Candida albicans/Macrophage Biointerface on Human and Porcine Decellularized Adipose Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Human and Porcine Adipose Tissue Decellularization
2.2. Characterization of DAMs and Coating Preparation
2.3. Culture of RAW-264.7 Macrophages on DAMs
2.4. Morphological Studies of Macrophages by Scanning Electron Microscopy (SEM)
2.5. Candida albicans Strain
2.6. Confocal Microscopy Studies of Candida albicans/Macrophage Biointerface on Human and Porcine DAMs
2.7. Detection of TNF-α and IL-6 as Inflammatory Cytokines
2.8. Statistics
3. Results and Discussion
3.1. Characterization of DAMs
3.2. Adhesion of Macrophages on DAMs
3.3. Candida albicans/Macrophage Biointerface on Human or Porcine DAMs
3.4. TNFα and IL-6 Production by RAW-264.7 Macrophages Cultured on Human or Porcine DAMs after Candida albicans Interaction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hebecker, B.; Naglik, J.R.; Hube, B.; Jacobsen, I.D. Pathogenicity mechanisms and host response during oral Candida albicans infections. Expert Rev. Anti-Infect. Ther. 2014, 12, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 73, 1445–1456. [Google Scholar] [CrossRef]
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A. Vulvovaginal Candida albicans infections: Pathogenesis, immunity and vaccine prospects. BJOG. 2015, 122, 785–794. [Google Scholar] [CrossRef]
- Chaffin, W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008, 72, 495–544. [Google Scholar] [CrossRef]
- Moyes, D.L.; Richardson, J.P.; Naglik, J.R. Candida albicans-epithelial interactions and pathogenicity mechanisms: Scratching the surface. Virulence 2015, 6, 338–346. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Luig, M.; Kluger, M.A.; Goerke, B.; Meyer, M.; Nosko, A.; Yan, I.; Scheller, J.; Mittrücker, H.W.; Rose-John, S.; Stahl, R.A.; et al. Inflammation-Induced IL-6 Functions as a Natural Brake on Macrophages and Limits GN. J. Am. Soc. Nephrol. 2015, 26, 1597–1607. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Gorshkova, E.A.; Namakanova, O.A.; Zvartsev, R.V.; Hidalgo, J.; Drutskaya, M.S.; Tumanov, A.V.; Nedospasov, S.A. Non-redundant Functions of IL-6 Produced by Macrophages and Dendritic Cells in Allergic Airway Inflammation. Front. Immunol. 2018, 9, 2718. [Google Scholar] [CrossRef] [PubMed]
- Rougerie, P.; Miskolci, V.; Cox, D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: Role and regulation of the actin cytoskeleton. Immunol. Rev. 2013, 256, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Barger, S.R.; Reilly, N.S.; Shutova, M.S.; Li, Q.; Maiuri, P.; Heddleston, J.M.; Mooseker, M.S.; Flavell, R.A.; Svitkina, T.; Oakes, P.W.; et al. Membrane-cytoskeletal crosstalk mediated by myosin-I regulates adhesion turnover during phagocytosis. Nat. Commun. 2019, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.C.; Bender, J.A.; Fink, G.R. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 2004, 3, 1076–1087. [Google Scholar] [CrossRef]
- Fernández-Arenas, E.; Cabezón, V.; Bermejo, C.; Arroyo, J.; Nombela, C.; Diez-Orejas, R.; Gil, C. Integrated proteomics and genomics strategies bring new insight into Candida albicans response upon macrophage interaction. Mol. Cell. Proteomics 2007, 6, 460–478. [Google Scholar] [CrossRef] [PubMed]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Lewis, L.E.; Bain, J.M.; Lowes, C.; Gillespie, C.; Rudkin, F.M.; Gow, N.A.; Erwig, L.P. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012, 8, e1002578. [Google Scholar] [CrossRef]
- Jung, J.; Zeng, H.; Horng, T. Metabolism as a guiding force for immunity. Nat. Cell Biol. 2019, 21, 85–93. [Google Scholar] [CrossRef]
- Yan, J.; Horng, T. Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. 2020, 30, 979–989. [Google Scholar] [CrossRef]
- Huang, S.C.; Everts, B.; Ivanova, Y.; O’sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sassetti, C.M. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. USA 2008, 105, 4376–4380. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: Factors that influence the host response. Ann. Biomed. Eng. 2014, 42, 517–1527. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, S.; Chameettachal, S.; Cromer, B.; Pati, F.; Kingshott, P. Decellularized extracellular matrix hydrogels—Cell behavior as a function of matrix stiffness. Curr. Opin. Biomed. Eng. 2019, 10, 123–133. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Chaubal, M.; Hotchkiss, K.M.; Olivares-Navarrete, R. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials 2020, 243, 119920. [Google Scholar] [CrossRef]
- Yu, C.; Kornmuller, A.; Brown, C.; Hoare, T.; Flynn, L. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 2017, 120, 66–80. [Google Scholar] [CrossRef]
- Ibsirlioglu, T.; Elçin, A.E.; Elçin, Y.M. Decellularized biological scaffold and stem cells from autologous human adipose tissue for cartilage tissue engineering. Methods 2020, 171, 97–107. [Google Scholar] [CrossRef]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef]
- Dominic, M.; Handleton, R.; Shazly, T.; Matthews, M. A novel supercritical CO2-based decellularization method for maintaining scaffold hydration and mechanical properties. J. Supercrit. Fluids 2018, 131, 72–81. [Google Scholar]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Dembo, M.; Torney, D.; Saxman, K.; Hammer, D. The kinetics of membrane-to-surface adhesión and detachment. Proc. R. Soc. 1988, 234, 55–83. [Google Scholar]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed]
- Sagvolden, G.; Giaever, I.; Pettersen, E.O.; Feder, J. Cell adhesion force microscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Cicuéndez, M.; Casarrubios, L.; Feito, M.J.; Madarieta, I.; Garcia-Urkia, N.; Murua, O.; Olalde, B.; Briz, N.; Diez-Orejas, R.; Portolés, M.T. Effects of Human and Porcine Adipose Extracellular Matrices Decellularized by Enzymatic or Chemical Methods on Macrophage Polarization and Immunocompetence. Int. J. Mol. Sci. 2021, 22, 3847. [Google Scholar] [CrossRef]
- Gillum, A.M.; Tsay, E.Y.; Kirsch, D.R. Isolation of the Candida albicans gene for orotidine-5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef]
- Prieto, D.; Román, E.; Correia, I.; Pla, J. The HOG pathway is critical for the colonization of the mouse gastrointestinal tract by Candida albicans. PLoS ONE 2014, 9, e87128. [Google Scholar] [CrossRef]
- Yaoa, Q.; Zheng, Y.-W.; Lan, Q.-H.; Kou, L.; Xu, H.L.; Zhao, Y.-Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C 2019, 104, 109942. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol. Life Sci. 2015, 72, 1303–1316. [Google Scholar] [CrossRef]
- Mattila, P.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Niedergang, F.; Chavrier, P. Signaling and membrane dynamics during phagocytosis: Many roads lead to the phagos(R)ome. Curr. Opin. Cell Biol. 2004, 16, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kress, H.; Stelzer, E.H.; Holzer, D.; Buss, F.; Griffiths, G.; Rohrbach, A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc. Natl. Acad. Sci. USA 2007, 104, 11633–11638. [Google Scholar] [CrossRef]
- Vonna, L.; Wiedemann, A.; Aepfelbacher, M.; Sackmann, E. Micromechanics of filopodia mediated capture of pathogens by macrophages. Eur. Biophys. J. 2007, 36, 145–151. [Google Scholar] [CrossRef]
- Koyama, Y.; Norose-Toyoda, K.; Hirano, S.; Kobayashi, M.; Ebihara, T.; Someki, I.; Fijisaki, H.; Irie, S. Type I collagen is a non-adhesive extracellular matrix for macrophages. Arch. Histol. Cytol. 2000, 63, 71–79. [Google Scholar] [CrossRef]
- Gowen, B.B.; Borg, T.K.; Ghaffar, A.; Mayer, E.P. Selective adhesion of macrophages to denatured forms of type I collagen is mediated by scavenger receptors. Matrix Biol. 2000, 19, 61–71. [Google Scholar] [CrossRef]
- Mazur, A.; Holthoff, E.; Vadali, S.; Kelly, T.; Post, S.R. Cleavage of Type I Collagen by Fibroblast Activation Protein-α Enhances Class A Scavenger Receptor Mediated Macrophage Adhesion. PLoS ONE 2016, 11, e0150287. [Google Scholar] [CrossRef]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Bahr, J.C.; Weiss, S.J. Macrophage-Dependent Tracking and Remodeling of the Basement Membrane-Interstitial Matrix Interface. BioRxiv 2018, 364422. [Google Scholar]
- Etich, J.; Koch, M.; Wagener, R.; Zaucke, F.; Fabri, M.; Brachvogel, B. Gene Expression Profiling of the Extracellular Matrix Signature in Macrophages of Different Activation Status: Relevance for Skin Wound Healing. Int. J. Mol. Sci. 2019, 20, 5086. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Bromberg, J.S. Regulation of the immune system by laminins. Trends Immunol. 2017, 38, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M.; Messier, J.M.; Mercurio, A.M. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the α6β1 integrin. J. Cell Biol. 1990, 110, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Iorio, V.; Troughton, L.D.; Hamill, K.J. Laminins: Roles and Utility in Wound Repair. Adv. Wound Care 2015, 4, 250. [Google Scholar] [CrossRef]
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 2163. [Google Scholar] [CrossRef]
- Schiavinato, A.; Keene, D.R.; Wohl, A.P.; Corallo, D.; Colombatti, A.; Wagener, R.; Paulsson, M.; Bonaldo, P.; Sengle, G. Targeting of EMILIN-1 and EMILIN-2 to Fibrillin Microfibrils Facilitates their Incorporation into the Extracellular Matrix. J. Investig. Dermatol. 2016, 136, 1150–1160. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Davis, E.C.; Starcher, B.C.; Ouchi, T.; Yanagisawa, M.; Richardson, J.A.; Olson, E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 2002, 415, 168–171. [Google Scholar] [CrossRef]
- Wohner, N.; Muczynski, V.; Mohamadi, A.; Legendre, P.; Proulle, V.; Aymé, G.; Christophe, O.D.; Lenting, P.J.; Denis, C.V.; Casari, C. Macrophage scavenger receptor SR-AI contributes to the clearance of von Willebrand factor. Haematologica 2018, 103, 728–737. [Google Scholar] [CrossRef]
- Adair-Kirk, T.L.; Senior, R.M. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 2008, 40, 1101–1110. [Google Scholar] [CrossRef]
- Boyd, D.F.; Thomas, P.G. Towards integrating extracellular matrix and immunological pathways. Cytokine 2017, 98, 79–86. [Google Scholar] [CrossRef]
- Gerisch, G.; Ecke, M.; Schroth-Diez, B.; Gerwig, S.; Engel, U.; Maddera, L.; Clarke, M. Self-organizing actin waves as planar phagocytic cup structures. Cell Adhes. Migr. 2009, 3, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Prashar, A.; Bhatia, S.; Gigliozzi, D.; Martin, T.; Duncan, C.; Guyard, C.; Terebiznik, M.R. Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages. J. Cell Biol. 2013, 203, 1081–1097. [Google Scholar] [CrossRef]
- Ashwini, N.A.; Sachin, V.S.; Yogesh, S.S.; Jomon, J.; Milind, S.P.; Rajendra, L.D. Association of small Rho GTPases and actin ring formation in epithelial cells during the invasion by Candida albicans. FEMS Immunol. Med. Microbiol. 2009, 55, 74–84. [Google Scholar]
- Tsarfaty, I.; Sandovsky-Losica, H.; Mittelman, L.; Berdicevsky, I.; Segal, E. Cellular actin is affected by interaction with Candida albicans. FEMS Microbiol. Lett. 2000, 189, 225–232. [Google Scholar] [CrossRef]
- Sandovsky-Losica, H.; Segal, E. Infection of Hep2 epithelial cells with Candida albicans: Adherence and postadherence events. FEMS Immunol. Med. Microbiol. 2006, 46, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Maxson, M.E.; Naj, X.; O’Meara, T.R.; Plumb, J.D.; Cowen, L.E.; Grinstein, S. Integrin-based diffusion barrier separates membrane domains enabling the formation of microbiostatic frustrated phagosomes. ELife 2018, 7, e34798. [Google Scholar] [CrossRef]
- Gerlitz, G.; Bustin, M. Efficient cell migration requires global chromatin condensation. J. Cell Sci. 2010, 123, 2207–2217. [Google Scholar] [CrossRef]
- Chua, T.K.; Ramdas, N.M.; Shivashankar, G.V. Actin cytoskeleton differentially alters the dynamics of lamin A, HP1α and H2B core histone proteins to remodel chromatin condensation state in living cells. Integr. Biol. 2015, 7, 1309–1317. [Google Scholar]
- Xie, S.X.; Mahmood, R.; Gjorgjieva, T.; Percipalle, P. Emerging roles of cytoskeletal proteins in regulating gene expression and genoma organization during differentiation. Nucleus 2020, 11, 53–65. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Choy, E.; Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef]
- Luu, T.U.; Liu, W.F. Regulation of Macrophages by Extracellular Matrix Composition and Adhesion Geometry. Regen. Eng. Transl. Med. 2018, 4, 238–246. [Google Scholar] [CrossRef]
- Reales-Calderón, J.A.; Aguilera-Montilla, N.; Corbí, A.L.; Molero, G.; Gil, C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics 2014, 14, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, U.; Yang, M.; Matundan, H.H.; Hirose, S.; Shah, P.K.; Sharifi, B.G.; Ghiasiet, H. Increased phagocytosis in the presence of enhanced M2-like macrophage responses correlates with increased primary and latent HSV-1 infection. PLoS Pathog. 2020, 16, e1008971. [Google Scholar] [CrossRef]
- Tomlin, H.; Piccinini, A.M. A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology 2018, 155, 186–201. [Google Scholar] [CrossRef] [PubMed]
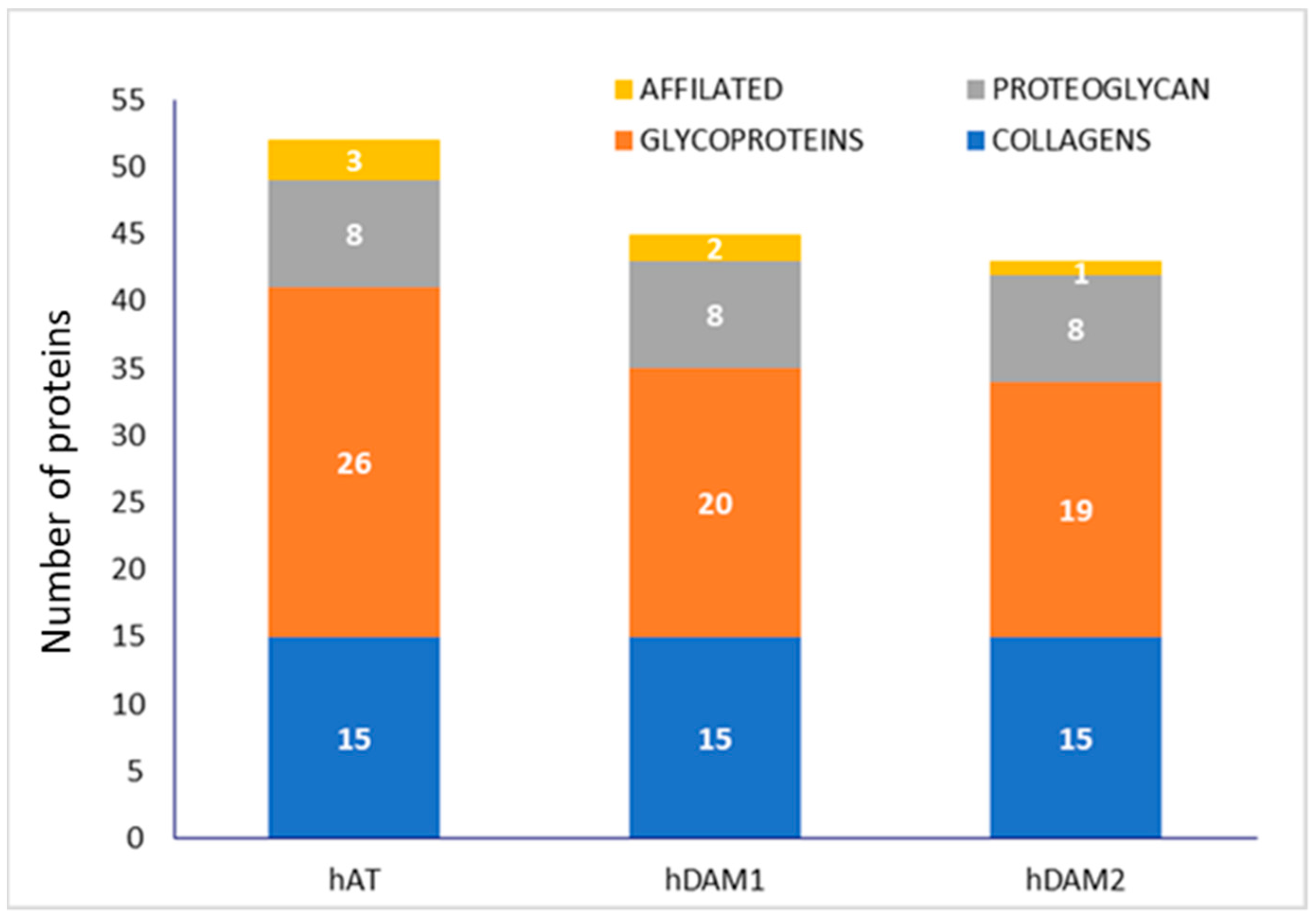
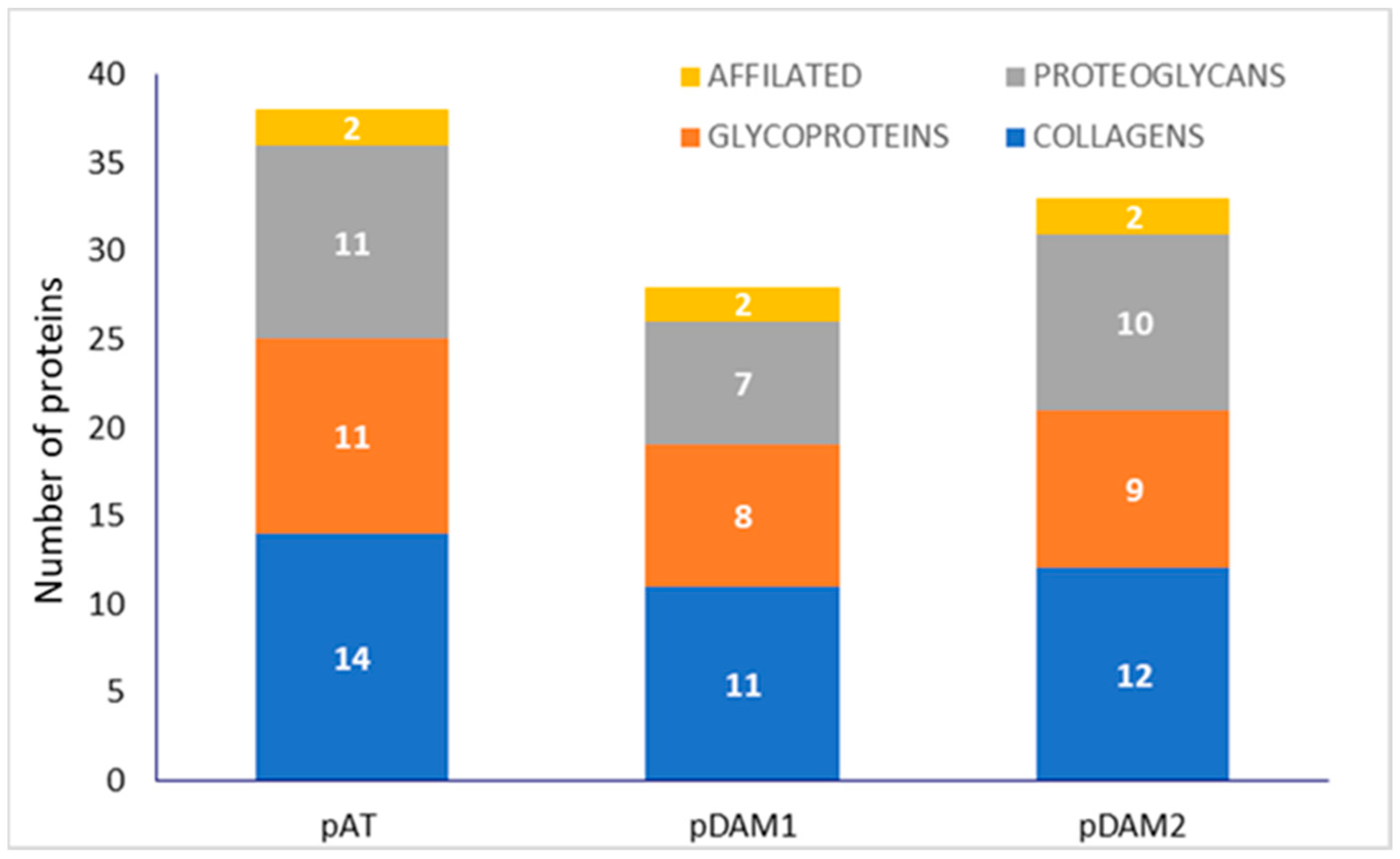
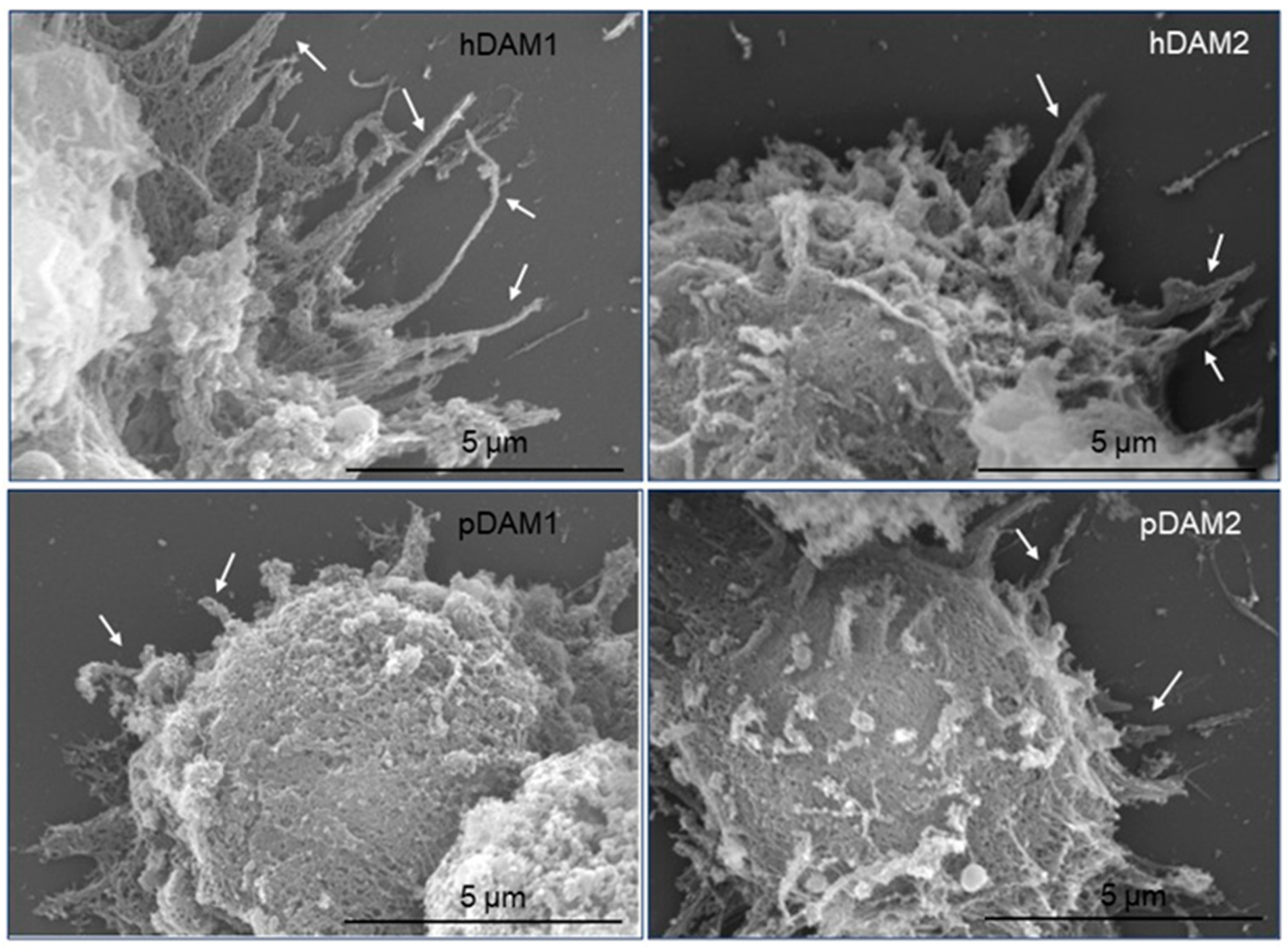


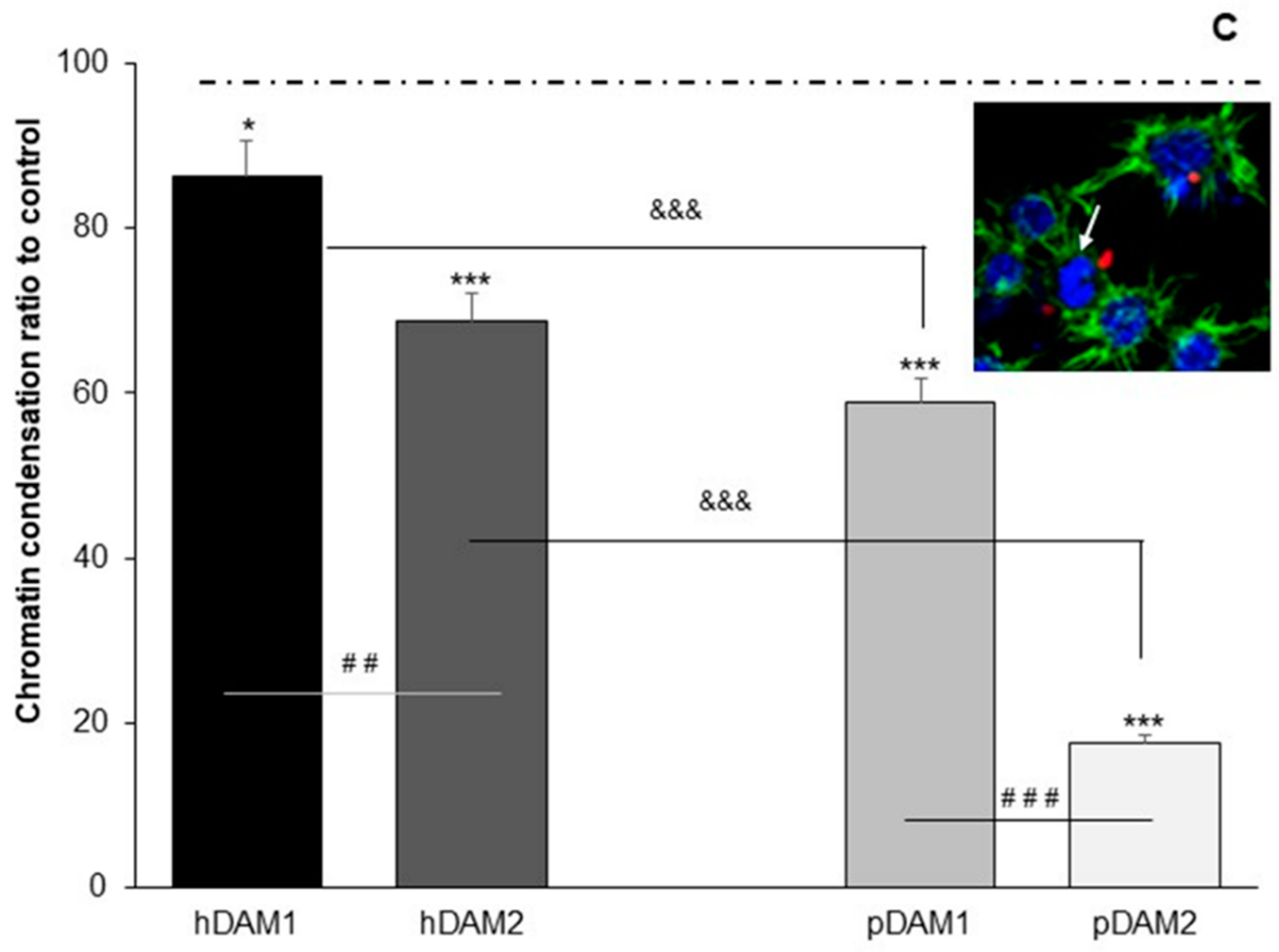

| PROTEINS | DAMs | |||
|---|---|---|---|---|
| hDAM1 | hDAM2 | pDAM1 | pDAM2 | |
| Collagens | ||||
| Collagen α-1(I) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-1(III) chain | ✓ | ✓ | ||
| Collagen α-1(IV) chain | ✓ | ✓ | ||
| Collagen α-1(V) chain | ✓ | ✓ | ||
| Collagen α-1(VI) chain | ✓ | ✓ | ✓ | |
| Collagen α-1(XII) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-1(XIV) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-1(XV) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-1(XVIII) chain | ✓ | ✓ | ||
| Collagen α-2 (I) chain | ✓ | ✓ | ||
| Collagen α-2 (IV) chain | ✓ | ✓ | ✓ | |
| Collagen α-2 (V) chain | ✓ | ✓ | ✓ | |
| Collagen α-2(VI) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-3 (V) chain | ✓ | ✓ | ||
| Collagen α-3 (VI) chain | ✓ | ✓ | ✓ | ✓ |
| Laminins | ||||
| Laminin α-2 | ✓ | ✓ | ||
| Laminin α-3 | ✓ | |||
| Laminin α-4 | ✓ | ✓ | ||
| Laminin α-5 | ✓ | ✓ | ||
| Laminin β-1 | ✓ | ✓ | ✓ | ✓ |
| Laminin β-2 | ✓ | ✓ | ✓ | ✓ |
| Laminin γ-1 | ✓ | ✓ | ✓ | |
| EMILIN | ||||
| Emilin-1 | ✓ | ✓ | ||
| Fibulins | ||||
| Fibulin-1 | ✓ | |||
| Fibulin-2 | ✓ | ✓ | ||
| Fibulin-5 | ✓ | ✓ | ✓ | |
| von Willebrand factor | ✓ | ✓ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicuéndez, M.; Casarrubios, L.; Feito, M.J.; Madarieta, I.; Garcia-Urkia, N.; Murua, O.; Olalde, B.; Briz, N.; Diez-Orejas, R.; Portolés, M.T. Candida albicans/Macrophage Biointerface on Human and Porcine Decellularized Adipose Matrices. J. Fungi 2021, 7, 392. https://doi.org/10.3390/jof7050392
Cicuéndez M, Casarrubios L, Feito MJ, Madarieta I, Garcia-Urkia N, Murua O, Olalde B, Briz N, Diez-Orejas R, Portolés MT. Candida albicans/Macrophage Biointerface on Human and Porcine Decellularized Adipose Matrices. Journal of Fungi. 2021; 7(5):392. https://doi.org/10.3390/jof7050392
Chicago/Turabian StyleCicuéndez, Mónica, Laura Casarrubios, María José Feito, Iratxe Madarieta, Nerea Garcia-Urkia, Olatz Murua, Beatriz Olalde, Nerea Briz, Rosalía Diez-Orejas, and María Teresa Portolés. 2021. "Candida albicans/Macrophage Biointerface on Human and Porcine Decellularized Adipose Matrices" Journal of Fungi 7, no. 5: 392. https://doi.org/10.3390/jof7050392
APA StyleCicuéndez, M., Casarrubios, L., Feito, M. J., Madarieta, I., Garcia-Urkia, N., Murua, O., Olalde, B., Briz, N., Diez-Orejas, R., & Portolés, M. T. (2021). Candida albicans/Macrophage Biointerface on Human and Porcine Decellularized Adipose Matrices. Journal of Fungi, 7(5), 392. https://doi.org/10.3390/jof7050392







