Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis
Abstract
:1. Introduction
2. Materials and Methods
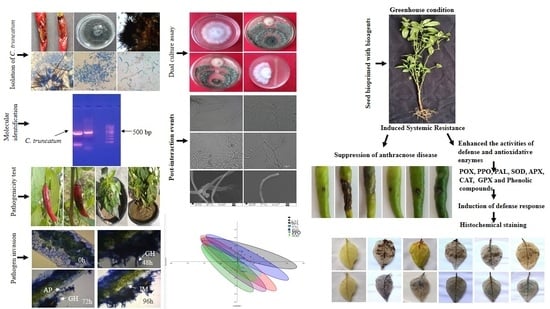
2.1. Isolation, Morphological and Cultural Characterization of the Pathogen
2.2. Molecular Identification of the Pathogen
2.2.1. Fungal Genomic DNA Extraction
2.2.2. PCR Amplification and Sequencing
2.3. Plant Growth Promoting Bacteria (PGPB) Isolates
2.3.1. Selection of Plant Growth Promoting Bacterium
2.3.2. Identification of the Selected Plant Growth Promoting Bacterium
2.4. Collection of Plant Growth Promoting Fungi
2.5. Preparation of Bacterial and Fungal Inocula
2.6. Plant Material and Growth
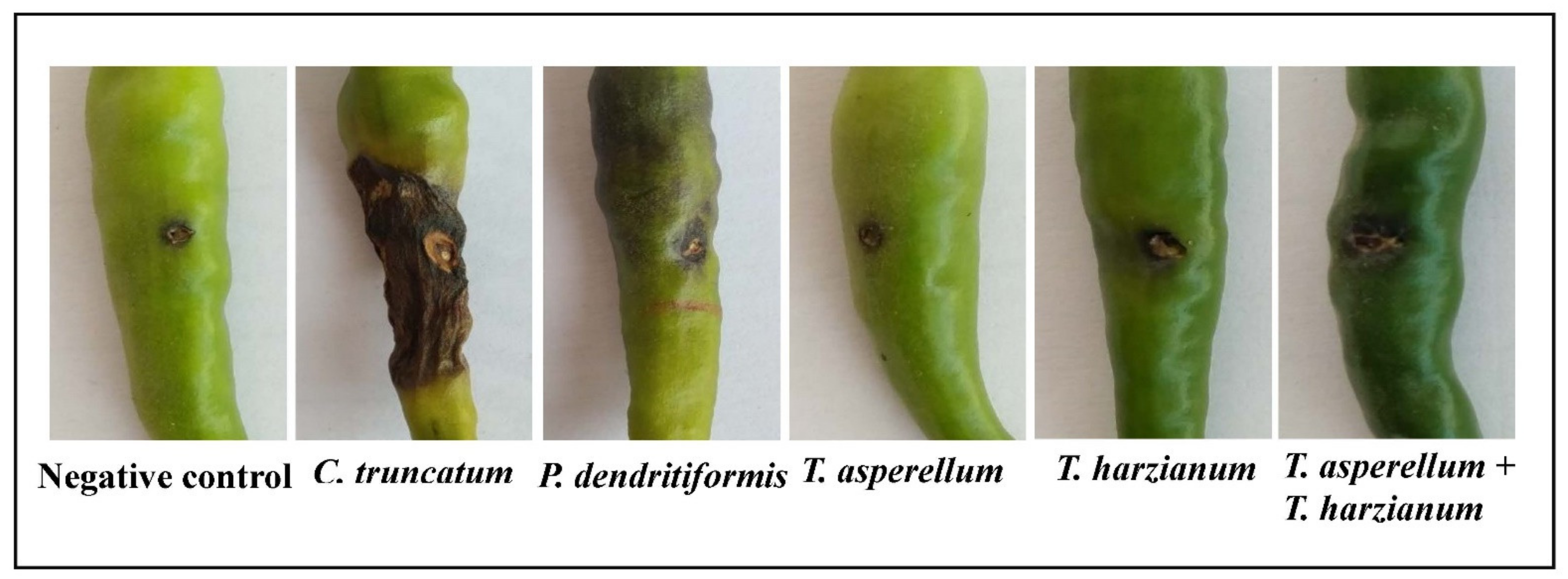
2.7. Pathogenicity Test on Leaves and Fruits
Measurement of Disease Index (DI)
- 0 = No visible disease symptom on the fruit.
- 1 = Slight infection, with small spots (≤1 mm).
- 2 = Moderate infection, with medium spots (1–2 mm).
- 3 = Severe infection, with large spots (>2 mm).
2.8. In Vitro Antagonism of P. dendritiformis, T. harzianum and T. asperellum against C. truncatum
2.9. Scanning Electron Microscopy to Study the Interaction between P. dendritiformis and C. truncatum
2.10. Experimental Design for Chilli Seed Treatments
Seed Priming with P. dendritiformis, T. asperellum, T. harzianum, T. asperellum + T. harzianum
2.11. Treatment of Chilli Plants with Biocontrol Agents under Greenhouse Condition
2.12. Assessment of P. dendritiformis, T. asperellum, T. harzianum, and T. asperellum + T. harzianum on Fruit Anthracnose Protection against C. truncatum under Greenhouse Conditions
2.13. Chlorophyll Estimation
2.14. Biochemical Studies
2.14.1. Fruit Harvest and Analysis
2.14.2. Defence Enzyme Assay
Assessment of Peroxidase (POX) Activity
Assessment of Phenylalanine Ammonia-Lyase (PAL) Activity
Assessment of Polyphenol Oxidase (PPO) Activity
2.14.3. Antioxidant Enzyme Activities
Assessment of Superoxide Dismutase (SOD) Activity
Assessment of Catalase (CAT) Activity
Assessment of Ascorbate Peroxidase (APx) Activity
Assessment of Guaiacol Peroxidase (GPx) Activity
2.14.4. Estimation of Total Phenolic Compound
2.14.5. Histochemical Analysis
Detection of Hydrogen Peroxide Accumulation
Nitroblue Tetrazolium (NBT) Staining to Detect O2− Deposition
2.15. Statistical Analysis
3. Results
3.1. Morphological, Cultural and Molecular Characterization of the Pathogen
3.2. Pathogenicity Test
3.3. Disease Index
3.4. Dual Culture Assay
3.5. Scanning Electron Microscope Observation
3.6. Assessment of P. dendritiformis, T. harzianum, T. asperellum, and T. harzianum + T. asperellum on Fruit Anthracnose Protection against C. truncatum under Greenhouse Conditions
3.7. Effect of P. dendritiformis, T. harzianum, T. asperellum and T. harzianum + T. asperellum on Chlorophyll Content
3.8. Defense Enzyme Assay
3.8.1. Peroxidase (POX) Activity
3.8.2. PAL Activity
3.8.3. Polyphenol Oxidase (PPO) Activity
3.9. Antioxidant Enzyme Assay
3.9.1. Superoxide Dismutase (SOD) Activity
3.9.2. Catalase (CAT) Activity
3.9.3. Ascorbate Peroxidase (APX) Activity
3.9.4. Guaiacol Peroxidase (GPX) Activity
3.9.5. Estimation of Total Phenolic Content
3.10. Histochemical Studies
3.10.1. Detection of Hydrogen Peroxide Accumulation in Leaves
3.10.2. Detection of O2− Deposition in Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosland, P.W.; Votava, E.J.; Votava, E.M. Peppers: Vegetable and Spice Capsicums; CABI Publishing: New York, NY, USA, 2012. [Google Scholar]
- Liu, F.; Tang, G.; Zheng, X.; Li, Y.; Sun, X.; Qi, X.; Gong, G. Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province. China Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Raghuwanshi, R.; Gupta, V.K.; Singh, H.B. Chilli Anthracnose: The Epidemiology and Management. Front. Microbiol. 2016, 7, 1527. [Google Scholar] [CrossRef] [Green Version]
- Kommula, S.K.; Reddy, G.P.D.; Undrajavarapu, P.; Kanchana, K.S. Effect of Various Factors (Temperature, pH and Light Inten-835 sity) on Growth of Colletotrichum capsici Isolated from Infected Chilli. Int. J. Pure App. Biosci. 2017, 5, 535–543. [Google Scholar] [CrossRef]
- Nbsp; FAOSTAT Production—Crops. Available online: http://www.fao.org/faostat/en/#compare (accessed on 11 March 2019).
- Dubey, M.K.; Zehra, A.; Aamir, M.; Yadav, M.; Samal, S.; Upadhyay, R.S. Isolation, identification, carbon utilization profile and control of Pythium graminicola, the causal agent of chilli damping-off. J. Phytopathol. 2019, 168, 88–102. [Google Scholar] [CrossRef]
- Pakdeevaraporn, P.; Wasee, S.; Taylor, P.W.J.; Mongkolporn, O. Inheritance of resistance to anthracnose caused by Colleto-trichum capsici in Capsicum. Plant Breed. 2005, 124, 206–208. [Google Scholar] [CrossRef]
- Than, P.P.; Jeewon, R.; Hyde, K.D.; Pongsupasamit, S.; Mongkolporn, O.; Taylor, P.W.J. Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand. Plant Pathol. 2008, 57, 562–572. [Google Scholar] [CrossRef]
- Thind, T.S.; Jhooty, J.S. Relative prevalence of fungal diseases of chilli fruits in Punjab. J. Mycol. Plant Pathol. 1985, 15, 305–307. [Google Scholar]
- De Silva, D.D.; Groenewald, J.Z.; Crous, P.W.; Ades, P.K.; Nasruddin, A.; Mongkolporn, O.; Taylor, P.W.J. Identification, prevalence and pathogenicity of Colletotrichum species causing anthracnose of Capsicum annuum in Asia. IMA Fungus 2019, 10, 1–32. [Google Scholar] [CrossRef]
- Mishra, A.; Ratan, V.; Trivedi, S.; Dabbas, M.R.; Shankar, K.; Singh, A.K.; Srivastava, Y. Survey of anthracnose and wilt of chilli: A potential threat to chilli crop in central Uttar Pradesh. Int. J. Pharmacogn. Phytochem. 2018, 7, 1970–1976. [Google Scholar]
- Oo, M.M.; Oh, S.-K. Chilli anthracnose (Colletotrichum spp.) disease and its management approach. Korean J. Agric. Sci. 2016, 43, 153–162. [Google Scholar] [CrossRef]
- Ray, S.; Singh, S.; Sarma, B.K.; Singh, H.B. Endophytic Alcaligenes Isolated from Horticultural and Medicinal Crops Promotes Growth in Okra (Abelmoschus esculentus). J. Plant Growth Regul. 2015, 35, 401–412. [Google Scholar] [CrossRef]
- Chowdappa, P.; Kumar, S.M.; Lakshmi, M.J.; Upreti, K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB. Biol. Control 2013, 65, 109–117. [Google Scholar] [CrossRef]
- Islam, S.; Akanda, A.M.; Prova, A.; Islam, M.T.; Hossain, M.M. Isolation and identification of plant growth promoting rhizo-bacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front. Microbiol. 2016, 6, 1360. [Google Scholar] [CrossRef] [Green Version]
- Gowtham, H.; Murali, M.; Singh, S.B.; Lakshmeesha, T.; Murthy, K.N.; Amruthesh, K.; Niranjana, S. Plant growth promoting rhizobacteria- Bacillus amyloliquefaciens improves plant growth and induces resistance in chilli against anthracnose disease. Biol. Control 2018, 126, 209–217. [Google Scholar] [CrossRef]
- Labuschagne, N.; Labuschagne, N.; Pretorius, T.; Pretorius, T.; Idris, A.H.; Idris, A.H. Plant Growth Promoting Rhizobacteria as Biocontrol Agents Against Soil-Borne Plant Diseases. In Beneficial Microorganisms in Food and Nutraceuticals; Springer Science and Business Media LLC: Berlin, Germany, 2010; pp. 211–230. [Google Scholar]
- Chet, I. Trichoderma: Ap plication, mode of action, and potential as biocontrol agent of soil borne plant pathogenic fungi. Innov. Approaches Plant Dis. Control 1987, 137–160. [Google Scholar]
- Howell, C.R. Cotton Seedling Preemergence Damping-Off Incited by Rhizopus oryzae and Pythium spp. and Its Biological Control with Trichoderma spp. Phytopathology 2002, 92, 177–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, A.; Mishra, S.; Ray, S.; Raghuwanshi, R.; Singh, H.B. Differential Reprogramming of Defense Network in Capsicum annum L. Plants Against Colletotrichum truncatum Infection by Phyllospheric and Rhizospheric Trichoderma Strains. J. Plant Growth Regul. 2020, 39, 751–763. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Del-Val, E.; Macías-Rodríguez, L.; Alarcón, A.; González-Esquivel, C.E.; Larsen, J. Trichoderma atroviride, a maize root associated fungus, increases the parasitism rate of the fall armyworm Spodoptera frugiperda by its natural enemy Campoletis sonorensis. Soil Biol. Biochem. 2018, 122, 196–202. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Raguchander, T.; Samiyappan, R. Enhancing Resistance of Tomato and Hot Pepper to Pythium Diseases by Seed Treatment with Fluorescent Pseudomonads. Eur. J. Plant Pathol. 2002, 108, 429–441. [Google Scholar] [CrossRef]
- Abhayashree, M.; Murali, M.; Amruthesh, K. Abiotic elicitors mediated resistance and enhanced defense related enzymes in Capsicum annuum L. against anthracnose disease. Sci. Hortic. 2016, 204, 172–178. [Google Scholar] [CrossRef]
- Mahesh, H.; Murali, M.; Pal, M.A.C.; Melvin, P.; Sharada, M. Salicylic acid seed priming instigates defense mechanism by inducing PR-Proteins in Solanum melongena L. upon infection with Verticillium dahliae Kleb. Plant Physiol. Biochem. 2017, 117, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Creissen, G.P.; Edwards, E.A.; Mullineaux, P.M. Glutathione reductase and ascorbate peroxidase. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants; CRC Press: Boca Raton, FL, USA, 1994; pp. 343–364. [Google Scholar]
- Scandalios, J.G.; Guan, L.; Polidoros, A.N. Catalases in plants: Gene structure, properties, regulation and expression. Cold Spring Harb. Monogr. Ser. 1997, 34, 343–406. [Google Scholar]
- Naziya, B.; Murali, M.; Amruthesh, K.N. Plant Growth-Promoting Fungi (PGPF) Instigate Plant Growth and Induce Disease Resistance in Capsicum annuum L. upon Infection with Colletotrichum capsici (Syd.) Butler & Bisby. Biomolecules 2019, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Gong, G.S.; Xu, Q.; Zhang, M.; Yang, J.Z.; Chen, H.B.; Shen, S.A.; Tang, T.F. A simple method for single fungal spore isolation. J. Maize Sci. 2010, 18, 126–127. [Google Scholar]
- Sharma, G.; Pinnaka, A.K.; Shenoy, B.D. Infra-specific diversity of Colletotrichum truncatum associated with chilli anthracnose in India based on microsatellite marker analysis. Arch. Phytopathol. Plant Prot. 2014, 47, 2509–2523. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Shinsky, J., White, T., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Zinniel, D.K.; Lambrecht, P.; Harris, N.B.; Feng, Z.; Kuczmarski, D.; Higley, P.; Ishimaru, C.A.; Arunakumari, A.; Barletta, R.G.; Vidaver, A.K. Isolation and Characterization of Endophytic Colonizing Bacteria from Agronomic Crops and Prairie Plants. Appl. Environ. Microbiol. 2002, 68, 2198–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, R.S.; Rai, B. Studies on antagonism between F. udum Butler and root region microflora of pigeonpea. Plant Soil 1987, 101, 79–93. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.; Irizarry, I.; Bergen, M.; Kharwar, R.N.; White, J.F., Jr. Seed-vectored endophytic bacteria modulate development of rice seedlings. J. Appl. Microbiol. 2017, 122, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Mukherjee, K.; Sarkar, A.; Acharya, K. Interaction between Bean and Colletotrichum gloeosporioides: Under-standing through a biochemical approach. Plants 2019, 8, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, D.D.; Ades, P.K.; Crous, P.W.; Taylor, P.W.J. Colletotrichum species associated with chili anthracnose in Australia. Plant Pathol. 2017, 66, 254–267. [Google Scholar] [CrossRef]
- Anaruma, N.D.; Schmidt, F.L.; Duarte, M.C.T.; Figueira, G.M.; Delarmelina, C.; Benato, E.A.; Sartoratto, A. Control of Colle-totrichum gloeosporioides (penz.) Sacc. in yellow passion fruit using Cymbopogon citratus essential oil. Braz. J. Microbiol. 2010, 41, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, S.D. Biology of Root-Infecting Fungi; Cambridge University Press: New York, NY, USA, 1956; Volume 97. [Google Scholar]
- Yuan, W.M.; Crawford, D.L. Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Appl. Environ. Microbiol. 1995, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Llorca, L.V.; Carbonell, T.; Salinas, J. Colonization of plant waste substrates by entomopathogenic and mycoparasitic fungi—A SEM study. Micron 1999, 30, 325–333. [Google Scholar] [CrossRef]
- Jain, A.; Singh, S.; Sarma, B.K.; Singh, H.B. Microbial consortium-mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. J. Appl. Microbiol. 2011, 112, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.S.A.; Romeiro, R.D.S.; Mounteer, A. Development of a Root Colonization Bioassay for Rapid Screening of Rhizobacteria for Potential Biocontrol Agents. J. Phytopathol. 2003, 151, 42–46. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Orlando, FL, USA, 1987; pp. 350–382. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Dickerson, D.P.; Pascholati, S.F.; Hagerman, A.E.; Butler, L.G.; Nicholson, R.L. Phenylalanine ammonia-lyase and hy-droxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol. Plant Pathol. 1984, 25, 111–123. [Google Scholar] [CrossRef]
- Arora, Y.K.; Bajaj, K.L. Peroxidase and Polyphenol Oxidase Associated with, Induced Resistance of Mung Bean to Rhizoctonia solani Kuhn. J. Phytopathol. 1985, 114, 325–331. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Orlando, FL, USA, 1984; pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Academic Press: Orlando, FL, USA, 1974; pp. 685–690. [Google Scholar]
- Zieslin, N.; Ben Zaken, R. Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol. Biochem. 1993, 31, 333–339. [Google Scholar]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Wang, C.-F.; Huang, L.-L.; Buchenauer, H.; Han, Q.-M.; Zhang, H.-C.; Kang, Z.-S. Histochemical studies on the accumulation of reactive oxygen species (O2− and H2O2) in the incompatible and compatible interaction of wheat—Puccinia striiformis f. sp. tritici. Physiol. Mol. Plant Pathol. 2007, 71, 230–239. [Google Scholar] [CrossRef]
- Murali, M.; Amruthesh, K.N. Plant growth-promoting fungus Penicillium oxalicum enhances plant growth and induces re-sistance in pearl millet against downy mildew disease. J. Phytopathol. 2015, 163, 743–754. [Google Scholar] [CrossRef]
- Williams, G.; Asher, M. Selection of rhizobacteria for the control of Pythium ultimum and Aphanomyces cochlioides on sugar-beet seedlings. Crop. Prot. 1996, 15, 479–486. [Google Scholar] [CrossRef]
- Wei, G.; Kloepper, J.W.; Tuzun, S. Induced systemic resistance to cucumber diseases and increased plant growth by plant growth-promoting rhizobacteria under field conditions. Phytopathology 1996, 86, 221–224. [Google Scholar] [CrossRef]
- Babu, A.N.; Jogaiah, S.; Ito, S.I.; Nagaraj, A.K.; Tran, L.S.P. Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of anti-oxidant peroxidase and polyphenol oxidase. Plant Sci. 2015, 231, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Abhayashree, M.S.; Murali, M.; Thriveni, M.C.; Sindhu, G.M.; Amruthesh, K.N. Crude oligosaccharides mediated resistance and histochemical changes in Capsicum annuum against anthracnose disease caused by Colletotrichum capsici. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016, 151, 221–233. [Google Scholar] [CrossRef]
- Radwan, D.E.M. Salicylic acid induced alleviation of oxidative stress caused by clethodim in maize (Zea mays L.) leaves. Pestic. Biochem. Physiol. 2012, 102, 182–188. [Google Scholar] [CrossRef]
- Lubaina, A.S.; Murugan, K. Ultrastructural changes and oxidative stress markers in wild and cultivar Sesamum orientale L. following Alternaria sesami (Kawamura) Mohanty and Behera. inoculation. Indian J. Exp. Boil. 2013, 51, 670–680. [Google Scholar]
- Saxena, A.; Raghuwanshi, R.; Singh, H.B. Elevation of defense network in chili against Colletotrichum capsici by phyllospheric Trichoderma strain. J. Plant Growth Regul. 2016, 35, 377–389. [Google Scholar] [CrossRef]















| Antagonists | Percent Inhibition of Radial Growth |
|---|---|
| Trichoderma harzianum | 75.46 ± 0.45 |
| Trichoderma asperellum | 73.09 ± 0.56 |
| Paenibacillus dendritiformis | 71.56 ± 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, M.; Dubey, M.K.; Upadhyay, R.S. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi 2021, 7, 307. https://doi.org/10.3390/jof7040307
Yadav M, Dubey MK, Upadhyay RS. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. Journal of Fungi. 2021; 7(4):307. https://doi.org/10.3390/jof7040307
Chicago/Turabian StyleYadav, Mukesh, Manish Kumar Dubey, and Ram Sanmukh Upadhyay. 2021. "Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis" Journal of Fungi 7, no. 4: 307. https://doi.org/10.3390/jof7040307
APA StyleYadav, M., Dubey, M. K., & Upadhyay, R. S. (2021). Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. Journal of Fungi, 7(4), 307. https://doi.org/10.3390/jof7040307