplasma 1990, 154, 172–178. [Google Scholar] [CrossRef]
- Gorain, P.C.; Bagchi, S.K.; Mallick, N. Effects of calcium, magnesium and sodium chloride in enhancing lipid accumulation in two green microalgae. Environ. Technol. 2013, 34, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-A.; Liu, W.-X.; Durnaoglu, S.; Lee, S.-K.; Lian, J.; Lehner, R.; Ahnn, J.; Agellon, L.B.; Michalak, M. Loss of calreticulin uncovers a critical role for calcium in regulating cellular lipid homeostasis. Sci. Rep. 2017, 7, 5941. [Google Scholar] [CrossRef]
- Cifuentes, M.; Rojas, C.V. Antilipolytic effect of calcium-sensing receptor in human adipocytes. Mol. Cell. Biochem. 2008, 319, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Kurat, C.F.; Natter, K.; Petschnigg, J.; Wolinski, H.; Scheuringer, K.; Scholz, H.; Zimmermann, R.; Leber, R.; Zechner, R.; Kohlwein, S.D. Obese yeast: Triglyceride lipolysis is functionally conserved from mammals to yeast. J. Biol. Chem. 2006, 281, 491–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bien, C.M.; Espenshade, P.J. Sterol regulatory element binding proteins in fungi: Hypoxic transcription factors linked to pathogenesis. Eukaryot. Cell 2010, 9, 352–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosa, G.; Zimmermann, B.; Kohler, A.; Ekeberg, D.; Afseth, N.K.; Mounier, J.; Shapaval, V. High-throughput screening of Mucoromycota fungi for production of low-and high-value lipids. Biotechnol. Biofuels 2018, 11, 66. [Google Scholar] [CrossRef]
- Kosa, G.; Vuoristo, K.S.; Horn, S.J.; Zimmermann, B.; Afseth, N.K.; Kohler, A.; Shapaval, V. Assessment of the scalability of a microtiter plate system for screening of oleaginous microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 4915–4925. [Google Scholar] [CrossRef] [Green Version]
- Kavadia, A.; Komaitis, M.; Chevalot, I.; Blanchard, F.; Marc, I.; Aggelis, G. Lipid and γ-linolenic acid accumulation in strains of Zygomycetes growing on glucose. J. Am. Oil Chem. Soc. 2001, 78, 341–346. [Google Scholar] [CrossRef]
- Kosa, G.; Kohler, A.; Tafintseva, V.; Zimmermann, B.; Forfang, K.; Afseth, N.K.; Tzimorotas, D.; Vuoristo, K.S.; Horn, S.J.; Mounier, J. Microtiter plate cultivation of oleaginous fungi and monitoring of lipogenesis by high-throughput FTIR spectroscopy. Microb. Cell Factories 2017, 16, 101. [Google Scholar] [CrossRef] [Green Version]
- ISO. Animal Feeding Stuffs—Determination of Phosphorus Content—Spectrometric Method; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- Staal, L.B.; Petersen, A.B.; Jørgensen, C.A.; Nielsen, U.G.; Nielsen, P.H.; Reitzel, K. Extraction and quantification of polyphosphates in activated sludge from waste water treatment plants by 31P NMR spectroscopy. Water Res. 2019, 157, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.; Nichols, P.D.; McMeekin, T.A. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J. Microbiol. Meth. 2000, 43, 107–116. [Google Scholar] [CrossRef]
- Langseter, A.M.; Dzurendova, S.; Shapaval, V.; Kohler, A.; Ekeberg, D.; Zimmermann, B. Evaluation and optimisation of direct transesterification methods for the assessment of lipid accumulation in oleaginous filamentous fungi. Microb. Cell Factories 2021, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, Č.; Hočevar, T.; Milutinovič, M.; Možina, M.; Polajnar, M.; Toplak, M.; Starič, A. Orange: Data mining toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Kosa, G.; Shapaval, V.; Kohler, A.; Zimmermann, B. FTIR spectroscopy as a unified method for simultaneous analysis of intra-and extracellular metabolites in high-throughput screening of microbial bioprocesses. Microb. Cell Factories 2017, 16, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Zhang, B.; Zheng, W.; Xing, L.; Li, M. Alkaline stress triggers an immediate calcium fluctuation in Candida albicans mediated by Rim101p and Crz1p transcription factors. FEMS Yeast Res. 2011, 11, 430–439. [Google Scholar]
- Speake, T.; Elliott, A.C. Modulation of calcium signals by intracellular pH in isolated rat pancreatic acinar cells. J. Physiol. 1998, 506, 415–430. [Google Scholar] [CrossRef]
- Ramos, I.B.; Miranda, K.; Pace, D.A.; Verbist, K.C.; Lin, F.-Y.; Zhang, Y.; Oldfield, E.; Machado, E.A.; De Souza, W.; Docampo, R. Calcium-and polyphosphate-containing acidic granules of sea urchin eggs are similar to acidocalcisomes, but are not the targets for NAADP. Biochem. J. 2010, 429, 485–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, J.W.; Quinn, J.P. Intracellular accumulation of polyphosphate by the yeast Candida humicola G-1 in response to acid pH. Appl. Environ. Microbiol. 2000, 66, 4068–4073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, G.; Gaillardin, C. Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol. Rev. 1997, 19, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Kawazoe, N.; Kimata, Y.; Izawa, S. Acetic acid causes endoplasmic reticulum stress and induces the unfolded protein response in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1192. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; El-Shanawany, A.-R.; Shah, A.M.; Nazir, Y.; Naz, T.; Ullah, S.; Mustafa, K.; Song, Y. Comparative analysis of different isolated oleaginous Mucoromycota fungi for their γ-linolenic acid and carotenoid production. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef]
- Fraser, P.D.; Ruiz-Hidalgo, M.J.; Lopez-Matas, M.A.; Alvarez, M.I.; Eslava, A.P.; Bramley, P.M. Carotenoid biosynthesis in wild type and mutant strains of Mucor circinelloides. Biochim. Biophys. Acta (BBA) Gen. Subj. 1996, 1289, 203–208. [Google Scholar] [CrossRef]
- Enrique, A.; Papp, T.; Breum, J.; Arnau, J.; Arturo, P. Strain and culture conditions improvement for β-carotene production with Mucor. In Microbial Processes and Products; Springer: Berlin, Germany, 2005; pp. 239–256. [Google Scholar]
- Khanafari, A.; Tayari, K.; Emami, M. Light requirement for the carotenoids production by Mucor hiemalis. Ir. J. Med. Sci. 2008, 11, 25–32. [Google Scholar]





| Fungal Strain | Collection № | Short Name |
|---|---|---|
| Amylomyces rouxii | CCM F220 | AR |
| Mucor circinelloides | VI 04473 | MC1 |
| Mucor circinelloides | FRR 5020 | MC2 |
| Mucor racemosus | UBOCC A 102007 | MR |
| Rhizopus stolonifer | CCM F445 | RS |
| Umbelopsis vinacea | CCM F539 | UV |
| Sample | Pi4 | Pi1 | Pi05 | |
|---|---|---|---|---|
| Amylomyces rouxii | Ca1 | 30.37 ± 0.28 | 46.76 ± 1.80 | 27.36 ± 0.14 |
| Ca0 | 31.24 ± 0.49 | 40.02 ± 0.32 | 37.48 ± 1.59 | |
| Mucor circinelloides | Ca1 | 42.80 ± 0.00 | 47.85 ± 0.48 | 22.67 ± 2.96 |
| VI 04473 | Ca0 | 47.42 ± 1.74 | 54.01 ± 1.56 | 48.05 ± 0.36 |
| Mucor circinelloides | Ca1 | 34.37 ± 0.24 | 47.62 ± 2.48 | 37.11 ± 0.70 |
| FRR 5020 | Ca0 | 41.01 ± 1.68 | 48.60 ± 2.07 | 35.79 ± 0.24 |
| Mucor racemosus | Ca1 | 31.10 ± 0.83 | 37.85 ± 0.08 | 22.83 ± 3.42 |
| Ca0 | 30.86 ± 3.09 | 35.04 ± 0.62 | 39.63 ± 1.82 | |
| Rhizopus stolonifer | Ca1 | 25.33 ± 1.06 | 24.27 ± 0.51 | 22.78 ± 0.55 |
| Ca0 | 27.40 ± 1.70 | 26.75 ± 1.71 | 27.90 ± 0.37 | |
| Umbelopsis vinacea | Ca1 | 69.90 ± 1.66 | 81.04 ± 1.93 | 52.36 ± 3.21 |
| Ca0 | 58.43 ± 1.08 | 84.18 ± 2.94 | 66.70 ± 0.21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzurendova, S.; Zimmermann, B.; Kohler, A.; Reitzel, K.; Nielsen, U.G.; Dupuy--Galet, B.X.; Leivers, S.; Horn, S.J.; Shapaval, V. Calcium Affects Polyphosphate and Lipid Accumulation in Mucoromycota Fungi. J. Fungi 2021, 7, 300. https://doi.org/10.3390/jof7040300
Dzurendova S, Zimmermann B, Kohler A, Reitzel K, Nielsen UG, Dupuy--Galet BX, Leivers S, Horn SJ, Shapaval V. Calcium Affects Polyphosphate and Lipid Accumulation in Mucoromycota Fungi. Journal of Fungi. 2021; 7(4):300. https://doi.org/10.3390/jof7040300
Chicago/Turabian StyleDzurendova, Simona, Boris Zimmermann, Achim Kohler, Kasper Reitzel, Ulla Gro Nielsen, Benjamin Xavier Dupuy--Galet, Shaun Leivers, Svein Jarle Horn, and Volha Shapaval. 2021. "Calcium Affects Polyphosphate and Lipid Accumulation in Mucoromycota Fungi" Journal of Fungi 7, no. 4: 300. https://doi.org/10.3390/jof7040300
APA StyleDzurendova, S., Zimmermann, B., Kohler, A., Reitzel, K., Nielsen, U. G., Dupuy--Galet, B. X., Leivers, S., Horn, S. J., & Shapaval, V. (2021). Calcium Affects Polyphosphate and Lipid Accumulation in Mucoromycota Fungi. Journal of Fungi, 7(4), 300. https://doi.org/10.3390/jof7040300





