Candida albicans/Macrophage Biointerface on Human and Porcine Decellularized Adipose Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human and Porcine Adipose Tissue Decellularization
2.2. Characterization of DAMs and Coating Preparation
2.3. Culture of RAW-264.7 Macrophages on DAMs
2.4. Morphological Studies of Macrophages by Scanning Electron Microscopy (SEM)
2.5. Candida albicans Strain
2.6. Confocal Microscopy Studies of Candida albicans/Macrophage Biointerface on Human and Porcine DAMs
2.7. Detection of TNF-α and IL-6 as Inflammatory Cytokines
2.8. Statistics
3. Results and Discussion
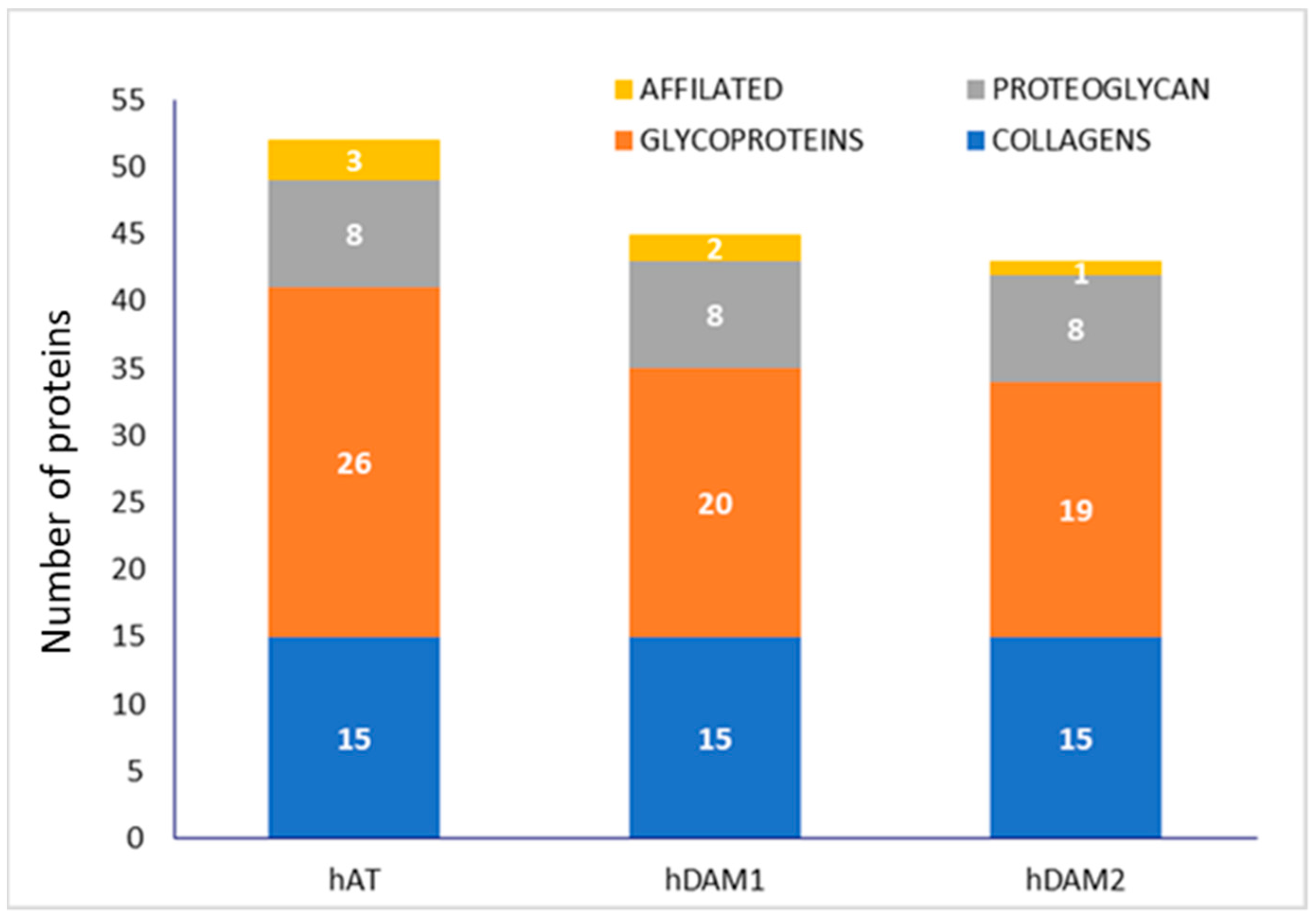
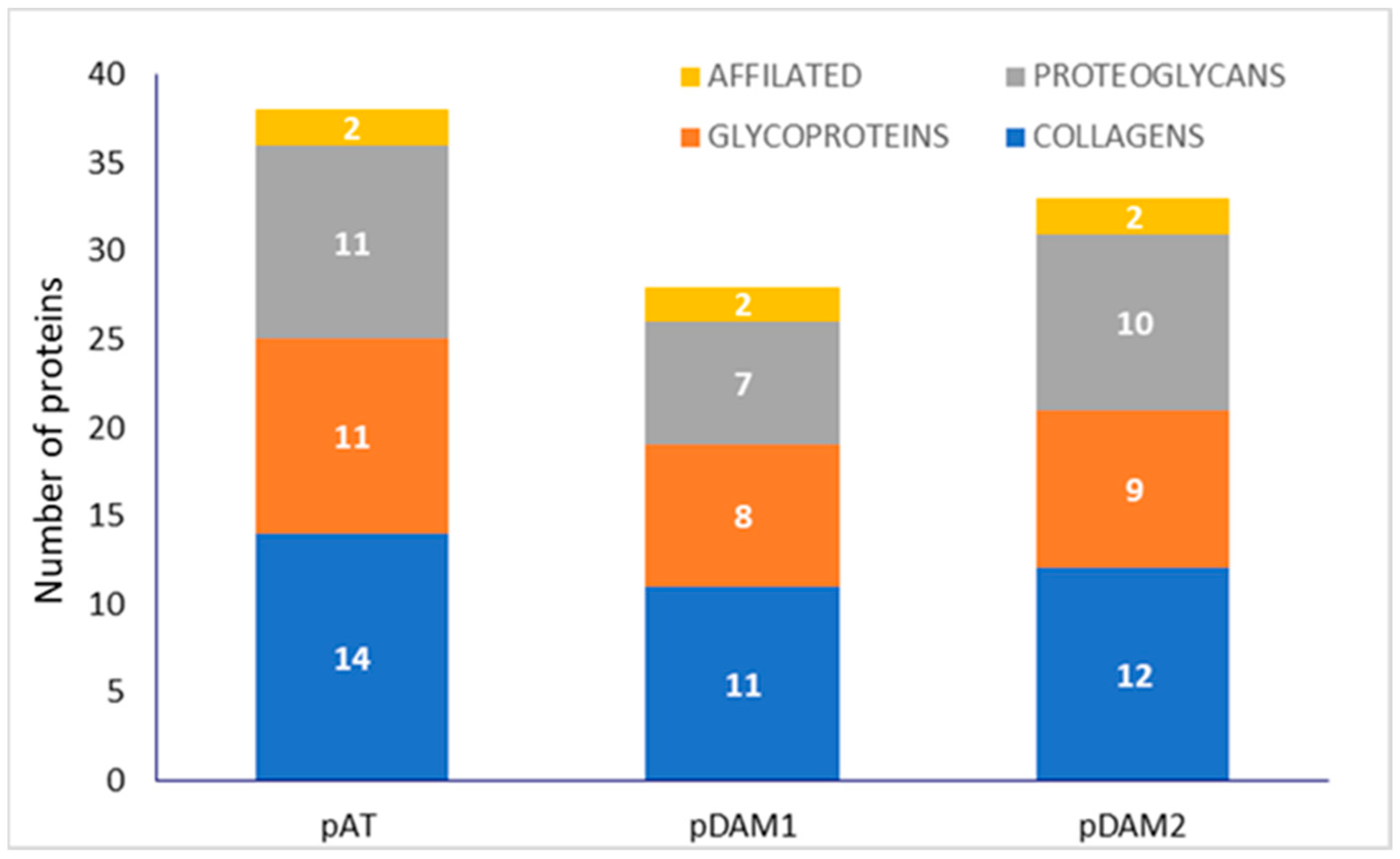
3.1. Characterization of DAMs
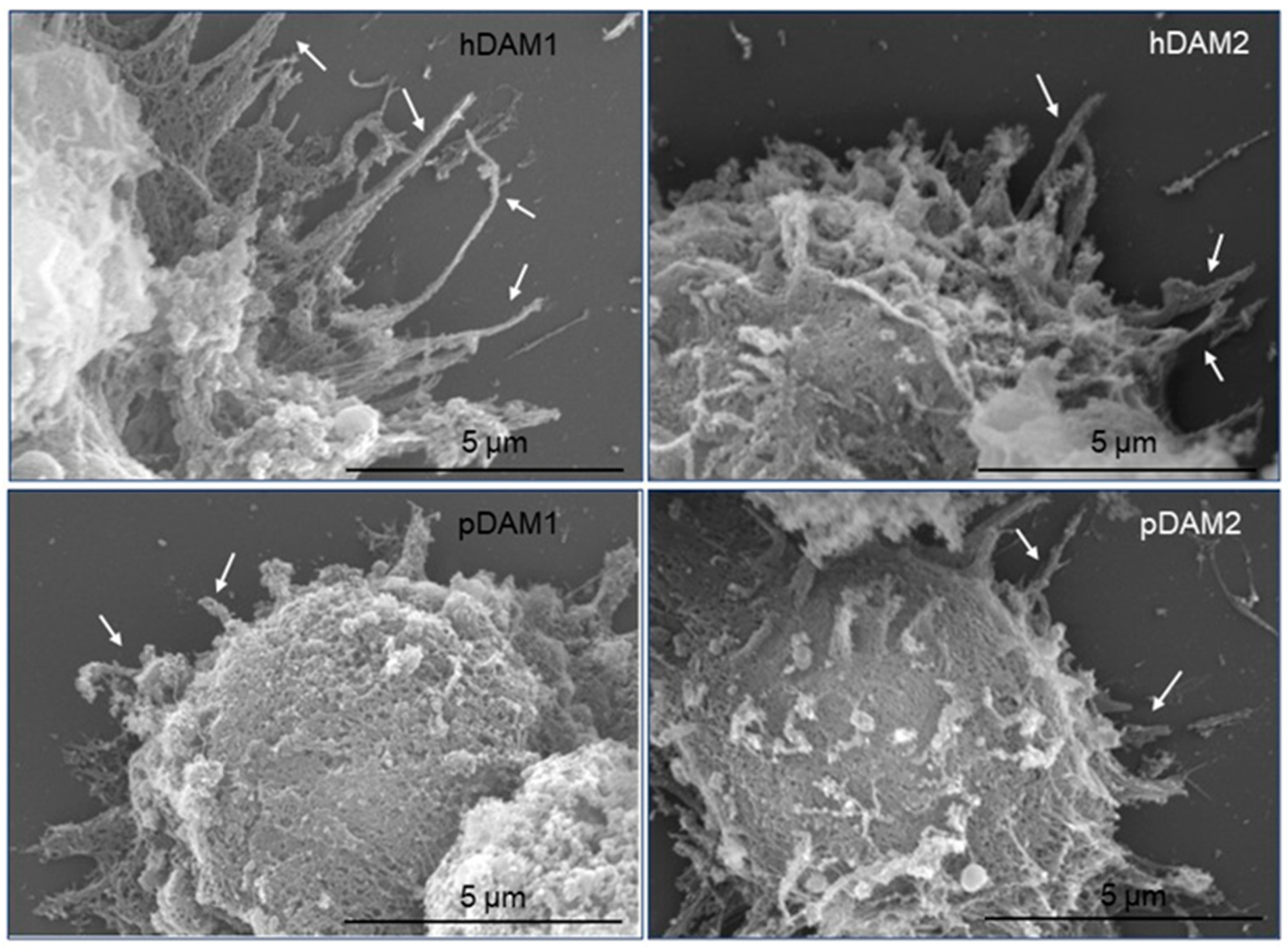
3.2. Adhesion of Macrophages on DAMs
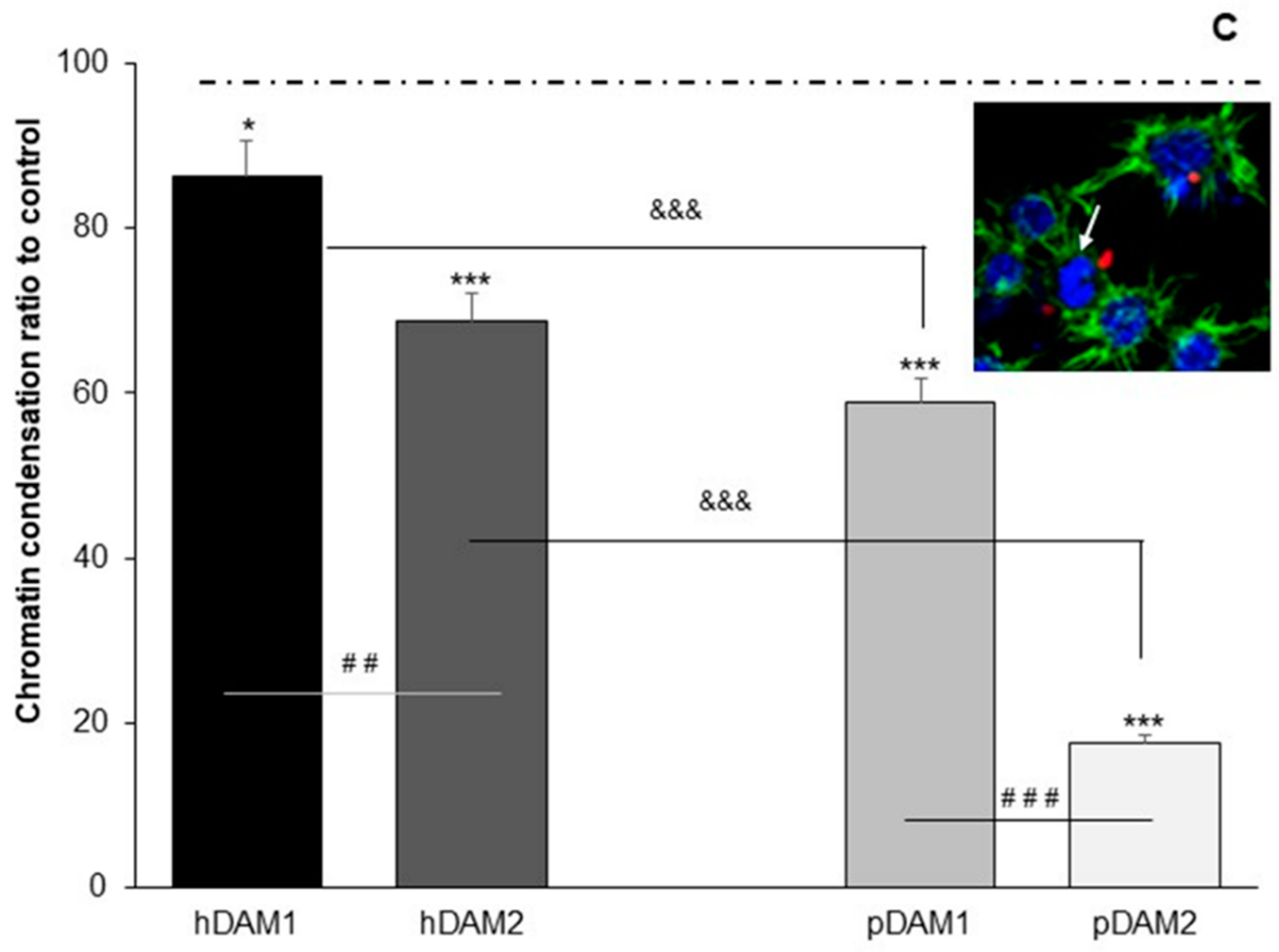
3.3. Candida albicans/Macrophage Biointerface on Human or Porcine DAMs
3.4. TNFα and IL-6 Production by RAW-264.7 Macrophages Cultured on Human or Porcine DAMs after Candida albicans Interaction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hebecker, B.; Naglik, J.R.; Hube, B.; Jacobsen, I.D. Pathogenicity mechanisms and host response during oral Candida albicans infections. Expert Rev. Anti-Infect. Ther. 2014, 12, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 73, 1445–1456. [Google Scholar] [CrossRef] [Green Version]
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A. Vulvovaginal Candida albicans infections: Pathogenesis, immunity and vaccine prospects. BJOG. 2015, 122, 785–794. [Google Scholar] [CrossRef]
- Chaffin, W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008, 72, 495–544. [Google Scholar] [CrossRef] [Green Version]
- Moyes, D.L.; Richardson, J.P.; Naglik, J.R. Candida albicans-epithelial interactions and pathogenicity mechanisms: Scratching the surface. Virulence 2015, 6, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Luig, M.; Kluger, M.A.; Goerke, B.; Meyer, M.; Nosko, A.; Yan, I.; Scheller, J.; Mittrücker, H.W.; Rose-John, S.; Stahl, R.A.; et al. Inflammation-Induced IL-6 Functions as a Natural Brake on Macrophages and Limits GN. J. Am. Soc. Nephrol. 2015, 26, 1597–1607. [Google Scholar] [CrossRef] [Green Version]
- Gubernatorova, E.O.; Gorshkova, E.A.; Namakanova, O.A.; Zvartsev, R.V.; Hidalgo, J.; Drutskaya, M.S.; Tumanov, A.V.; Nedospasov, S.A. Non-redundant Functions of IL-6 Produced by Macrophages and Dendritic Cells in Allergic Airway Inflammation. Front. Immunol. 2018, 9, 2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rougerie, P.; Miskolci, V.; Cox, D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: Role and regulation of the actin cytoskeleton. Immunol. Rev. 2013, 256, 222–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barger, S.R.; Reilly, N.S.; Shutova, M.S.; Li, Q.; Maiuri, P.; Heddleston, J.M.; Mooseker, M.S.; Flavell, R.A.; Svitkina, T.; Oakes, P.W.; et al. Membrane-cytoskeletal crosstalk mediated by myosin-I regulates adhesion turnover during phagocytosis. Nat. Commun. 2019, 10, 1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenz, M.C.; Bender, J.A.; Fink, G.R. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 2004, 3, 1076–1087. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Arenas, E.; Cabezón, V.; Bermejo, C.; Arroyo, J.; Nombela, C.; Diez-Orejas, R.; Gil, C. Integrated proteomics and genomics strategies bring new insight into Candida albicans response upon macrophage interaction. Mol. Cell. Proteomics 2007, 6, 460–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Lewis, L.E.; Bain, J.M.; Lowes, C.; Gillespie, C.; Rudkin, F.M.; Gow, N.A.; Erwig, L.P. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012, 8, e1002578. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Zeng, H.; Horng, T. Metabolism as a guiding force for immunity. Nat. Cell Biol. 2019, 21, 85–93. [Google Scholar] [CrossRef]
- Yan, J.; Horng, T. Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. 2020, 30, 979–989. [Google Scholar] [CrossRef]
- Huang, S.C.; Everts, B.; Ivanova, Y.; O’sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.K.; Sassetti, C.M. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. USA 2008, 105, 4376–4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badylak, S.F. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: Factors that influence the host response. Ann. Biomed. Eng. 2014, 42, 517–1527. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, S.; Chameettachal, S.; Cromer, B.; Pati, F.; Kingshott, P. Decellularized extracellular matrix hydrogels—Cell behavior as a function of matrix stiffness. Curr. Opin. Biomed. Eng. 2019, 10, 123–133. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Chaubal, M.; Hotchkiss, K.M.; Olivares-Navarrete, R. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials 2020, 243, 119920. [Google Scholar] [CrossRef]
- Yu, C.; Kornmuller, A.; Brown, C.; Hoare, T.; Flynn, L. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 2017, 120, 66–80. [Google Scholar] [CrossRef]
- Ibsirlioglu, T.; Elçin, A.E.; Elçin, Y.M. Decellularized biological scaffold and stem cells from autologous human adipose tissue for cartilage tissue engineering. Methods 2020, 171, 97–107. [Google Scholar] [CrossRef]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Dominic, M.; Handleton, R.; Shazly, T.; Matthews, M. A novel supercritical CO2-based decellularization method for maintaining scaffold hydration and mechanical properties. J. Supercrit. Fluids 2018, 131, 72–81. [Google Scholar]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Dembo, M.; Torney, D.; Saxman, K.; Hammer, D. The kinetics of membrane-to-surface adhesión and detachment. Proc. R. Soc. 1988, 234, 55–83. [Google Scholar]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagvolden, G.; Giaever, I.; Pettersen, E.O.; Feder, J. Cell adhesion force microscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 471–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicuéndez, M.; Casarrubios, L.; Feito, M.J.; Madarieta, I.; Garcia-Urkia, N.; Murua, O.; Olalde, B.; Briz, N.; Diez-Orejas, R.; Portolés, M.T. Effects of Human and Porcine Adipose Extracellular Matrices Decellularized by Enzymatic or Chemical Methods on Macrophage Polarization and Immunocompetence. Int. J. Mol. Sci. 2021, 22, 3847. [Google Scholar] [CrossRef]
- Gillum, A.M.; Tsay, E.Y.; Kirsch, D.R. Isolation of the Candida albicans gene for orotidine-5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef]
- Prieto, D.; Román, E.; Correia, I.; Pla, J. The HOG pathway is critical for the colonization of the mouse gastrointestinal tract by Candida albicans. PLoS ONE 2014, 9, e87128. [Google Scholar] [CrossRef] [Green Version]
- Yaoa, Q.; Zheng, Y.-W.; Lan, Q.-H.; Kou, L.; Xu, H.L.; Zhao, Y.-Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C 2019, 104, 109942. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [Green Version]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol. Life Sci. 2015, 72, 1303–1316. [Google Scholar] [CrossRef] [Green Version]
- Mattila, P.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Niedergang, F.; Chavrier, P. Signaling and membrane dynamics during phagocytosis: Many roads lead to the phagos(R)ome. Curr. Opin. Cell Biol. 2004, 16, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kress, H.; Stelzer, E.H.; Holzer, D.; Buss, F.; Griffiths, G.; Rohrbach, A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc. Natl. Acad. Sci. USA 2007, 104, 11633–11638. [Google Scholar] [CrossRef] [Green Version]
- Vonna, L.; Wiedemann, A.; Aepfelbacher, M.; Sackmann, E. Micromechanics of filopodia mediated capture of pathogens by macrophages. Eur. Biophys. J. 2007, 36, 145–151. [Google Scholar] [CrossRef]
- Koyama, Y.; Norose-Toyoda, K.; Hirano, S.; Kobayashi, M.; Ebihara, T.; Someki, I.; Fijisaki, H.; Irie, S. Type I collagen is a non-adhesive extracellular matrix for macrophages. Arch. Histol. Cytol. 2000, 63, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Gowen, B.B.; Borg, T.K.; Ghaffar, A.; Mayer, E.P. Selective adhesion of macrophages to denatured forms of type I collagen is mediated by scavenger receptors. Matrix Biol. 2000, 19, 61–71. [Google Scholar] [CrossRef]
- Mazur, A.; Holthoff, E.; Vadali, S.; Kelly, T.; Post, S.R. Cleavage of Type I Collagen by Fibroblast Activation Protein-α Enhances Class A Scavenger Receptor Mediated Macrophage Adhesion. PLoS ONE 2016, 11, e0150287. [Google Scholar] [CrossRef] [Green Version]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahr, J.C.; Weiss, S.J. Macrophage-Dependent Tracking and Remodeling of the Basement Membrane-Interstitial Matrix Interface. BioRxiv 2018, 364422. [Google Scholar]
- Etich, J.; Koch, M.; Wagener, R.; Zaucke, F.; Fabri, M.; Brachvogel, B. Gene Expression Profiling of the Extracellular Matrix Signature in Macrophages of Different Activation Status: Relevance for Skin Wound Healing. Int. J. Mol. Sci. 2019, 20, 5086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, T.; Bromberg, J.S. Regulation of the immune system by laminins. Trends Immunol. 2017, 38, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M.; Messier, J.M.; Mercurio, A.M. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the α6β1 integrin. J. Cell Biol. 1990, 110, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Iorio, V.; Troughton, L.D.; Hamill, K.J. Laminins: Roles and Utility in Wound Repair. Adv. Wound Care 2015, 4, 250. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 2163. [Google Scholar] [CrossRef] [Green Version]
- Schiavinato, A.; Keene, D.R.; Wohl, A.P.; Corallo, D.; Colombatti, A.; Wagener, R.; Paulsson, M.; Bonaldo, P.; Sengle, G. Targeting of EMILIN-1 and EMILIN-2 to Fibrillin Microfibrils Facilitates their Incorporation into the Extracellular Matrix. J. Investig. Dermatol. 2016, 136, 1150–1160. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, H.; Davis, E.C.; Starcher, B.C.; Ouchi, T.; Yanagisawa, M.; Richardson, J.A.; Olson, E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 2002, 415, 168–171. [Google Scholar] [CrossRef]
- Wohner, N.; Muczynski, V.; Mohamadi, A.; Legendre, P.; Proulle, V.; Aymé, G.; Christophe, O.D.; Lenting, P.J.; Denis, C.V.; Casari, C. Macrophage scavenger receptor SR-AI contributes to the clearance of von Willebrand factor. Haematologica 2018, 103, 728–737. [Google Scholar] [CrossRef] [Green Version]
- Adair-Kirk, T.L.; Senior, R.M. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 2008, 40, 1101–1110. [Google Scholar] [CrossRef] [Green Version]
- Boyd, D.F.; Thomas, P.G. Towards integrating extracellular matrix and immunological pathways. Cytokine 2017, 98, 79–86. [Google Scholar] [CrossRef]
- Gerisch, G.; Ecke, M.; Schroth-Diez, B.; Gerwig, S.; Engel, U.; Maddera, L.; Clarke, M. Self-organizing actin waves as planar phagocytic cup structures. Cell Adhes. Migr. 2009, 3, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prashar, A.; Bhatia, S.; Gigliozzi, D.; Martin, T.; Duncan, C.; Guyard, C.; Terebiznik, M.R. Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages. J. Cell Biol. 2013, 203, 1081–1097. [Google Scholar] [CrossRef] [Green Version]
- Ashwini, N.A.; Sachin, V.S.; Yogesh, S.S.; Jomon, J.; Milind, S.P.; Rajendra, L.D. Association of small Rho GTPases and actin ring formation in epithelial cells during the invasion by Candida albicans. FEMS Immunol. Med. Microbiol. 2009, 55, 74–84. [Google Scholar]
- Tsarfaty, I.; Sandovsky-Losica, H.; Mittelman, L.; Berdicevsky, I.; Segal, E. Cellular actin is affected by interaction with Candida albicans. FEMS Microbiol. Lett. 2000, 189, 225–232. [Google Scholar] [CrossRef]
- Sandovsky-Losica, H.; Segal, E. Infection of Hep2 epithelial cells with Candida albicans: Adherence and postadherence events. FEMS Immunol. Med. Microbiol. 2006, 46, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Maxson, M.E.; Naj, X.; O’Meara, T.R.; Plumb, J.D.; Cowen, L.E.; Grinstein, S. Integrin-based diffusion barrier separates membrane domains enabling the formation of microbiostatic frustrated phagosomes. ELife 2018, 7, e34798. [Google Scholar] [CrossRef]
- Gerlitz, G.; Bustin, M. Efficient cell migration requires global chromatin condensation. J. Cell Sci. 2010, 123, 2207–2217. [Google Scholar] [CrossRef] [Green Version]
- Chua, T.K.; Ramdas, N.M.; Shivashankar, G.V. Actin cytoskeleton differentially alters the dynamics of lamin A, HP1α and H2B core histone proteins to remodel chromatin condensation state in living cells. Integr. Biol. 2015, 7, 1309–1317. [Google Scholar]
- Xie, S.X.; Mahmood, R.; Gjorgjieva, T.; Percipalle, P. Emerging roles of cytoskeletal proteins in regulating gene expression and genoma organization during differentiation. Nucleus 2020, 11, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Choy, E.; Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef] [Green Version]
- Luu, T.U.; Liu, W.F. Regulation of Macrophages by Extracellular Matrix Composition and Adhesion Geometry. Regen. Eng. Transl. Med. 2018, 4, 238–246. [Google Scholar] [CrossRef]
- Reales-Calderón, J.A.; Aguilera-Montilla, N.; Corbí, A.L.; Molero, G.; Gil, C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics 2014, 14, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, U.; Yang, M.; Matundan, H.H.; Hirose, S.; Shah, P.K.; Sharifi, B.G.; Ghiasiet, H. Increased phagocytosis in the presence of enhanced M2-like macrophage responses correlates with increased primary and latent HSV-1 infection. PLoS Pathog. 2020, 16, e1008971. [Google Scholar] [CrossRef]
- Tomlin, H.; Piccinini, A.M. A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology 2018, 155, 186–201. [Google Scholar] [CrossRef] [PubMed]



| PROTEINS | DAMs | |||
|---|---|---|---|---|
| hDAM1 | hDAM2 | pDAM1 | pDAM2 | |
| Collagens | ||||
| Collagen α-1(I) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-1(III) chain | ✓ | ✓ | ||
| Collagen α-1(IV) chain | ✓ | ✓ | ||
| Collagen α-1(V) chain | ✓ | ✓ | ||
| Collagen α-1(VI) chain | ✓ | ✓ | ✓ | |
| Collagen α-1(XII) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-1(XIV) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-1(XV) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-1(XVIII) chain | ✓ | ✓ | ||
| Collagen α-2 (I) chain | ✓ | ✓ | ||
| Collagen α-2 (IV) chain | ✓ | ✓ | ✓ | |
| Collagen α-2 (V) chain | ✓ | ✓ | ✓ | |
| Collagen α-2(VI) chain | ✓ | ✓ | ✓ | ✓ |
| Collagen α-3 (V) chain | ✓ | ✓ | ||
| Collagen α-3 (VI) chain | ✓ | ✓ | ✓ | ✓ |
| Laminins | ||||
| Laminin α-2 | ✓ | ✓ | ||
| Laminin α-3 | ✓ | |||
| Laminin α-4 | ✓ | ✓ | ||
| Laminin α-5 | ✓ | ✓ | ||
| Laminin β-1 | ✓ | ✓ | ✓ | ✓ |
| Laminin β-2 | ✓ | ✓ | ✓ | ✓ |
| Laminin γ-1 | ✓ | ✓ | ✓ | |
| EMILIN | ||||
| Emilin-1 | ✓ | ✓ | ||
| Fibulins | ||||
| Fibulin-1 | ✓ | |||
| Fibulin-2 | ✓ | ✓ | ||
| Fibulin-5 | ✓ | ✓ | ✓ | |
| von Willebrand factor | ✓ | ✓ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicuéndez, M.; Casarrubios, L.; Feito, M.J.; Madarieta, I.; Garcia-Urkia, N.; Murua, O.; Olalde, B.; Briz, N.; Diez-Orejas, R.; Portolés, M.T. Candida albicans/Macrophage Biointerface on Human and Porcine Decellularized Adipose Matrices. J. Fungi 2021, 7, 392. https://doi.org/10.3390/jof7050392
Cicuéndez M, Casarrubios L, Feito MJ, Madarieta I, Garcia-Urkia N, Murua O, Olalde B, Briz N, Diez-Orejas R, Portolés MT. Candida albicans/Macrophage Biointerface on Human and Porcine Decellularized Adipose Matrices. Journal of Fungi. 2021; 7(5):392. https://doi.org/10.3390/jof7050392
Chicago/Turabian StyleCicuéndez, Mónica, Laura Casarrubios, María José Feito, Iratxe Madarieta, Nerea Garcia-Urkia, Olatz Murua, Beatriz Olalde, Nerea Briz, Rosalía Diez-Orejas, and María Teresa Portolés. 2021. "Candida albicans/Macrophage Biointerface on Human and Porcine Decellularized Adipose Matrices" Journal of Fungi 7, no. 5: 392. https://doi.org/10.3390/jof7050392







