Investigating Host Preference of Root Endophytes of Three European Tree Species, with a Focus on Members of the Phialocephala fortinii—Acephala applanata Species Complex (PAC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Design
2.3. Isolation of Fungal Cultures
2.4. Classification of Fungal Cultures
2.5. Microsatellite Genotyping of DSE Isolates
2.6. Sequencing of Non-PAC DSE and Non-DSE Cultures
2.7. Statistical Analyses
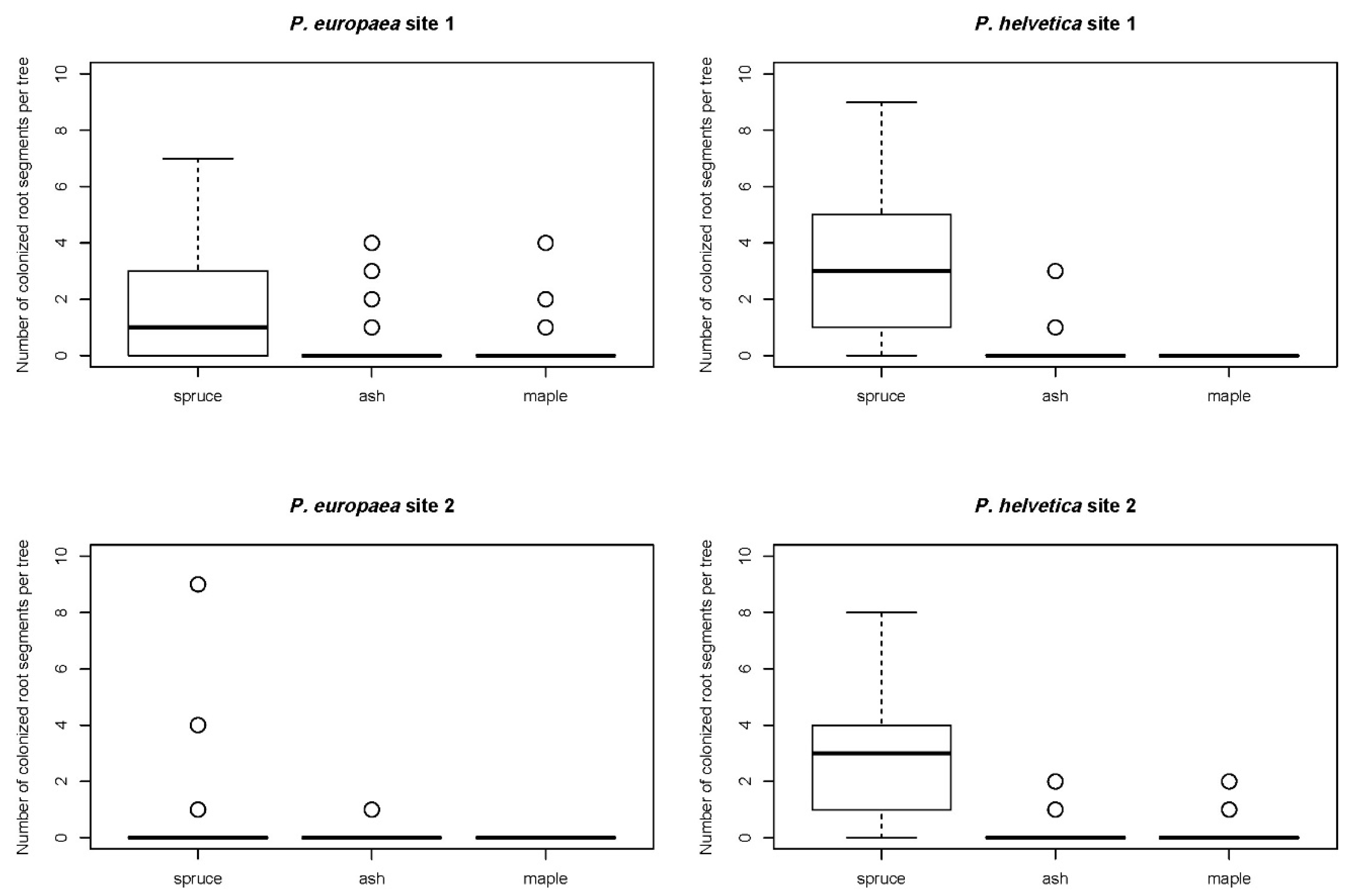
3. Results
3.1. PAC and Non-PAC DSE
3.1.1. PAC
3.1.2. Non-PAC DSE
3.2. Non-DSE
4. Discussion
4.1. PAC and Non-PAC DSE
4.1.1. PAC
4.1.2. Non-PAC DSE
4.2. Non-DSE
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Horton, T.R.; Bruns, T.D. Multiple-Host Fungi Are the Most Frequent and Abundant Ectomycorrhizal Types in a Mixed Stand of Douglas Fir (Pseudotsuga menziesii) and Bishop Pine (Pinus muricata). New Phytol. 1998, 139, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, P.G.; Izzo, A.D.; Bruns, T.D. There Is High Potential for the Formation of Common Mycorrhizal Networks between Understorey and Canopy Trees in a Mixed Evergreen Forest: Mycorrhizal Networks in Mixed Evergreen Forest. J. Ecol. 2003, 91, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Molina, R.; Trappe, J.M. Patterns of Ectomycorrhizal Host Specificity and Potential among Pacific Northwest Conifers and Fungi. For. Sci. 1982, 28, 423–458. [Google Scholar]
- Holliday, P. A Dictionary of Plant. Pathology, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1998; ISBN 978-0-521-59453-0. [Google Scholar]
- Zhou, D.; Hyde, K.D. Host-Specificity, Host-Exclusivity, and Host-Recurrence in Saprobic Fungi. Mycol. Res. 2001, 105, 1449–1457. [Google Scholar] [CrossRef]
- Ishida, T.A.; Nara, K.; Hogetsu, T. Host Effects on Ectomycorrhizal Fungal Communities: Insight from Eight Host Species in Mixed Conifer–Broadleaf Forests. New Phytol. 2007, 174, 430–440. [Google Scholar] [CrossRef]
- Tedersoo, L.; Jairus, T.; Horton, B.M.; Abarenkov, K.; Suvi, T.; Saar, I.; Kõljalg, U. Strong Host Preference of Ectomycorrhizal Fungi in a Tasmanian Wet Sclerophyll Forest as Revealed by DNA Barcoding and Taxon-Specific Primers. New Phytol. 2008, 180, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.L.; Busby, R.R.; Hoeksema, J.D. Host Preference of Ectomycorrhizal Fungi in Mixed Pine–Oak Woodlands. Can. J. For. Res. 2018, 48, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Roy-Bolduc, A.; Laliberte, E.; Hijri, M. High Richness of Ectomycorrhizal Fungi and Low Host Specificity in a Coastal Sand Dune Ecosystem Revealed by Network Analysis. Ecol. Evol. 2015, 6, 349–362. [Google Scholar] [CrossRef]
- Stoyke, G.; Currah, R.S. Endophytic Fungi from the Mycorrhizae of Alpine Ericoid Plants. Can. J. Bot. 1991, 69, 347–352. [Google Scholar] [CrossRef]
- Ahlich, K.; Sieber, T.N. The Profusion of Dark Septate Endophytic Fungi in Non-Ectomycorrhizal Fine Roots of Forest Trees and Shrubs. New Phytol. 1996, 132, 259–270. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark Septate Endophytes: A Review of Facultative Biotrophic Root-Colonizing Fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Grünig, C.R.; Queloz, V.; Sieber, T.N.; Holdenrieder, O. Dark Septate Endophytes (DSE) of the Phialocephala fortinii s.l.–Acephala applanata Species Complex in Tree Roots: Classification, Population Biology, and Ecology. Botany 2008, 86, 1355–1369. [Google Scholar] [CrossRef]
- Grünig, C.R.; Duò, A.; Sieber, T.N.; Holdenrieder, O. Assignment of Species Rank to Six Reproductively Isolated Cryptic Species of the Phialocephala fortinii s.l.- Acephala applanata Species Complex. Mycologia 2008, 100, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Sieber, T.; Grünig, C.R. Fungal Root Endophytes. In Plant Roots-The Hidden Half; Eshel, A., Beeckman, T., Eds.; CRC Press; Taylor and Francis Group: Boca Raton, FL, USA, 2013; pp. 38.31–38.49. ISBN 978-1-4398-4648-3. [Google Scholar]
- Grünig, C.R.; McDonald, B.A.; Sieber, T.N.; Rogers, S.O.; Holdenrieder, O. Evidence for Subdivision of the Root-Endophyte Phialocephala fortinii into Cryptic Species and Recombination within Species. Fungal Genet. Biol. 2004, 41, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Jumpponen, A. Spatial Distribution of Discrete RAPD Phenotypes of a Root Endophytic fungus, Phialocephala fortinii, at a Primary Successional Site on a Glacier Forefront. New Phytol. 1999, 141, 333–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addy, H.D.; Hambleton, S.; Currah, R.S. Distribution and Molecular Characterization of the Root Endophyte Phialocephala fortinii along an Environmental Gradient in the Boreal Forest of Alberta. Mycol. Res. 2000, 104, 1213–1221. [Google Scholar] [CrossRef]
- Ruotsalainen, A.L. Dark Septate Endophytes (DSE) in Boreal and Subarctic Forests. In Endophytes of Forest Trees: Biology and Applications; Pirttilä, A.M., Frank, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 105–117. ISBN 978-3-319-89833-9. [Google Scholar]
- Mandyam, K.; Jumpponen, A. Seeking the Elusive Function of the Root-Colonising Dark Septate Endophytic Fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef] [Green Version]
- Spagnoletti, F.N.; Tobar, N.E.; Fernández Di Pardo, A.; Chiocchio, V.M.; Lavado, R.S. Dark Septate Endophytes Present Different Potential to Solubilize Calcium, Iron and Aluminum Phosphates. Appl. Soil Ecol. 2017, 111, 25–32. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Chiocchio, V.M. Tolerance of Dark Septate Endophytic Fungi (DSE) to Agrochemicals in Vitro. Rev. Argent. Microbiol. 2020, 52, 43–49. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.E.C.; Alves, L.S.; de Souza, S.R.; Santos, L.A.; Santa-Catarina, C.; da Silva, K.; Pereira, G.M.D.; Xavier, G.R.; Zilli, J.É. Contribution of Dark Septate Fungi to the Nutrient Uptake and Growth of Rice Plants. Braz. J. Microbiol. 2018, 49, 67–78. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Liu, G.; Smith, J.M.; Zhao, Z. Unraveling the Role of Dark Septate Endophyte (DSE) Colonizing Maize (Zea Mays) under Cadmium Stress: Physiological, Cytological and Genic Aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.K.; Wilcox, H.E. New Species of Ectendomycorrhizal and Pseudomycorrhizal Fungi: Phialophora finlandia, Chloridium paucisporum, and Phialocephala fortinii. Mycologia 1985, 77, 951–958. [Google Scholar] [CrossRef]
- Grünig, C.R.; Sieber, T.N.; Rogers, S.O.; Holdenrieder, O. Spatial Distribution of Dark Septate Endophytes in a Confined Forest Plot. Mycol. Res. 2002, 106, 832–840. [Google Scholar] [CrossRef]
- Summerbell, R.C. Root Endophyte and Mycorrhizosphere Fungi of Black Spruce, Picea Mariana, in a Boreal Forest Habitat: Influence of Site Factors on Fungal Distributions. Stud. Mycol. 2005, 53, 121–145. [Google Scholar] [CrossRef] [Green Version]
- Sieber, T.N.; Grünig, C.R. Biodiversity of Fungal Root-Endophyte Communities and Populations, in Particular of the Dark Septate Endophyte Phialocephala fortinii s. l. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 9, pp. 107–132. ISBN 978-3-540-33525-2. [Google Scholar]
- Read, D.J.; Haselwandter, K. Observations on the Mycorrhizal Status of Some Alpine Plant Communities. New Phytol. 1981, 88, 341–352. [Google Scholar] [CrossRef]
- Grünig, C.R.; Linde, C.C.; Sieber, T.N.; Rogers, S.O. Development of Single-Copy RFLP Markers for Population Genetic Studies of Phialocephala fortinii and Closely Related Taxa. Mycol. Res. 2003, 107, 1332–1341. [Google Scholar] [CrossRef]
- Grünig, C.R.; Sieber, T.N. Molecular and Phenotypic Description of the Widespread Root Symbiont Acephala Applanata Gen. et Sp. Nov., Formerly Known as Dark-Septate Endophyte Type 1. Mycologia 2005, 97, 628–640. [Google Scholar] [CrossRef]
- Grünig, C.R.; Brunner, P.C.; Duò, A.; Sieber, T.N. Suitability of Methods for Species Recognition in the Phialocephala fortinii–Acephala applanata Species Complex Using DNA Analysis. Fungal Genet. Biol. 2007, 44, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Queloz, V.; Duò, A.; Grünig, C.R. Isolation and Characterization of Microsatellite Markers for the Tree-Root Endophytes Phialocephala subalpina and Phialocephala fortinii s.s. Mol. Ecol. Resour. 2008, 8, 1322–1325. [Google Scholar] [CrossRef]
- Landolt, M.; Stroheker, S.; Queloz, V.; Gall, A.; Sieber, T.N. Does Water Availability Influence the Abundance of Species of the Phialocephala fortinii s.l.–Acephala applanata Complex (PAC) in Roots of Pubescent Oak (Quercus Pubescens) and Scots Pine (Pinus Sylvestris)? Fungal Ecol. 2020, 44, 100904. [Google Scholar] [CrossRef]
- Queloz, V.; Grunig, C.R.; Sieber, T.N.; Holdenrieder, O. Monitoring the Spatial and Temporal Dynamics of a Community of the Tree-Root Endophyte Phialocephala fortinii s.l. New Phytol. 2005, 168, 651–660. [Google Scholar] [CrossRef]
- Queloz, V.; Sieber, T.N.; Holdenrieder, O.; McDonald, B.A.; Grünig, C.R. No Biogeographical Pattern for a Root-Associated Fungal Species Complex: Biogeography of a Fungal Species Complex. Glob. Ecol. Biogeogr. 2011, 20, 160–169. [Google Scholar] [CrossRef]
- Stroheker, S.; Queloz, V.; Sieber, T.N. Spatial and Temporal Dynamics in the Phialocephala fortinii s.l.–Acephala applanata Species Complex (PAC). Plant Soil 2016, 407, 231–241. [Google Scholar] [CrossRef]
- Grünig, C.R.; Duò, A.; Sieber, T.N. Population Genetic Analysis of Phialocephala fortinii s.l. and Acephala applanata in Two Undisturbed Forests in Switzerland and Evidence for New Cryptic Species. Fungal Genet. Biol. 2006, 43, 410–421. [Google Scholar] [CrossRef]
- Brenn, N.; Menkis, A.; Grünig, C.R.; Sieber, T.N.; Holdenrieder, O. Community Structure of Phialocephala fortinii s. Lat. in European Tree Nurseries, and Assessment of the Potential of the Seedlings as Dissemination Vehicles. Mycol. Res. 2008, 112, 650–662. [Google Scholar] [CrossRef]
- Stroheker, S.; Dubach, V.; Queloz, V.; Sieber, T.N. Resilience of Phialocephala fortinii s.l.–Acephala applanata Communities–Effects of Disturbance and Strain Introduction. Fungal Ecol. 2018, 31, 19–28. [Google Scholar] [CrossRef]
- Halmschlager, E.; Kowalski, T. The Mycobiota in Nonmycorrhizal Roots of Healthy and Declining Oaks. Can. J. Bot. 2004, 82, 1446–1458. [Google Scholar] [CrossRef]
- Zheng, J.-H.; Meng, Z.-B.; Kang, J.-C.; Lei, B.-X.; Li, Q.-R.; Wen, T.C. Diversity of Endophytic Fungi Associated with Ginkgo Biloba. Mycosystema 2013, 32, 671–681. [Google Scholar]
- Haddadderafshi, N.; Halasz, K.; Posa, T.; Peter, G.; Gaspar, L.; Lukacs, N. Diversity of Endophytic Fungi Isolated from Cherry (Prunus Avium). J. Hortic. For. Biotechnol. 2011, 15, 1–6. [Google Scholar]
- Queloz, V.; Duò, A.; Sieber, T.N.; Grünig, C.R. Microsatellite Size Homoplasies and Null Alleles Do Not Affect Species Diagnosis and Population Genetic Analysis in a Fungal Species Complex. Mol. Ecol. Resour. 2010, 10, 348–367. [Google Scholar] [CrossRef]
- Piry, S.; Alapetite, A.; Cornuet, J.-M.; Paetkau, D.; Baudouin, L.; Estoup, A. GENECLASS2: A Software for Genetic Assignment and First-Generation Migrant Detection. J. Hered. 2004, 95, 536–539. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, S. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In PCR-Protocols and Applications-A Laboratory Manual; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Queloz, V.; Grünig, C.R.; Berndt, R.; Kowalski, T.; Sieber, T.N.; Holdenrieder, O. Cryptic Speciation in Hymenoscyphus Albidus: Speciation in Hymenoscyphus Albidus. For. Pathol. 2011, 41, 133–142. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Statist. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80. [Google Scholar] [CrossRef]
- Menkis, A.; Allmer, J.; Vasiliauskas, R.; Lygis, V.; Stenlid, J.; Finlay, R. Ecology and Molecular Characterization of Dark Septate Fungi from Roots, Living Stems, Coarse and Fine Woody Debris. Mycol. Res. 2004, 108, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Stroheker, S. Community Dynamics and Transmission within the Phialocephala fortinii s.l.–Acephala applanata Species Complex. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2017. [Google Scholar]
- Queloz, V. La face cachée du Creux du Van. Société Jura. d’Émulation 2007 2008, 11, 47–68. [Google Scholar]
- Tedersoo, L.; Sadam, A.; Zambrano, M.; Valencia, R.; Bahram, M. Low Diversity and High Host Preference of Ectomycorrhizal Fungi in Western Amazonia, a Neotropical Biodiversity Hotspot. ISME J. 2010, 4, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickie, I.A. Host Preference, Niches and Fungal Diversity. New Phytol. 2007, 174, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Otgonsuren, B.; Lee, M.-J. Pinus Sylvestris Can Form Ectomycorrhiza with Phialocephala fortinii. Taiwan J. For. Sci. 2012, 27, 265–281. [Google Scholar]
- Münzenberger, B.; Bubner, B.; Wöllecke, J.; Sieber, T.N.; Bauer, R.; Fladung, M.; Hüttl, R.F. The Ectomycorrhizal Morphotype Pinirhiza Sclerotia Is Formed by Acephala macrosclerotiorum Sp. Nov., a Close Relative of Phialocephala fortinii. Mycorrhiza 2009, 19, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Currah, R.S.; Sigler, L.; Hambleton, S. New Records and New Taxa of Fungi from the Mycorrhizae of Terrestrial Orchids of Alberta. Can. J. Bot. 1987, 65, 2473–2482. [Google Scholar] [CrossRef]
- Kohout, P.; Sýkorová, Z.; Čtvrtlíková, M.; Rydlová, J.; Suda, J.; Vohník, M.; Sudová, R. Surprising Spectra of Root-Associated Fungi in Submerged Aquatic Plants. FEMS Microbiol. Ecol. 2012, 80, 216–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Shen, J.; Xu, L.; Gao, J.; Zhang, C.; Wang, Y.; Chen, F. A Metabolite of Endophytic Fungus Cadophora Orchidicola from Kalimeris Indica Serves as a Potential Fungicide and TLR 4 Agonist. J. Appl. Microbiol. 2019, 126, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Upson, R.; Newsham, K.K.; Bridge, P.D.; Pearce, D.A.; Read, D.J. Taxonomic Affinities of Dark Septate Root Endophytes of Colobanthus Quitensis and Deschampsia Antarctica, the Two Native Antarctic Vascular Plant Species. Fungal Ecol. 2009, 2, 184–196. [Google Scholar] [CrossRef]
- Fernando, A.; Currah, R.S. Leptodontidium Orchidicola (Mycelium-Radicis-Atrovirens Complex)–Aspects of Its Conidiogenesis and Ecology. Mycotaxon 1995, 54, 287–294. [Google Scholar]
- Wu, L.; Guo, S. Interaction between an Isolate of Dark-Septate Fungi and Its Host Plant Saussurea Involucrata. Mycorrhiza 2008, 18, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Domsch, K.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi, 2nd ed.; IHW: Eching, Germany, 2007. [Google Scholar]
- Kowalski, S. Role of Mycorrhiza and Soil Fungi in Natural Regeneration of Fir (Abies Alba Mill.) in Polish Carpathians and Sudetes. For. Pathol. 1982, 12, 107–112. [Google Scholar] [CrossRef]
- Unestam, T.; Beyer-Ericson, L.; Strand, M. Involvement of Cylindrocarpon Destructans in Root Death of Pinus Sylvestris Seedlings: Pathogenic Behaviour and Predisposing Factors. Scand. J. For. Res. 1989, 4, 521–535. [Google Scholar] [CrossRef]
- Booth, C. The Genus Cylindrocarpon. Mycol. Pap. 1966, 104, 1–56. [Google Scholar]
- Lyr, H.; Kluge, E. Zusammenhänge Zwischen Pathogenität, Enzym- Und Toxinproduktion Bei Cylindrocarpon Radicicola. J. Phytopathol. 1968, 62, 220–231. [Google Scholar] [CrossRef]
- Seifert, K.A.; McMullen, C.R.; Yee, D.; Reeleder, R.D.; Dobinson, K.F. Molecular Differentiation and Detection of Ginseng-Adapted Isolates of the Root Rot Fungus Cylindrocarpon Destructans. Phytopathology 2003, 93, 1533–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalski, T.; Bartnik, C. Cryptosporiopsis radicicola Sp. Nov. from Roots of Quercus Robur. Mycol. Res. 1995, 99, 663–666. [Google Scholar] [CrossRef]
- Kowalski, T.; Halmschlager, E.; Schrader, K. Cryptosporiopsis melanigena Sp. Nov., a Root-Inhabiting Fungus of Quercus Robur and Q. Petraea. Mycol. Res. 1998, 102, 347–354. [Google Scholar] [CrossRef]
- Sigler, L.; Allan, T.; Lim, S.R.; Berch, S.; Berbee, M. Two New Cryptosporiopsis Species from Roots of Ericaceous Hosts in Western North America. Stud. Mycol. 2005, 53, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Sieber, T.N. Fungal Root Endophytes. In Plant Roots: The Hidden Half; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA; Basel, Switzerland, 2002. [Google Scholar]
- Tellenbach, C.; Sieber, T.N. Do Colonization by Dark Septate Endophytes and Elevated Temperature Affect Pathogenicity of Oomycetes? FEMS Microbiol. Ecol. 2012, 82, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Miles, L.A.; Lopera, C.A.; Gonzalez, S.; Cepero de Garcìa, M.; Franco, A.; Restrepo, S. Exploring the Biocontrol Potential of Fungal Endophytes from an Andean Colombian Paramo Ecosystem. BioControl 2012, 57, 697–710. [Google Scholar] [CrossRef]
| Host | Sterile | DSE 1 | Cylindrocarpon spp. | Cryptosporiopsis spp. | Phomopsis spp. | Others | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Site 1 | Spruce | 49 | 10.9 | 268 | 59.6 | 43 | 9.6 | 12 | 2.7 | 42 | 9.3 | 38 | 8.4 |
| Ash | 164 | 36.4 | 63 | 14.0 | 44 | 9.8 | 44 | 9.8 | 4 | 0.9 | 131 | 29.1 | |
| Maple | 82 | 18.2 | 51 | 11.3 | 151 | 33.6 | 13 | 2.9 | 1 | 0.2 | 156 | 34.7 | |
| Total | 295 | 21.9 | 382 | 28.3 | 238 | 17.6 | 69 | 5.1 | 47 | 3.5 | 325 | 24.1 | |
| Site 2 | Spruce | 58 | 12.9 | 210 | 46.7 | 41 | 9.1 | 47 | 10.4 | 20 | 4.4 | 78 | 17.3 |
| Ash | 201 | 44.7 | 19 | 4.2 | 66 | 14.7 | 16 | 3.6 | 0 | 0.0 | 155 | 34.4 | |
| Maple | 97 | 21.6 | 4 | 0.9 | 116 | 25.8 | 27 | 6.0 | 0 | 0.0 | 209 | 46.4 | |
| Total | 356 | 26.4 | 233 | 17.3 | 223 | 16.5 | 90 | 6.7 | 20 | 1.5 | 442 | 32.7 | |
| Total | all | 651 | 24.1 | 615 | 22.8 | 461 | 17.1 | 159 | 5.9 | 67 | 2.5 | 767 | 28.4 |
| TOTAL: 2720 2 (100.7%) | |||||||||||||
| Spruce | Ash | Maple | Totals | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAC 1 Species | Site 1 | Site 2 | Total | Site 1 | Site 2 | Total | Site 1 | Site 2 | Total | Total Isolates | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| P. turicensis (CSP11) | 9 | 2 | 6 | 1.3 | 15 | 1.7 | 0 | 0 | 1 | 0.2 | 1 | 0.1 | 2 | 0.4 | 0 | 0 | 2 | 0.2 | 18 | 0.67 |
| P. letzii (CSP2) | 3 | 0.7 | 13 | 2.9 | 16 | 1.8 | 0 | 0 | 1 | 0.2 | 1 | 0.1 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 18 | 0.67 |
| P. europaea (CSP3) | 80 | 17.8 | 21 | 4.7 | 101 | 11.2 | 18 | 4 | 1 | 0.2 | 19 | 2.1 | 21 | 4.7 | 0 | 0 | 21 | 2.3 | 141 | 5.22 |
| P. helvetica (CSP4) | 146 | 32.4 | 143 | 31.8 | 289 | 32.1 | 6 | 1.3 | 8 | 1.8 | 14 | 1.6 | 0 | 0 | 3 | 0.7 | 3 | 0.3 | 306 | 11.33 |
| P. subalpina (CSP6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 1 | 0.04 |
| P. fortinii s.s. (CSP7) | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.04 |
| CSP8 | 0 | 0 | 1 | 0.2 | 1 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.04 |
| CSP12 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 9 | 2 | 0 | 0 | 9 | 1 | 11 | 0.41 |
| CSP13 | 2 | 0.4 | 0 | 0 | 2 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.07 |
| CSP14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 1 | 0.04 |
| A. applanata | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.04 |
| Sum isolates | 242 | 53.8 | 184 | 40.9 | 426 | 47.3 | 26 | 5.8 | 11 | 2.4 | 37 | 4.1 | 35 | 7.8 | 3 | 0.7 | 38 | 4.2 | 501 | 18.56 |
| Sum species | 7 | 5 | 8 | 4 | 4 | 6 | 6 | 1 | 7 | 11 | ||||||||||
| Spruce | Ash | Maple | |
|---|---|---|---|
| Spruce | . | 0.0008 ** | 0.002 * |
| Ash | 0.0039 * | . | 0.7476 |
| Maple | 0.0009 ** | 0.3282 | . |
| Taxon | Site 1 | Site 2 | ||
|---|---|---|---|---|
| Ash | Maple | Ash | Maple | |
| PAC | 26 | 35 | 11 | 3 |
| C. orchidicola | 13 | - | 6 | 1 |
| Phomasp. | 27 | 6 | 1 | 7 |
| Rhexocercosporidiumsp. | 2 | - | - | - |
| Spruce | Ash | Maple | |
|---|---|---|---|
| Spruce | . | <0.0001 *** | <0.0001 *** |
| Ash | <0.0001 *** | . | 0.1596 |
| Maple | <0.0001 *** | 0.1478 | . |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroheker, S.; Dubach, V.; Vögtli, I.; Sieber, T.N. Investigating Host Preference of Root Endophytes of Three European Tree Species, with a Focus on Members of the Phialocephala fortinii—Acephala applanata Species Complex (PAC). J. Fungi 2021, 7, 317. https://doi.org/10.3390/jof7040317
Stroheker S, Dubach V, Vögtli I, Sieber TN. Investigating Host Preference of Root Endophytes of Three European Tree Species, with a Focus on Members of the Phialocephala fortinii—Acephala applanata Species Complex (PAC). Journal of Fungi. 2021; 7(4):317. https://doi.org/10.3390/jof7040317
Chicago/Turabian StyleStroheker, Sophie, Vivanne Dubach, Irina Vögtli, and Thomas N. Sieber. 2021. "Investigating Host Preference of Root Endophytes of Three European Tree Species, with a Focus on Members of the Phialocephala fortinii—Acephala applanata Species Complex (PAC)" Journal of Fungi 7, no. 4: 317. https://doi.org/10.3390/jof7040317
APA StyleStroheker, S., Dubach, V., Vögtli, I., & Sieber, T. N. (2021). Investigating Host Preference of Root Endophytes of Three European Tree Species, with a Focus on Members of the Phialocephala fortinii—Acephala applanata Species Complex (PAC). Journal of Fungi, 7(4), 317. https://doi.org/10.3390/jof7040317







