Mycorrhiza-Induced Resistance against Foliar Pathogens Is Uncoupled of Nutritional Effects under Different Light Intensities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material and AMF Inoculation
2.2. Experimental Design and Growing Conditions
2.3. Plant Harvesting and Determinations
2.4. Botrytis Cinerea Inoculation and Disease Assessment
2.5. Mineral Nutrients Analyses in Plant Tissues
2.6. Statistical Analyses
3. Results
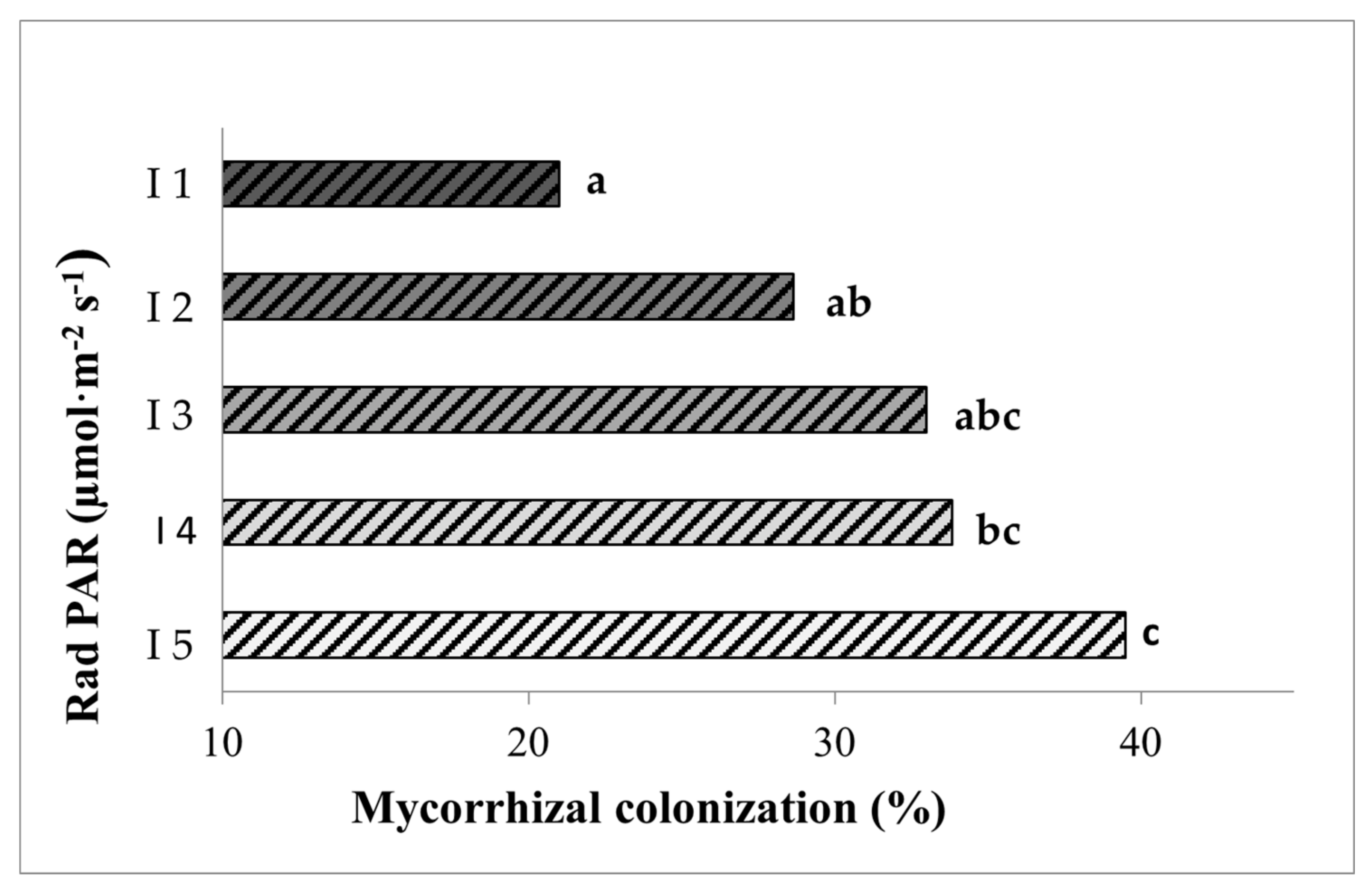
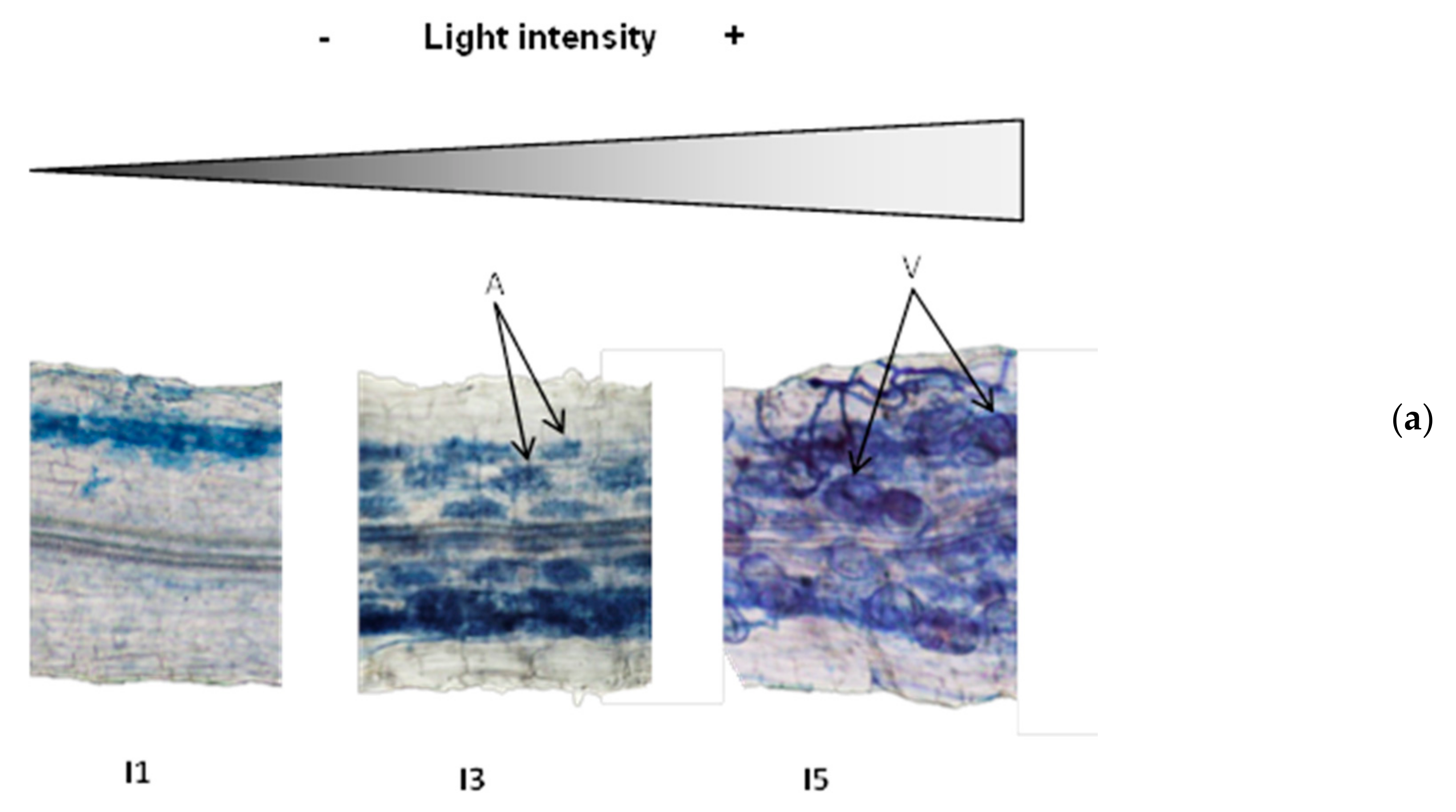
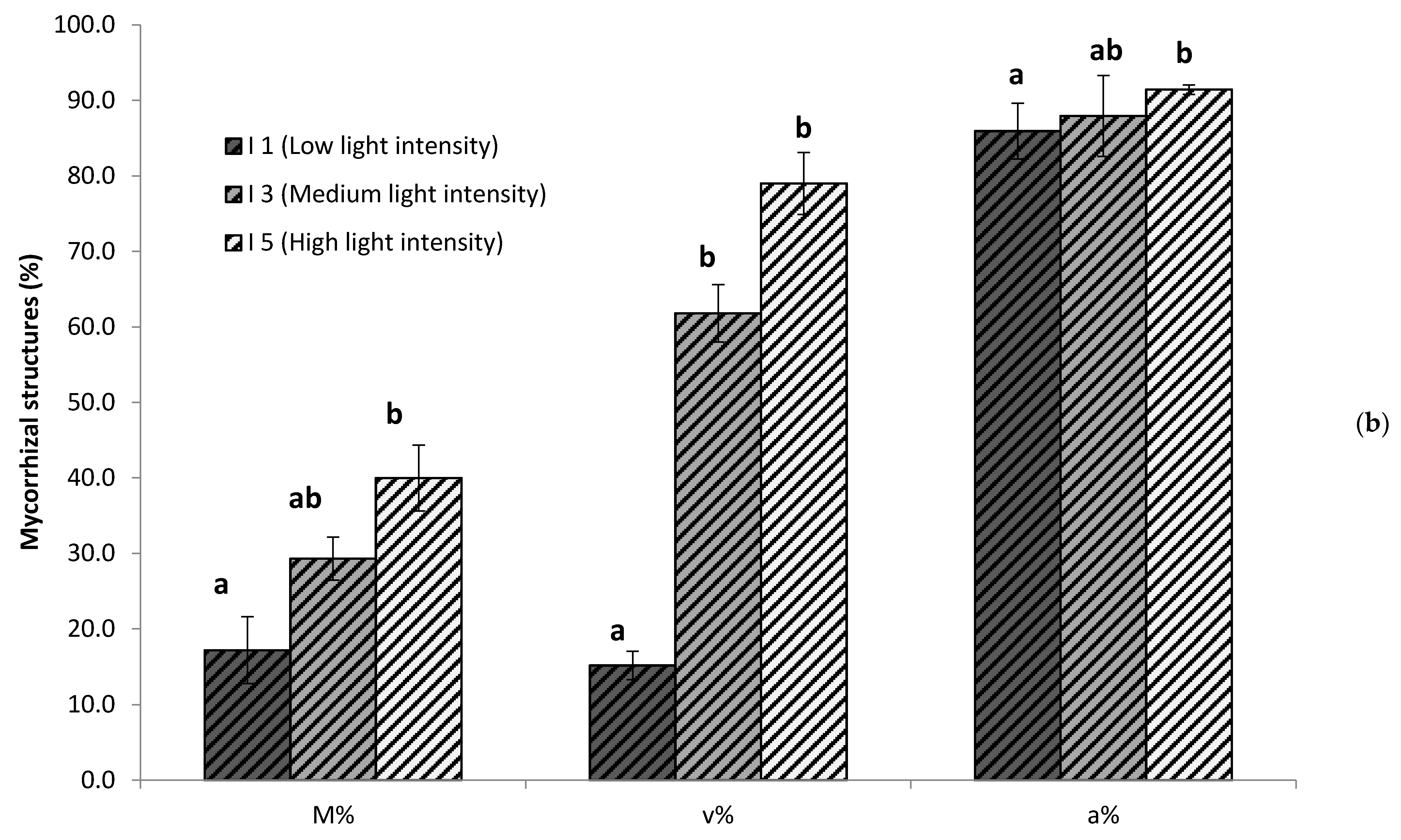
3.1. Light Intensity Determines Mycorrhizal Root Colonization and Vesicle Abundance
3.2. Light Intensity Impacts Photosynthesis, Plant Biomass and Mycorrhizal Growth Response
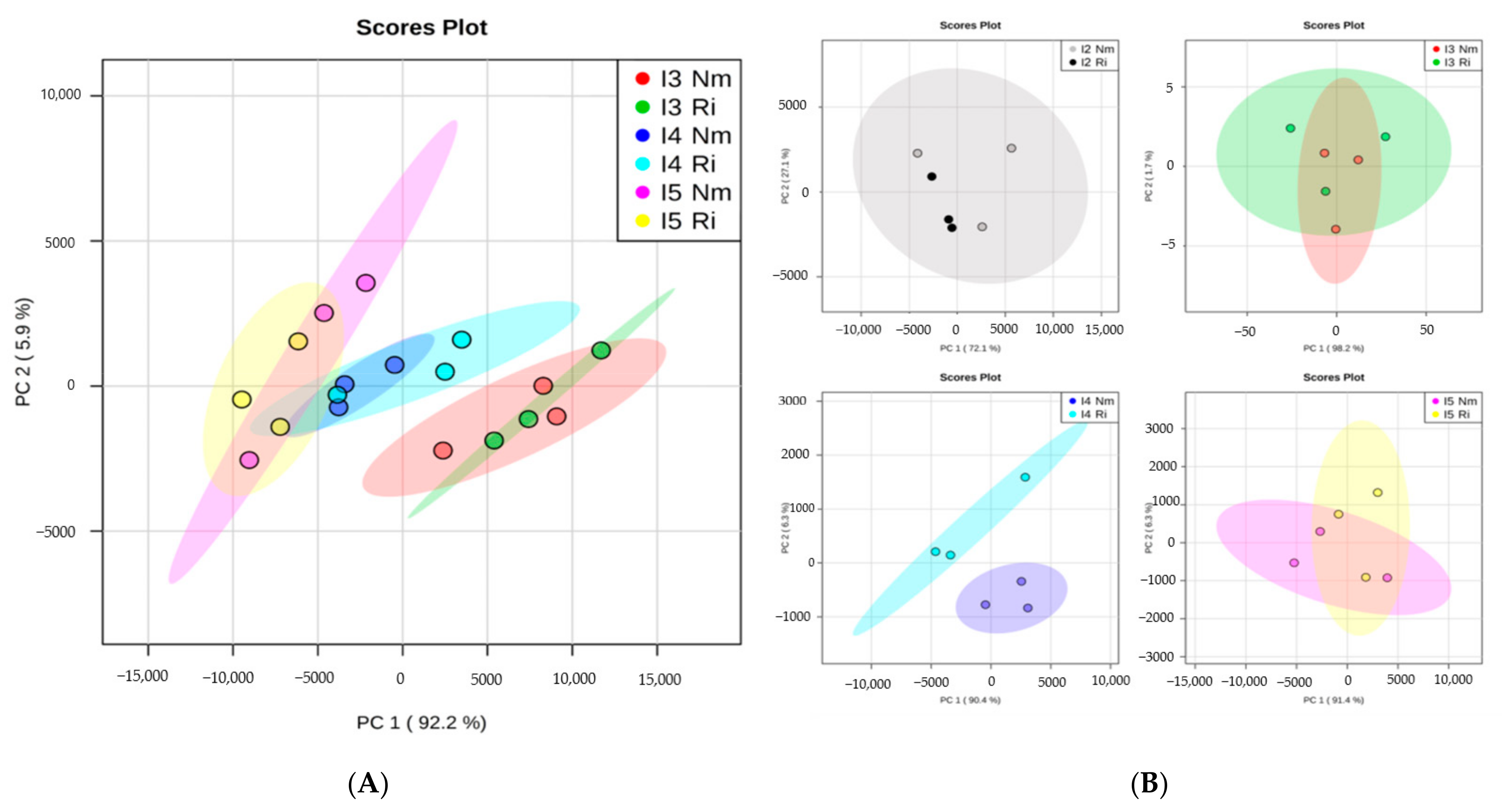
3.3. Light Intensity and Mycorrhizal Colonization Alters Plant Nutrient Contents
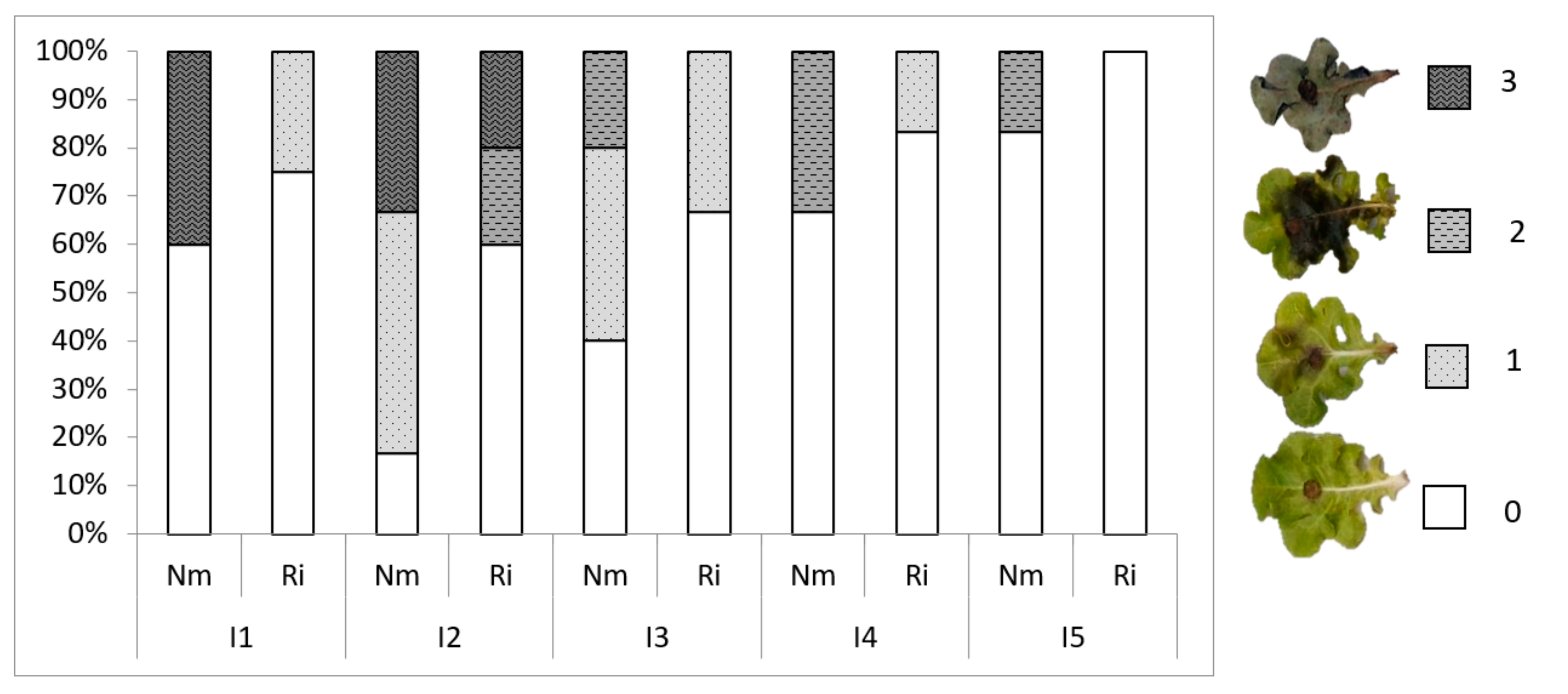
3.4. Light Intensity Impacts Plant Susceptibility to Botrytis cinerea and Mycorrhiza-Induced Resistance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finkel, O.M. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Mendes, R. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Zabalgogeazcoa, I.; Vazquez de Aldana, B.; Martinez-Medina, A. Untapping the potential of plant mycobiomes for applications in agriculture. Curr. Opin. Plant Biol. 2021, 60, 102034. [Google Scholar] [CrossRef]
- Pieterse, C.M.J. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruden, K.; Lidoy, J.; Petek, M.; Podpečan, V.; Flors, V.; Papadopoulou, K.K.; Pappas, M.L.; Martinez-Medina, A.; Bejarano, E.; Biere, A.; et al. Ménage à Trois: Unraveling the Mechanisms Regulating Plant–Microbe–Arthropod Interactions. Trends Plant Sci. 2020, 25, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agriculture: A roadmap for successful application. J. Exp. Bot. 2020, 1, 3878–3901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineda, A.; Dicke, M.; Pieterse, C.M.; Pozo, M.J. Beneficial microbes in a changing environment: Are they always helping plants to deal with insects? Funct Ecol. 2013, 27, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Barea, J.M.; Pozo, M.J.; López-Ráez, J.A.; Aroca, R.; Ruiz-Lozano, J.M.; Ferrol, N.; Azcón, R.; Azcon-Aguilar, C. Arbuscular mycorrhizas and their significance in promoting soil-plant system sustainability against environmental stresses. In Beneficial Plant-Microbial Interactions: Ecology and Applications; CRC: London, UK, 2013. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferrol, N.; Azcon-Aguilar, C.; Pérez-Tienda, J. Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: An overview on the mechanisms involved. Plant Sci. 2018, 280. [Google Scholar] [CrossRef] [Green Version]
- Pozo, M.J.; López-Ráez, J.A.; Azcon-Aguilar, C.; Garrido, J.M. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.; Álvarez, D.; Flors, V.; Azcon-Aguilar, C.; Pozo, M. Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol. 2018, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, A.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez JAPozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Song, Y.; Ye, M.; Li, C.Y.; Wang, R.L.; Wei, X.C.; Luo, S.M.; Zeng, R.S. Priming of anti-herbivore defense in tomato by arbuscular mycorrhizal fungus and involvement of the jasmonate pathway. J. Chem. Ecol. 2013, 39, 1036–1044. [Google Scholar] [CrossRef]
- Song, Y.; Chen, D.; Lu, K.; Sun, Z.; Zeng, R. Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front. Plant Sci. 2015, 6, 786. [Google Scholar] [CrossRef] [Green Version]
- Fiorilli, V.; Catoni, M.; Francia, D.; Cardinale, F.; Lanfranco, L. The arbuscular mycorrhizal simbiosis reduces disease severity in tomato plants infected by Botrytis cinerea. J. Plant Pathol. 2011, 93, 237–242. [Google Scholar]
- Fiorilli, V.; Vannini, C.; Ortolani, F.; Garcia-Seco, D.; Chiapello, M.; Novero, M.; Domingo, G.; Terzi, V.; Morcia, C.; Bagnaresi, P.; et al. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018, 8, 9625. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, N.; Sánchez-Bel, P.; Pastor, V.; Pastor-Fernández, J.; Mateu, D.; Pozo, M.J.; Cerezo, M.; Flors, V. Root-to-shoot signalling in mycorrhizal tomato plants upon Botrytis cinerea infection. Plant. Sci. 2020, 298, 110595. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, N.; Pastor, V.; Pastor-Fernández, J.; Flors, V.; Pozo, M.J.; Sánchez-Bel, P. Role and mechanisms of callose priming in mycorrhiza-induced resistance. J. Exp. Bot. 2020, 71, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Martín-Cardoso, H.; Olivé, M.; Pla, E.; Catala-Forner, M.; Martínez-Eixarch, M.; Segundo, B.S. Effect of root colonization by arbuscular mycorrhizal fungi on growth, productivity and blast resistance in rice. Rice 2020, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.; Lidoy, J.; Llopis-Giménez, Á.; Herrero, S.; Flors, V.; Pozo, M.J. Mycorrhizal symbiosis primes the accumulation of antiherbivore compounds and enhances herbivore mortality in tomato. J. Exp. Bot. 2021, erab171. [Google Scholar] [CrossRef]
- Tamayo, E.; Knight, S.; Valderas, A.; Dancis, A.; Ferrol, N. The arbuscular mycorrhizal fungus Rhizophagus irregularis uses a reductive iron assimilation pathway for high-affinity iron uptake: High-affinity Fe uptake in Rhizophagus irregularis. Environ. Microbiol. 2018, 20, 1857–1872. [Google Scholar] [CrossRef] [PubMed]
- Bago, B.; Pfeffer, P.E.; Shachar-Hill, Y. Carbon Metabolism and Transport in Arbuscular Mycorrhizas. Plant Physiol. 2000, 124, 949–958. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–586. [Google Scholar] [CrossRef]
- Olsson, P.A.; Rahm, J.; Aliasgharzad, N. Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol. Ecol. 2010, 72, 125–131. [Google Scholar] [CrossRef]
- Bitterlich, M.; Rouphael, Y.; Graefe, J.; Franken, P. Arbuscular mycorrhizas: A promising component of plant production systems provided favorable conditions for their growth. Front. Plant Sci. 2018, 9, 1329. [Google Scholar] [CrossRef]
- Konvalinková, T.; Jansa, J. Lights off for arbuscular mycorrhiza: On its symbiotic functioning under light deprivation. Front. Plant Sci. 2016, 7, 782. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Wang, M.; Biere, A. Interactive effects of mycorrhizae, soil phosphorus and light on growth and induction and priming of defense in Plantago lanceolata. Front. Plant Sci. 2021, 12, 647372. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ji, B.; Zhang, J.; Zhang, F.; Bever, J.D. Shading decreases plant carbon preferential allocation towards the most beneficial mycorrhizal mutualist. New Phytol. 2015, 205, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. 2010. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.; Galvão, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T.L. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [Green Version]
- Ballare, C. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Hua, J. Modulation of plant immunity by light, circadian rhythm, and temperature. Curr. Opin. Plant Biol. 2013, 16, 406–413. [Google Scholar] [CrossRef]
- Griebel, T.; Zeier, J. Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: Phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol. 2008, 147, 790–801. [Google Scholar] [CrossRef] [Green Version]
- Saijo, Y.; Loo, E.P. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [Green Version]
- Oberpichler, I. Light affects motility and infectivity of Agrobacterium tumefaciens. Environ. Microbiol. 2008, 10, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Tisch, D.; Schmoll, M. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 2010, 85, 1259–1277. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, J. How light affects the life of Botrytis. Fungal Genet. Biol. 2017, 106, 26–41. [Google Scholar] [CrossRef]
- Veloso, J.; van Kan, J.A.L. Many shades of grey in Botrytis–host plant interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Alsanius, B.; Karlsson, M.; Rosberg, A.K.; Dorais, M.; Naznin, T.K.; Sammar, K.; Bergstrand, K.J. Light and Microbial Lifestyle: The Impact of Light Quality on Plant–Microbe Interactions in Horticultural Production Systems—A Review. Horticulturae 2019, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Losi, A.; Gärtner, W. A light life together: Photosensing in the plant microbiota. Photochem. Photobiol. Sci. 2021, 20, 451–473. [Google Scholar] [CrossRef]
- Son, C.L.; Smith, F.A.; Smith, S.E. Effect of light intensity on root growth, mycorrhizal infection and phosphate uptake in onion (Allium cepa L.). In Structural and Functional Aspects of Transport in Roots; Springer: Dordrecht, The Netherland, 1989; pp. 107–110. [Google Scholar]
- Gehring, C.A. Growth responses to arbuscular mycorrhizae by rain forest seedlings vary with light intensity and tree species. Plant Ecol. 2003, 167, 127–139. [Google Scholar] [CrossRef]
- FAO. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 14 January 2021).
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.M.; Subbarao, K.V.; Raid, R.N.; Kurtz, E.A. Compendium of Lettuce Diseases; APS Press: St. Paul, MN, USA, 1997. [Google Scholar]
- Sanchez-Bel, P.; Troncho, P.; Gamir, J.; Pozo, M.J.; Camañes, G.; Cerezo, M.; Flors, V. The Nitrogen Availability Interferes with Mycorrhiza-Induced Resistance against Botrytis cinerea in Tomato. Front. Microbiol. 2016, 7, 1598. [Google Scholar] [CrossRef] [Green Version]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Arbuscular mycorrhizal fungi improved growth and nutritional quality of greenhouse-grown lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Pascual, I.; Sánchez-Díaz, M.; Erro, J.; García-Mina, J.M.; Goicoechea, N. Improvement of nutritional quality of greenhouse-grown lettuce by arbuscular mycorrhizal fungi is conditioned by the source of phosphorus nutrition. J. Agric Food Chem. 2011, 59, 11129–11140. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Garmendia, I.; Goicoechea, N. The arbuscular mycorrhizal symbiosis can overcome reductions in yield and nutritional quality in greenhouse-lettuces cultivated at inappropriate growing seasons. Sci. Hort 2013, 164, 145–154. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, A.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.; Gamir, J.M.; Aroca, R.; Pozo, M.J.; Flors, V. Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 2015, 6, 598. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition; Bureau of Horticulture and Plantation Crops: Maidstone, UK, 1966. [Google Scholar]
- Pozo, J.; Alvaro JEMorales, I.; Requena, J.; Mazuela, P.C.; Urrestarazu, M. A new local sustainable inorganic material for soilless culture in Spain: Granulated volcanic rock. HortScience 2014, 49, 1537–1541. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Mazuela, P.C.; Martínez, G.A. Effect of substrate reutilization on yield and properties of melon and tomato crops. J. Plant Nutr. 2008, 31, 2031–2043. [Google Scholar] [CrossRef]
- Vierheilig, H.; Schweiger PBrundrett, M. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots†. Physiol. Plant. 2005, 125, 393–404. [Google Scholar] [CrossRef]
- Giovannetti, M.; Avio, L.; Barale, R.; Ceccarelli, N.; Cristofani, R.; Iezzi, A.; Scarpato, R. Nutraceutical value and safety of tomato fruits produced by mycorrhizal plants. Br. J. Nutr. 2012, 107, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae: Proceedings of the 1st European Symposium on Mycorrhizae, Dijon, 1–5 July 1985; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 7, 14.10.1–14.10.91. [Google Scholar] [CrossRef]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next generation of microbial inoculants for agriculture and bioremediation. Microb. Biotechnol. 2016, 10, 19–21. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stechiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal phenotypes and the law of the minimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef]
- Morton, J.B.; Bentivenga, S.P.; Wheeler, W.W. Germ plasm in the international collection of arbuscular and vesicular-arbuscular mycorrhizal fungi (INVAM) and procedures for culture development, documentation and storage. Mycotaxon 1993, 48, 491–528. [Google Scholar]
- Graham, J.H.; Abbott, L.K. Wheat responses to aggressive and non-aggressive arbuscular mycorrhizal fungi. Plant Soil 2000, 220, 207–218. [Google Scholar] [CrossRef]
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.L.; Delaux, P.-M.; Klingl, V.; Von Röpenack-Lahaye, E.; Wang, T.L.; et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. Elife 2017, 6, e29107. [Google Scholar] [CrossRef]
- Jones, M.D.; Smith, S.E. Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? Can. J. Bot. 2004, 82, 1089–1109. [Google Scholar] [CrossRef] [Green Version]
- Diagne NNgom MDjighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Meng, L.; Höfte, M.; Van Labeke, M.C. Leaf age and light quality influence the basal resistance against Botrytis cinerea in strawberry leaves. Environ. Exp. Bot. 2019, 157, 35–45. [Google Scholar] [CrossRef]
- Lazzarin, M.; Mesenburg, M.; Meijer, D.; van Leperen, W.; Marcelis, L.F.M.; Kappers, I.F.; van der Krol, A.R.; van Loon, J.J.A.; Dicke, M. LEDs Make It Resilient: Effects on Plant Growth and Defense. Trends Plant Sci. 2020, 26, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; Pastor, V.; Robert, J.; Flors, V.; Mauch-Mani, B.; Ton, J. Callose deposition: A multifaceted plant defense response. Mol. Plant-Microbe Interact. 2011, 24, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
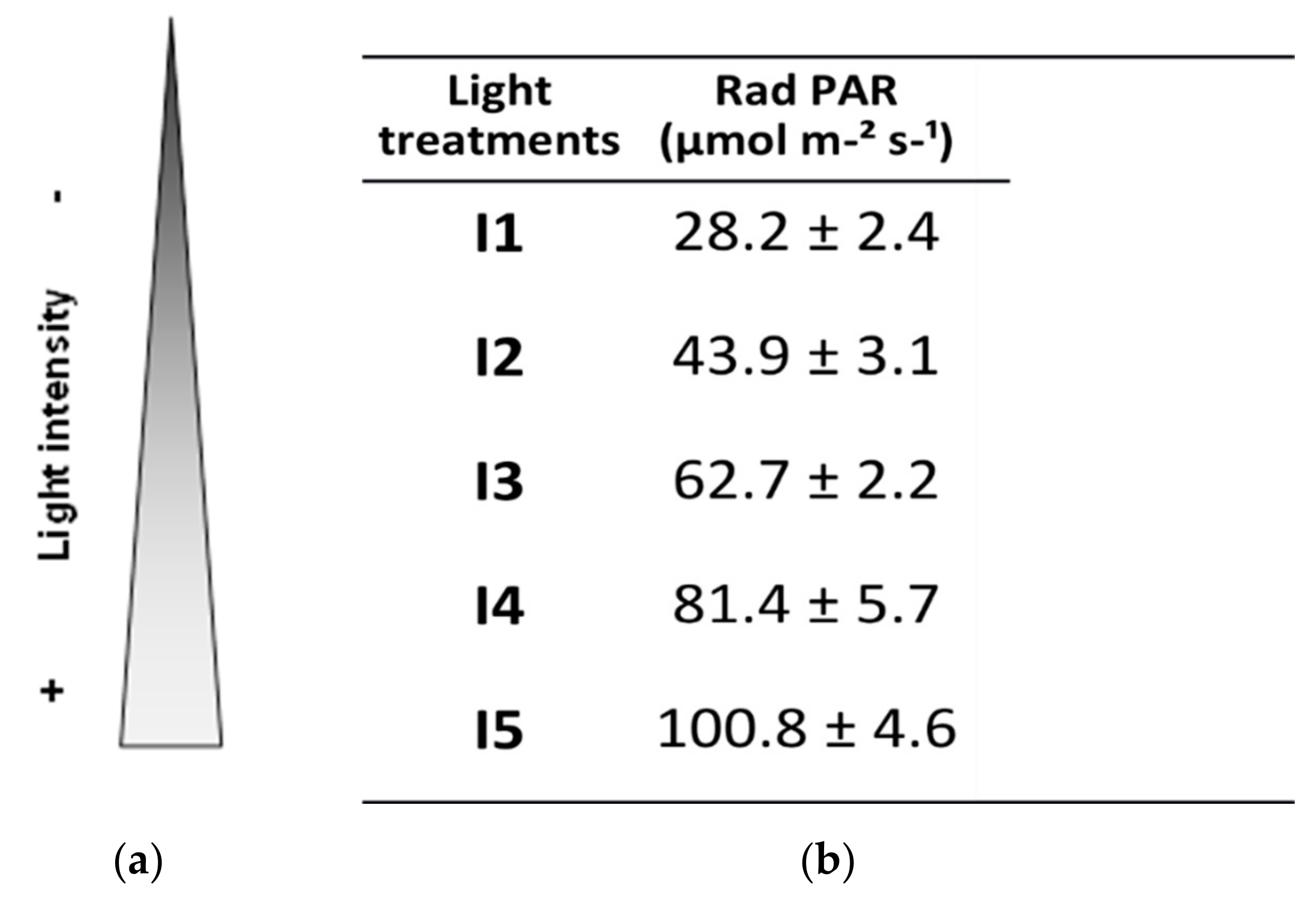
| Code | Power/Consumption | Voltage | Angle of Illuminated Beam | Power Factor | Measurements | Luminous Flux | Color |
|---|---|---|---|---|---|---|---|
| 120018B | 18 W | 100–250 V | 220o | >0.90 | 1200 × 22.5 mm | 1500 lm | 6500 k. |
| Light | AMF | Fotosyntetic Rate | FW (g) | DW (g) | Hypocotyl Length (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (µmol CO2m−2s−1) | Root | Shoot | Root | Shoot | |||||||||
| I 1 | Nm | 0.94 | ab | 0.3 | a | 1.9 | a | 0.02 | a | 0.07 | a | 2.0 | c |
| Ri | 0.60 | a | 0.3 | a | 1.7 | a | 0.03 | ab | 0.06 | a | 1.9 | c | |
| I 2 | Nm | 1.40 | bc | 1.1 | b | 2.9 | ab | 0.07 | abc | 0.11 | ab | 1.7 | bc |
| Ri | 1.20 | abc | 1.4 | b | 3.1 | b | 0.11 | cd | 0.13 | bc | 1.6 | bc | |
| I 3 | Nm | 1.41 | bc | 1.5 | b | 4.6 | c | 0.08 | bcd | 0,.8 | cd | 1.2 | ab |
| Ri | 1.74 | cde | 1.5 | b | 3.9 | bc | 0.09 | bcd | 0.15 | bc | 0.8 | a | |
| I 4 | Nm | 1.65 | cd | 3.5 | d | 6.6 | d | 0.2 | e | 0.27 | e | 1.0 | a |
| Ri | 2,.9 | e | 2.5 | c | 5.0 | c | 0.13 | d | 0.21 | d | 0.8 | a | |
| I 5 | Nm | 1.72 | cde | 3.5 | d | 6.3 | d | 0.29 | f | 0.38 | f | 0.9 | a |
| Ri | 2.22 | de | 2.6 | c | 4.8 | c | 0.2 | e | 0.3 | e | 0.9 | a | |
| I 1 | I 2 | I 3 | I 4 | I 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I1Nm | I1Ri | I2Nm | I2Ri | I3Nm | I3Ri | I4Nm | I4Ri | I5Nm | I5Ri | |
| P | 2370.8 b | 2614.1 b | 2176.6 b | 2514.7 b | 2256.7 b | 2229.1 b | 1528 a | 2380.9 b | 1505.3 a | 1571.5 a |
| Ca | 22,832 bc | 23,296 c | 22,612 c | 20,006 a | 22,837 c | 22,793 c | 20,211 ab | 19,781 a | 19,095 a | 19,473 a |
| Cu | 12.4 abcd | 11 ab | 14.7 d | 13.7 bcd | 11.6 abc | 13.6 bcd | 11 a | 13.8 cd | 10.9 a | 12 abc |
| Fe | 683 abc | 536 ab | 614 ab | 671 abc | 627 ab | 702 abc | 408 a | 973 c | 858 bc | 848 bc |
| K | 61,514 f | 58,983 ef | 54,200 cde | 56,190 def | 57,277 def | 58,963 ef | 48,741 abc | 51,876 bcd | 46,490 ab | 43,831 a |
| Mg | 5482 cd | 5958 d | 5534 cd | 5190 bc | 5471 c | 5535 cd | 4767 ab | 5378 c | 4747 a | 4785 ab |
| Mn | 92.9 c | 65.7 a | 86.7 c | 69.9 ab | 89.8 c | 81 bc | 73.3 ab | 73.9 ab | 87.9 c | 69.7 ab |
| Mo | 1.59 ab | 1.66 ab | 1.71 b | 1.26 a | 1.45 ab | 1.35 ab | 1.5 ab | 1.48 ab | 1.23 a | 1.31 a |
| Na | 12,556 f | 13,947 g | 10,937 cd | 10,697 bc | 11,890 ef | 11,743 def | 9879 b | 11,155 cde | 8070 a | 8806 a |
| Ni | 4.8 c | 4.7 c | 3.3 b | 3.2 ab | 2.9 ab | 3.7 b | 2.4 a | 3.6 b | 3.6 b | 3.1 ab |
| S | 4028 c | 4050 c | 3844 bc | 3750 bc | 3923 bc | 4009 c | 3660 b | 4009 c | 3357 a | 3827 bc |
| Si | 1241 ab | 1166 ab | 1228 ab | 1007 ab | 1043 ab | 1305 b | 894 a | 1074 ab | 1235 ab | 1239 ab |
| Zn | 144 ab | 196 c | 165 abc | 174 bc | 155 ab | 165 bc | 162 ab | 167 bc | 130 a | 144 ab |
| P Value | P | Ca | Cu | Fe | K | Mg | Mn | Mo | Na | Ni | S | Si | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light | 0.000 | 0.001 | 0.035 | 0.309 | 0.000 | 0.000 | 0.130 | 0.204 | 0.000 | 0.001 | 0.004 | 0.367 | 0.059 |
| AMF | 0.006 | 0.407 | 0.212 | 0.107 | 0.800 | 0.098 | 0.000 | 0.036 | 0.008 | 0.190 | 0.018 | 0.730 | 0.024 |
| L x AMF | 0.051 | 0.346 | 0.105 | 0.072 | 0.473 | 0.037 | 0.059 | 0.319 | 0.050 | 0.053 | 0.052 | 0.384 | 0.420 |
| Treatment | Disease | MIR | |
|---|---|---|---|
| Incidence (%) | |||
| I1 | Nm | 40 | 38% |
| Ri | 25 | ||
| I2 | Nm | 67 | 40% |
| Ri | 40 | ||
| I3 | Nm | 60 | 45% |
| Ri | 33 | ||
| I4 | Nm | 33 | 50% |
| Ri | 17 | ||
| I5 | Nm | 17 | 100% |
| Ri | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozo de la Hoz, J.; Rivero, J.; Azcón-Aguilar, C.; Urrestarazu, M.; Pozo, M.J. Mycorrhiza-Induced Resistance against Foliar Pathogens Is Uncoupled of Nutritional Effects under Different Light Intensities. J. Fungi 2021, 7, 402. https://doi.org/10.3390/jof7060402
Pozo de la Hoz J, Rivero J, Azcón-Aguilar C, Urrestarazu M, Pozo MJ. Mycorrhiza-Induced Resistance against Foliar Pathogens Is Uncoupled of Nutritional Effects under Different Light Intensities. Journal of Fungi. 2021; 7(6):402. https://doi.org/10.3390/jof7060402
Chicago/Turabian StylePozo de la Hoz, Judith, Javier Rivero, Concepción Azcón-Aguilar, Miguel Urrestarazu, and María J. Pozo. 2021. "Mycorrhiza-Induced Resistance against Foliar Pathogens Is Uncoupled of Nutritional Effects under Different Light Intensities" Journal of Fungi 7, no. 6: 402. https://doi.org/10.3390/jof7060402
APA StylePozo de la Hoz, J., Rivero, J., Azcón-Aguilar, C., Urrestarazu, M., & Pozo, M. J. (2021). Mycorrhiza-Induced Resistance against Foliar Pathogens Is Uncoupled of Nutritional Effects under Different Light Intensities. Journal of Fungi, 7(6), 402. https://doi.org/10.3390/jof7060402






