Environmental Status of Cryptococcus neoformans and Cryptococcus gattii in Colombia
Abstract
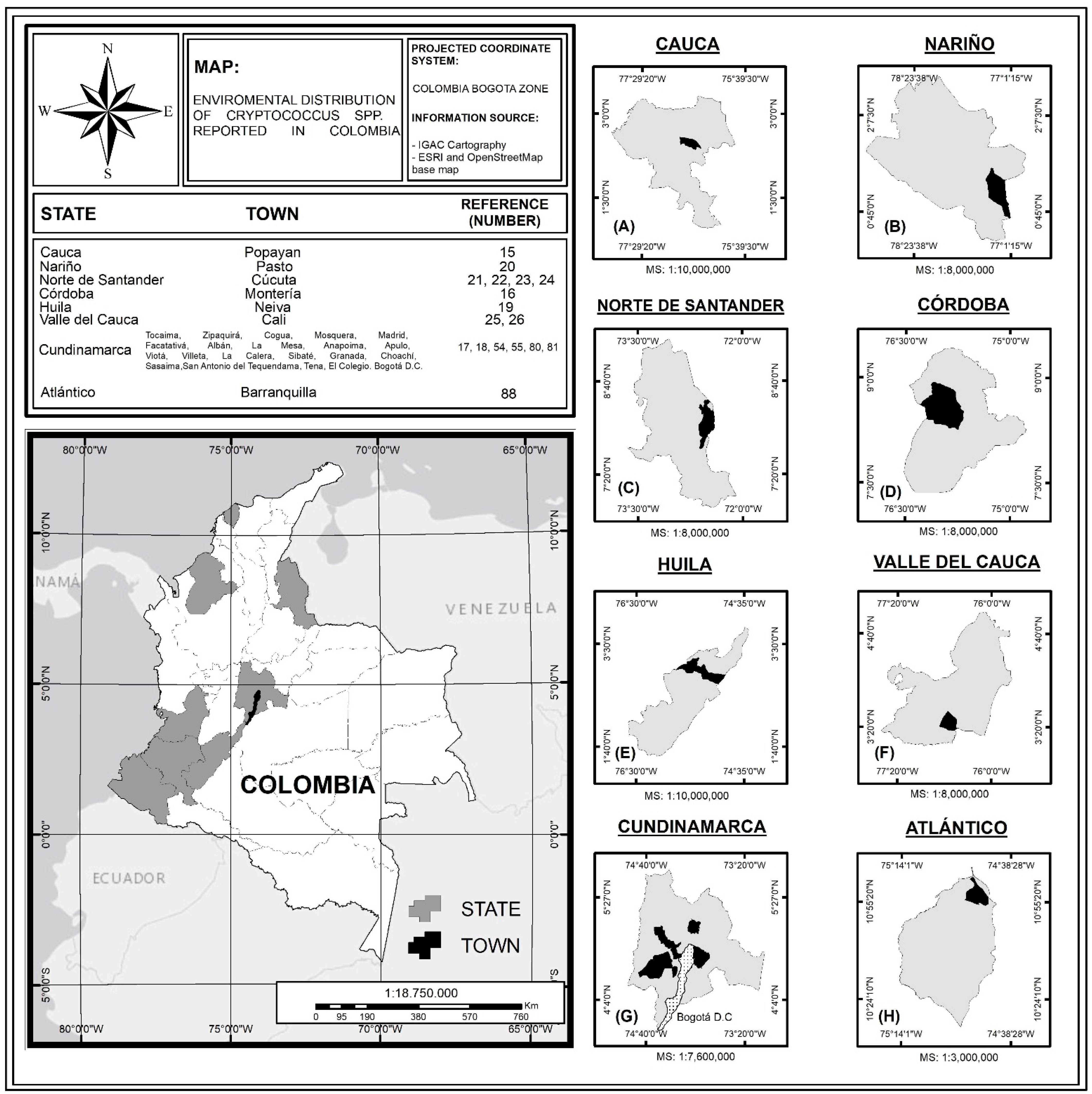
:1. Introduction
2. Materials and Methods
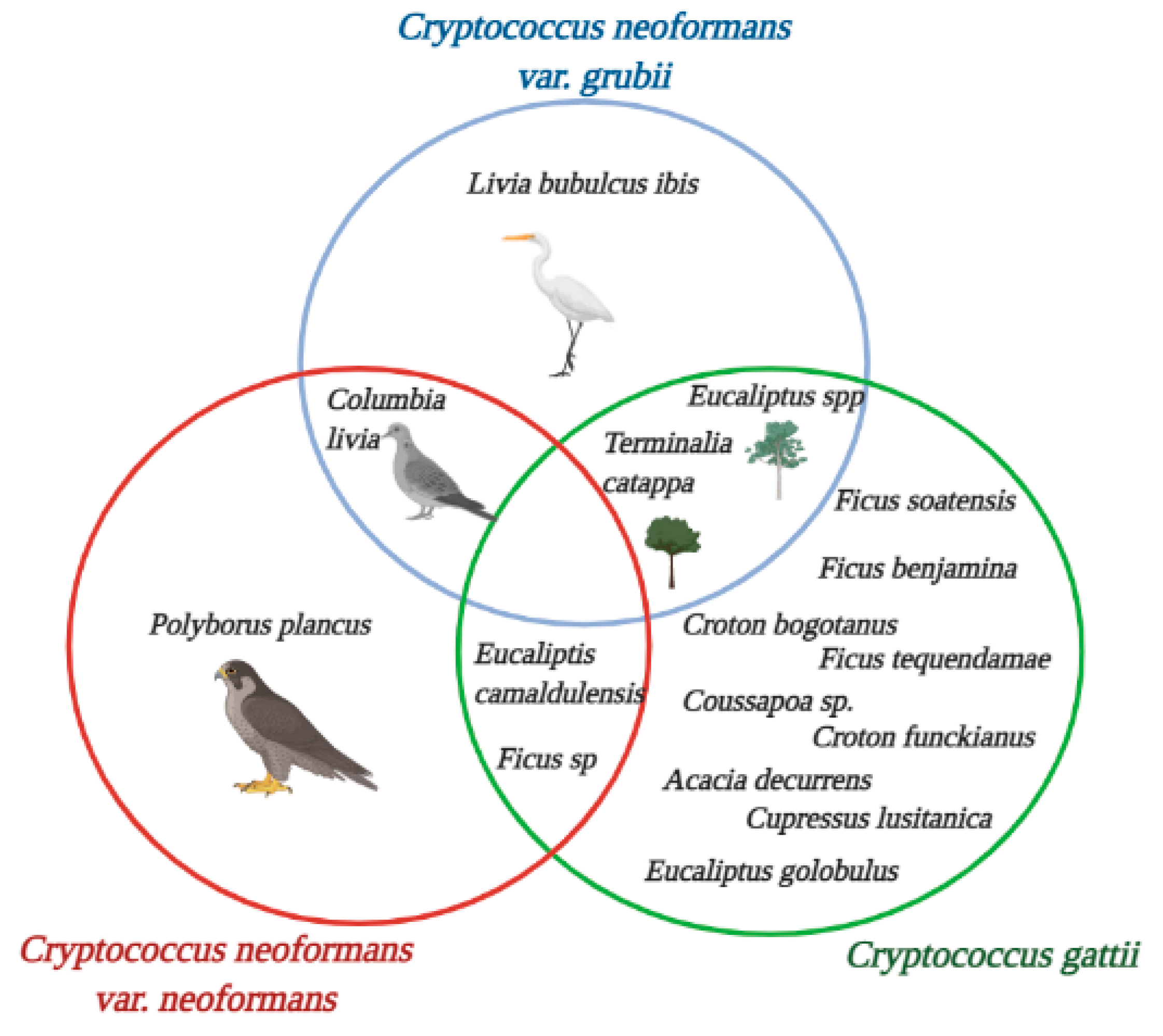
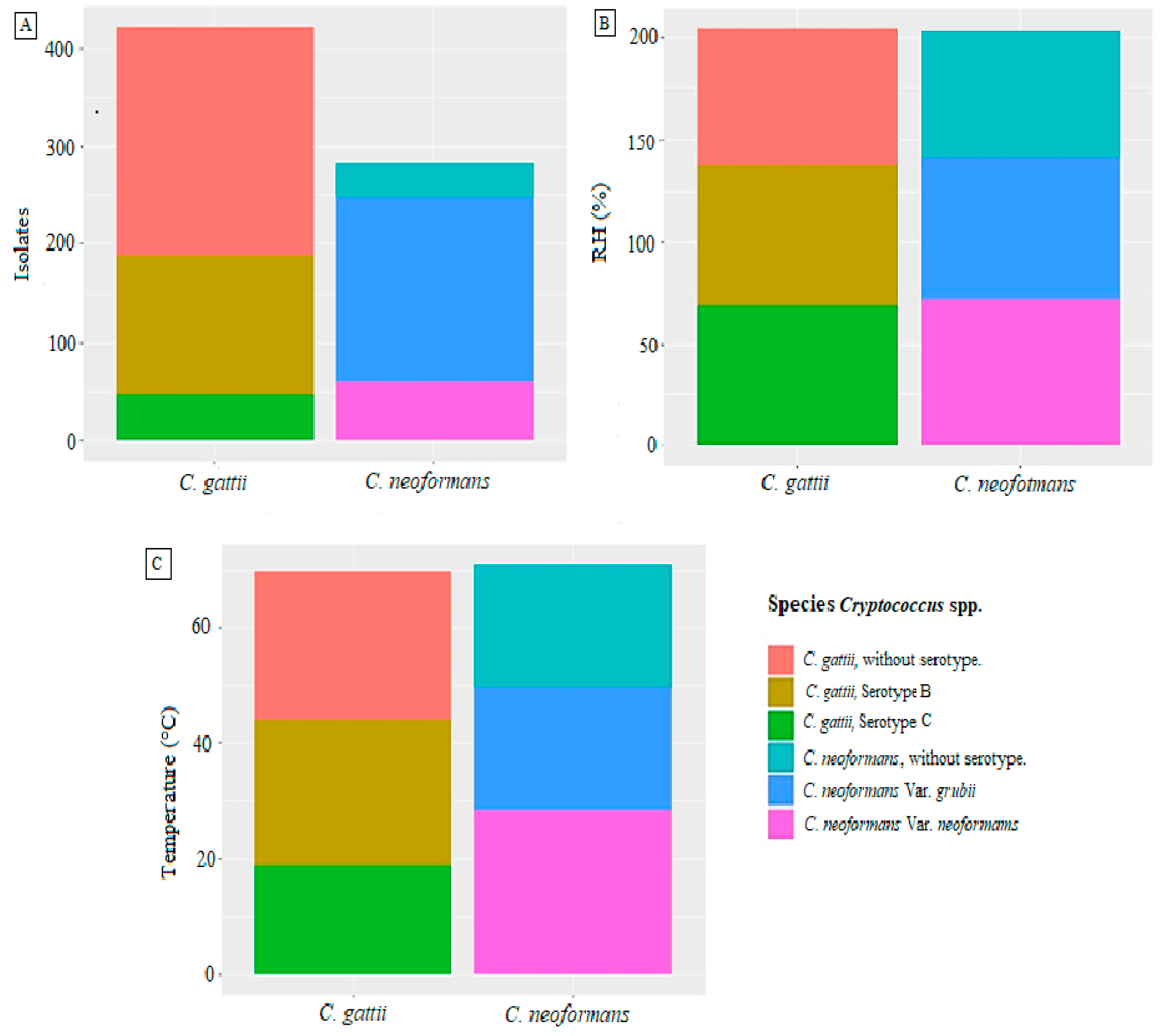
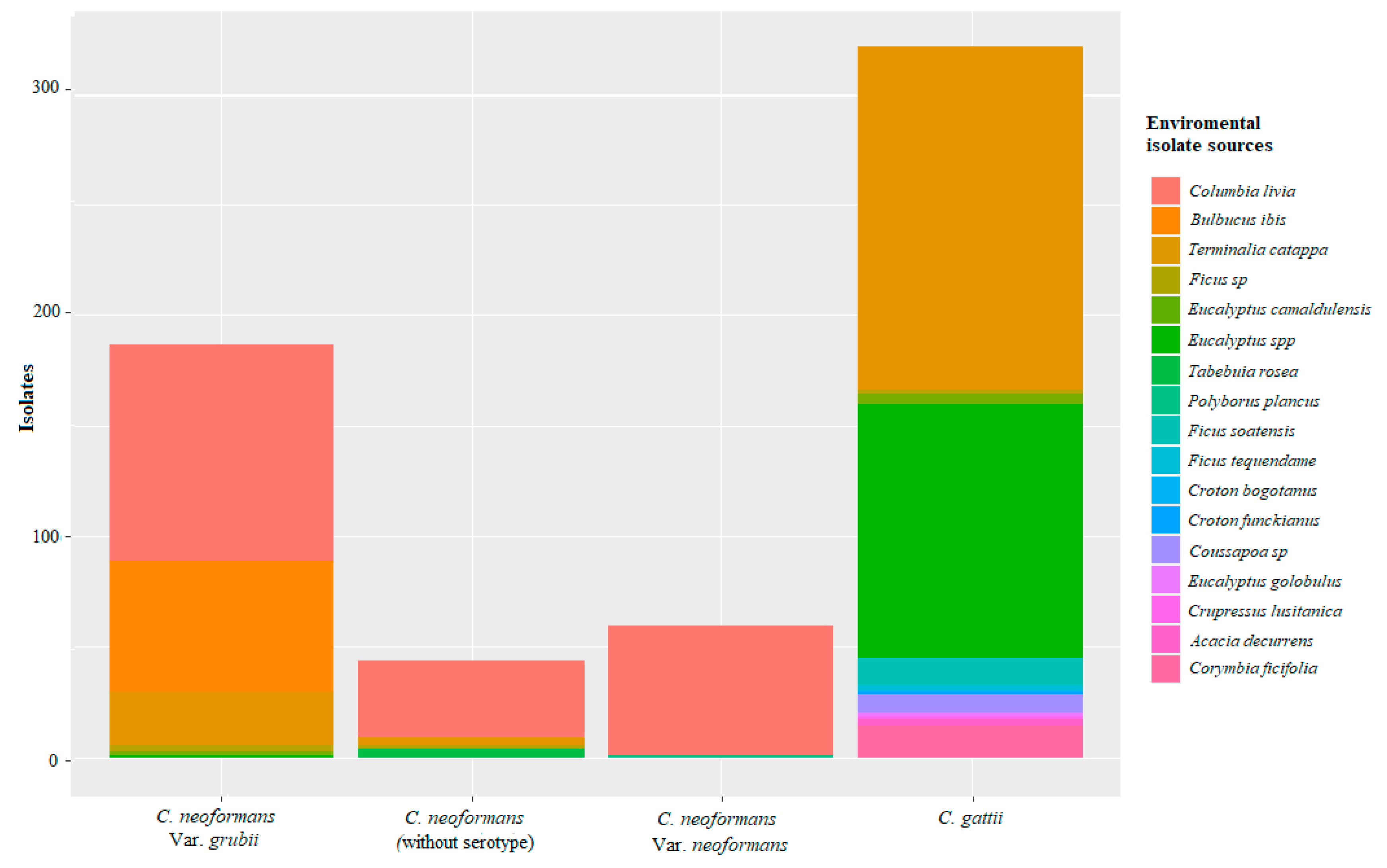
3. Results
3.1. Atlántico
3.2. Bogotá
3.3. Cali
3.4. Cúcuta
3.5. Cundinamarca
3.6. Huila
3.7. Monteria
3.8. Pasto
3.9. Popayán
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanbreuseghem, R.; Takashio, M. An atypical strain of Cryptococcus neoformans (San Felice) Vuillemin 1894. Part II. Cryptococcus neoformans var. gattii. nov. Med. Trop. 1970, 50, 695–702. [Google Scholar]
- Kwon-Chung, K.J.; Boekhout, T.; Fell, J.W.; Diaz, M. (1557) Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. bacillisporus (Basidiomycota, Hymenomycetes, Tremellomycetidae). Taxon 2002, 51, 804–806. [Google Scholar] [CrossRef]
- Xue, C.; Tada, Y.; Dong, X.; Heitman, J. The Human Fungal Pathogen Cryptococcus Can Complete Its Sexual Cycle during a Pathogenic Association with Plants. Cell Host Microbe 2007, 1, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R.; et al. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef]
- Fell, J.W.; Boekhout, T.; Fonseca, A.; Scorzetti, G.; Statzell-Tallman, A. Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int. J. Syst. Evol. Microbiol. 2000, 50, 1351–1371. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Bennett, J.E.; Wickes, B.L.; Meyer, W.; Cuomo, C.A.; Wollenburg, K.R.; Bicanic, T.A.; Castañeda, E.; Chang, Y.C.; Chen, J.; et al. The Case for Adopting the “Species Complex” Nomenclature for the Etiologic Agents of Cryptococcosis. mSphere 2017, 2, e00357-16. [Google Scholar] [CrossRef] [Green Version]
- Hagen, F.; Lumbsch, H.T.; Arsenijevic, V.A.; Badali, H.; Bertout, S.; Billmyre, R.B.; Bragulat, M.R.; Cabañes, F.J.; Carbia, M.; Chakrabarti, A.; et al. Importance of Resolving Fungal Nomenclature: The Case of Multiple Pathogenic Species in the Cryptococcus Genus. mSphere 2017, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.R.D. The Primary Pulmonary Lymph Node Complex of Cryptococcosis. Am. J. Clin. Pathol. 1976, 65, 83–92. [Google Scholar] [CrossRef]
- May, R.C.; Stone, N.; Wiesner, D.L.; Bicanic, T.; Nielsen, K.V. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Genet. 2016, 14, 106–117. [Google Scholar] [CrossRef]
- Iii, E.J.B.; Bildfell, R.J.; Frank, S.A.; Mitchell, T.G.; Marr, K.A.; Heitman, J. Molecular Evidence That the Range of the Vancouver Island Outbreak of Cryptococcus gattii Infection Has Expanded into the Pacific Northwest in the United States. J. Infect. Dis. 2009, 199, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Springer, D.J.; Billmyre, R.B.; Filler, E.E.; Voelz, K.; Pursall, R.; Mieczkowski, P.; Larsen, R.A.; Dietrich, F.S.; May, R.C.; Filler, S.G.; et al. Cryptococcus gattii VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal. PLoS Pathog. 2014, 10, e1004285. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, M.M.; Teixeira, F.M.; Schalcher, T.R.; De Brito, M.T.F.M.; Valerio, E.S.; Monteiro, M.C. Cryptococcosis, A Risk for Immunocompromised and Immunocompetent Individuals. Open Epidemiol. J. 2013, 6, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Tintelnot, K.; Lemmer, K.; Losert, H.; Schar, G.; Polak, A. Follow-up of epidemiological data of cryptococcosis in Austria, Germany and Switzerland with special focus on the characterization of clinical isolates. Fortfuhrung der Datenerhebung zur Cryptococcose in Osterreich, Deutschland und der Schweiz unter besonderer Berucksichtigung der Charakterisierung der klinischen Isolate. Mycoses 2004, 47, 455–464. [Google Scholar] [CrossRef]
- Kang, R.; Quah, J.; Low, T.; Chong, C. Cryptococcus gattii infection with pulmonary and CNS involvement in an immu-nocompetent patient. Chest 2016, 149, A117. [Google Scholar] [CrossRef]
- Tello, M.; Gutiérrez, E.; Béjar, V.; Galarza, C.; Ramos, W.; Ortega, A. Criptococosis. Rev. Méd. Risaralda 2013, 19, 147–153. [Google Scholar]
- Galanis, E. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg. Infect. Dis. 2010, 16, 251–257. [Google Scholar] [CrossRef]
- Li, Y.; Zou, M.; Yin, J.; Liu, Z.; Lu, B. Microbiological, Epidemiological, and Clinical Characteristics of Patients with Cryptococcal Meningitis at a Tertiary Hospital in China: A 6-Year Retrospective Analysis. Front. Microbiol. 2020, 11, 1837. [Google Scholar] [CrossRef]
- Anacona, C.; Vásquez, A.L.R.; Escandón, P. First isolation and molecular characterization of Cryptococcus neoformans var. grubii in excreta of birds in the urban perimeter of the Municipality of Popayán, Colombia. Rev. Iberoam. De Micol. 2018, 35, 123–129. [Google Scholar] [CrossRef]
- Martínez, O.I.C.; Morinelli, M.P.A.; Furnieles, J.L.A.; Ramos, A.M.J. Presumptive identification of Cryptococcus gattii isolated from Terminalia catappa in Montería, Córdoba, Colombia. [Identificación presuntiva de Cryptococcus gattii aislado de Terminalia catappa en Montería, Córdoba, Colombia. Rev. Cubana Med. Trop. 2011, 63, 117–122. [Google Scholar]
- Castañeda, A.; Castañeda, E. Isolation of Cryptococcus species associated with Eucalyptus in a park in Bogotá. Biomédica 2011, 21, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.; Ordoñez, N.; Castañeda, E. Association of yeasts of the genus Cryptococcus with Eucalyptus species in Santafe de Bogota. [Asociacion de leveduras del genero Cryptococcus con especies de Eucalyptus en Santafe de Bogota]. Rev. Inst. Med. Trop. São Paulo 1994, 36, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Virviescas, C.; Aragón, M.; González, F.; Escandón, P.; Castro, H.; Vasquez, L.R. Molecular characterization of Cryptococcus neoformans recovered from pigeon droppings in Rivera and Neiva, Colombia. Rev. MVZ Córdoba 2018, 23, 6991–6997. [Google Scholar] [CrossRef] [Green Version]
- Timarán, D.A.V.; Melo, C.J.B.; Caicedo, M.I.M.; Ceballos, A.M.C.; Vallejo, D.; Velásquez, C.A.C. Aislamiento de Cryptococcus neoformans en heces de palomas (Columba livia) en el casco urbano del municipio de Pasto, Colombia. Biosalud 2016, 15, 62–71. [Google Scholar] [CrossRef]
- Castañeda, A.; Huérfano, S.; Rodríguez, M.C.; Castañeda, E. Recovery of Cryptococcus neoformans var. gattii serotype C from almond tree debris. [Recuperación de Cryptococcus neoformans var. gattii serotipo C a partir de detritos de almendros]. Biomédica 2001, 21, 70–74. [Google Scholar] [CrossRef]
- Callejas, A.; Ordoñez, N.; Rodriguez, M.C.; Castañeda, E. First isolation of Cryptococcus neoformans var gattii, serotype C, from the environment in Colombia. Med. Mycol. 1998, 36, 341–344. [Google Scholar] [CrossRef]
- Firacative, C.; Torres, G.; Rodríguez, M.C.; Escandón, P. First environmental isolation of Cryptococcus gattii serotype B, from Cúcuta, Colombia. Biomédica 2011, 31, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angarita-Sánchez, A.; Cárdenas-Sierra, D.; Parra-Giraldo, C.; Diaz-Carvajal, C.; Escandon-Hernandez, P. Recovery of environmental Cryptococcus neoformans and Cryptococcus gattii and their association with clinical isolates in Cúcuta, Colom-bia. [Recuperación de Cryptococcus neoformans y Cryptococcus gattii ambientales y su asociación con aislados clínicos en Cú-cuta, Colombia]. MVZ Córdoba 2019, 24, 7137–7144. [Google Scholar]
- Caicedo, B.L.D.; Alvarez, V.M.I.; Llanos, C.E.; Molina, D. Cryptococcus neoformans in pigeon excreta from the urban perimeter of Cali. [Cryptococcus neoformans en excretas de palomas del perímetro urbano de Cali]. Colomb. Med. 1996, 27, 106–109. [Google Scholar]
- Caicedo, L.D.; Alvarez, M.I.; Delgado, M.; Cárdenas, A. Cryptococcus neoformans in bird excreta in the city zoo of Cali, Colombia. Mycopathol. 1999, 147, 121–124. [Google Scholar] [CrossRef]
- Archibald, L.K.; Tuohy, M.J.; Wilson, D.A.; Nwanyanwu, O.; Kazembe, P.N.; Tansuphasawadikul, S.; Eampokalap, B.; Chaovavanich, A.; Reller, L.; Jarvis, W.R.; et al. Antifungal Susceptibilities of Cryptococcus neoformans. Emerg. Infect. Dis. 2004, 10, 143–145. [Google Scholar] [CrossRef]
- Nnadi, N.; Enweani, I.; Cogliati, M.; Ayanbimpe, G.; Okolo, M.; Kim, E.; Sabitu, M.; Criseo, G.; Romeo, O.; Scordino, F. Molecular characterization of environmental Cryptococcus neoformans VNII isolates in Jos, Plateau State, Nigeria. J. Mycol. Méd. 2016, 26, 306–311. [Google Scholar] [CrossRef]
- Irokanulo, E.O.A.; Makinde, A.A.; Akuesgi, C.O.; Ekwonu, M. Cryptococcus neoformans var neoformans Isolated from Droppings of Captive Birds in Nigeria. J. Wildl. Dis. 1997, 33, 343–345. [Google Scholar] [CrossRef]
- Dou, H.; Wang, H.; Xie, S.; Chen, X.; Xu, Z.; Xu, Y. Molecular characterization of Cryptococcus neoformans isolated from the environment in Beijing, China. Med. Mycol. 2017, 55, 737–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mseddi, F.; Sellami, A.; Jarboui, M.A.; Makni, F.; Ayadi, A. First Environmental Isolations of Cryptococcus neoformans and Cryptococcus gattii in Tunisia and Review of Published Studies on Environmental Isolations in Africa. Mycopathologia 2011, 171, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.M.; Lazera, M.S.; Barbosa, G.G.; Trilles, L.; Balassiano, B.R.; Macedo, R.C.L.; Bezerra, C.C.F.; Pérez, M.A.; Cardarelli, P.; Wanke, B. Serotyping of 467 Cryptococcus neoformans Isolates from Clinical and Environmental Sources in Brazil: Analysis of Host and Regional Patterns. J. Clin. Microbiol. 2003, 41, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, C.C.B.A.; Souza, L.K.H.E.; Fernandes, O.D.F.L.; De Brito, S.C.A.; Silva, A.C.; De Sousa, E.D.; Silva, M.D.R.R. Characterization of Cryptococcus neoformans isolated from urban environmental sources in Goiânia, Goiás State, Brazil. Rev. Inst. Med. Trop. São Paulo 2005, 47, 203–207. [Google Scholar] [CrossRef]
- Kidd, S.E.; Chow, Y.; Mak, S.; Bach, P.; Chen, H.; Hingston, A.O.; Kronstad, J.W.; Bartlett, K.H. Characterization of Environmental Sources of the Human and Animal Pathogen Cryptococcus gattii in British Columbia, Canada, and the Pacific Northwest of the United States. Appl. Environ. Microbiol. 2006, 73, 1433–1443. [Google Scholar] [CrossRef] [Green Version]
- Montagna, M.T.; De Donno, A.; Caggiano, G.; Serio, F.; De Giglio, O.; Bagordo, F.; D’Amicis, R.; Lockhart, S.R.; Cogliati, M. Molecular characterization of Cryptococcus neoformans and Cryptococcus gattii from environmental sources and genetic comparison with clinical isolates in Apulia, Italy. Environ. Res. 2018, 160, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Laurenson, I.F.; Lalloo, D.G.; Naraqi, S.; Seaton, R.A.; Trevett, A.J.; Matuka, A.; Kevau, I.H. Cryptococcus neoformans in Papua New Guinea: A common pathogen but an elusive source. [Cryptococcus neoformans en Papua Nueva Guinea: Un patógeno común pero una fuente evasiva]. J. Med. Vet. Mycol. 1997, 35, 437–440. [Google Scholar] [CrossRef] [Green Version]
- Refojo, N.; Perrotta, D.; Brudny, R.; Abrantes, A.; Hevia, I.; Davel, G. Isolation of Cryptococcus neoformans and Cryptococ-cus gattii from trunk hollows of living trees in Buenos Aires City, Argentina. Med. Mycol. 2009, 47, 177–184. [Google Scholar] [CrossRef]
- Cattana, M.E.; de los Ángeles Sosa, M.; Fernández, M.; Rojas, F.; Mangiaterra, M.; Giusiano, G. Native trees of the Northeast Argentine: Natural hosts of the Cryptococcus neoformans-Cryptococcus gattii species complex. Rev. Iberoam. Micol. 2014, 31, 188–192. [Google Scholar] [CrossRef]
- Pedroso, R.S.; Lavrador, M.A.; Ferreira, J.C.; Candido, R.C.; Maffei, C.M. Cryptococcus neoformans var.grubii—Pathogenicity of environmental isolates correlated to virulence factors, susceptibility to fluconazole and molecular profile. Memórias Inst. Oswaldo Cruz 2010, 105, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Meyer, W.; Castañeda, A.; Jackson, S.; Huynh, M.; Castañeda, E.; The IberoAmerican Cryptococcal Study Group Molecular Typing of IberoAmerican. Cryptococcus neoformans Isolates. Emerg. Infect. Dis. 2003, 9, 189–195. [Google Scholar] [CrossRef]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.P.G.; Prabu, D.; Mitani, H.; Mikami, Y.; Menon, T. Environmental isolation of Cryptococcus neoformans and Cryptococcus gattii from living trees in Guindy National Park, Chennai, South India. Mycoses 2010, 53, 262–264. [Google Scholar] [CrossRef]
- Toro Zúñiga, V. Presumptive isolation and characterization of Cryptococcus neoformans and Cryptococcus gattii from trees in the O’Higgins and Maule region, Chile. [Aislamiento presuntivo y caracterización de Cryptococcus neoformans y Cryptococcus gattii desde árboles en]. Boletín Micológico 2015, 30, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Kwon-Chung, K.J.; Bennett, J.E. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am. J. Epidemiol. 1984, 120, 123–130. [Google Scholar] [CrossRef]
- Bauwens, L.; Swinne, D.; De Vroey, C.; De Meurichy, W. Isolation of Cryptococcus neoformans var. neoformans in the aviaries of the Antwerp Zoological Gardens. Mykosen 1986, 29, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Salim, R.; Runco, R. Presence of Cryptococcus neoformans in urban pigeons excreta from San Miguel de Tu-cumán—Argentina. [Presencia de Cryptococcus neoformans en excretas de palomas urbanas en San Miguel de Tucumán—Ar-gentina]. Boletín Micológico 2010, 25, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Rosario, I.; Acosta, B.; Colom, F. The pigeon and other birds as reservoirs for Cryptococcus spp. [La paloma y otras aves como reservorio de Cryptococcus spp]. Rev. Iberoam. Micol. 2008, 25, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Afshari, S.A.K.; Shokohi, T.; Aghili, S.R.; Badali, H. Epidemiology and molecular characterization of Cryptococcus neoformans isolated from pigeon excreta in Mazandaran province, northern Iran. J. Mycol. Méd. 2012, 22, 160–166. [Google Scholar] [CrossRef]
- Abulreesh, H.H.; Organji, S.R.; Elbanna, K.; Osman, G.E.H.; Almalki, M.H.K.; Abdel-Mallek, A.Y. First report of en-vironmental isolation of Cryptococcus neoformans and other fungi from pigeon droppings in Makkah, Saudi Arabia and in vitro susceptibility testing. Asian Pac. J. Trop. Dis. 2015, 5, 622–626. [Google Scholar] [CrossRef]
- Huamán, A.; Béjar, V.; Sáez, G.; Guevara, J.; Sevilla, R.; Tapia, M.; Castillo, E.; Valencia, E.; Marocho, L.; Pareja, E.; et al. Cryptococcus neoformans en heces de palomas (Columba livia) en Lima Metropolitana. Rev. Med. Hered. 2018, 29, 85. [Google Scholar] [CrossRef]
- Cabañes, F.J. Mycoses and zoonoses: Cryptococcus spp. Rev. Iberoam. Micol. 2008, 25, S1–S3. [Google Scholar] [CrossRef]
- Cogliati, M.; D’Amicis, R.; Zani, A.; Montagna, M.T.; Caggiano, G.; De Giglio, O.; Balbino, S.; De Donno, A.; Serio, F.; Susever, S.; et al. Environmental distribution of Cryptococcus neoformans and C. gattii around the Mediterranean basin. FEMS Yeast Res. 2016, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Quintero, E.; Castañeda, E.; Ruiz, A. Environmental distribution of Cryptococcus neoformans in the department of Cun-dinamarca-Colombia. [Distribución ambiental de Cryptococcus neoformans en el departamento de Cundinamarca-Colombia]. Rev. Iberoam. Micol. 2005, 22, 93–98. [Google Scholar] [CrossRef]
- Ergin, Ç.; Şengül, M.; Aksoy, L.; Döğen, A.; Sun, S.; Averette, A.F.; Cuomo, C.A.; Seyedmousavi, S.; Heitman, J.; Ilkit, M. Cryptococcus neoformans Recovered from Olive Trees (Olea europaea) in Turkey Reveal Allopatry With African and South American Lineages. Front. Cell. Infect. Microbiol. 2019, 9, 384. [Google Scholar] [CrossRef] [Green Version]
- Springer, D.J.; Chaturvedi, V. Projecting Global Occurrence of Cryptococcus gattii. Emerg. Infect. Dis. 2010, 16, 14–20. [Google Scholar] [CrossRef]
- Escandón, P.; Sánchez, A.; Firacative, C.; Castañeda, E. Isolation of Cryptococcus gattii molecular type VGIII, from Corymbia ficifolia detritus in Colombia. Med. Mycol. 2010, 48, 675–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escandón, P.; Quintero, E.; Granados, D.; Huérfano, S.; Ruiz, A.; Castañeda, E. Aislamiento de Cryptococcus gattii serotipo B a partir de detritos de Eucalyptus spp. en Colombia. Biomédica 2005, 25, 390. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.T.; Fraser, J.A.; Nichols, C.B.; Dietrich, F.S.; Carter, D.; Heitman, J. Clinical and Environmental Isolates of Cryptococcus gattii from Australia That Retain Sexual Fecundity. Eukaryot. Cell 2005, 4, 1410–1419. [Google Scholar] [CrossRef] [Green Version]
- Chowdhary, A.; Randhawa, H.S.; Prakash, A.; Meis, J.F. Environmental prevalence of Cryptococcus neoformans and Cryptococcus gattii in India: An update. Crit. Rev. Microbiol. 2012, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Bartlett, K.H.; Baer, R.; Byrnes, E.; Galanis, E.; Heitman, J.; Hoang, L.; Leslie, M.J.; MacDougall, L.; Magill, S.S.; et al. Spread of Cryptococcus gattii into Pacific Northwest Region of the United States. Emerg. Infect. Dis. 2009, 15, 1185–1191. [Google Scholar] [CrossRef]
- Hurst, S.; Lysen, C.; Cooksey, G.; Vugia, D.J.; Litvintseva, A.P.; Lockhart, S.R. Molecular typing of clinical and environ-mental isolates of Cryptococcus gattii species complex from southern California, United States. Mycoses 2019, 62, 1029–1034. [Google Scholar] [CrossRef]
- Linares, C.; Colom, M.F.; Torreblanca, M.; Esteban, V.; Álvaro, R.; Hagen, F. Environmental sampling of Ceratonia siliqua (carob) trees in Spain reveals the presence of the rare Cryptococcus gattii genotype AFLP7/VGIV. Rev. Iberoam. Micol. 2015, 32, 269–272. [Google Scholar] [CrossRef]
- Sorrell, T.C. Cryptococcus neoformans variety gattii. Med Mycol. 2001, 39, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Davel, G.; Abrantes, R.; Brudny, M.; Cordoba, S.; Rodero, L.; Canteros, C.E.; Perrotta, D. First environmental isolation of Cryptococcus neoformans var. gattii in Argentina. [Primer aislamiento ambi-ental de Cryptococcus neoformans var. gattii en Argentina]. Rev. Argent. Microbiol. 2003, 35, 110–112. [Google Scholar]
- Andrade-Silva, L. Susceptibility profile of clinical and environmental isolates of Cryptococcus neoformans and Crypto-coccus gattii in Uberaba, Minas Gerais, Brazil. Med. Mycol. 2013, 51, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Brito-Santos, F.; Barbosa, G.G.; Trilles, L.; Nishikawa, M.M.; Wanke, B.; Meyer, W.; Carvalho-Costa, F.A.; Lazéra, M.D.S. Environmental Isolation of Cryptococcus gattii VGII from Indoor Dust from Typical Wooden Houses in the Deep Amazonas of the Rio Negro Basin. PLoS ONE 2015, 10, e0115866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassi, F.K.; Bellet, V.; Drakulovski, P.; Krasteva, D.; Roger, F.; Valérie, B.T.A.; Aboubakar, T.; Doumbia, A.; Kouakou, G.A.; Delaporte, E.; et al. Comparative typing analyses of clinical and environmental strains of the Cryptococcus neofor-mans/Cryptococcus gattii species complex from ivory coast. J. Med. Microbiol. 2018, 67, 87–96. [Google Scholar] [CrossRef]
- Randhawa, H.S.; Kowshik, T.; Preeti Sinha, K.; Chowdhary, A.; Khan, Z.U.; Yan, Z.; Xu, J.; Kumar, A. Distribution of Cryptococcus gattii and Cryptococcus neoformans in decayed trunk wood of Syzygium cumini trees in north-western India. Med. Mycol. 2006, 44, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Casadevall, A.; Perfect, J.R. Cryptococcus Neoformans; ASM Press: Washington, DC, USA, 1998; Volume 595. [Google Scholar] [CrossRef]
- Ellis, D.H.; Pfeiffer, T.J. Natural habitat of Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 1990, 28, 1642–1644. [Google Scholar] [CrossRef] [Green Version]
- Byrnes, E.J.; Bartlett, K.H.; Perfect, J.R.; Heitman, J. Cryptococcus gattii: An emerging fungal pathogen infecting humans and animals. Microbes Infect. 2011, 13, 895–907. [Google Scholar] [CrossRef] [Green Version]
- Firacative, C.; Lizarazo, J.; Illnait-Zaragozí, M.T.; Castañeda, E. The status of cryptococcosis in Latin America. Memórias Inst. Oswaldo Cruz 2018, 113, e170554. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, K.H.; Kidd, S.E.; Kronstad, J.W. The emergence of Cryptococcus gattii in British Columbia and the Pacific Northwest. Curr. Infect. Dis. Rep. 2008, 10, 58–65. [Google Scholar] [CrossRef]
- Upton, A.; Fraser, J.A.; Kidd, S.E.; Bretz, C.; Bartlett, K.H.; Heitman, J.; Marr, K.A. First Contemporary Case of Human Infection with Cryptococcus gattii in Puget Sound: Evidence for Spread of the Vancouver Island Outbreak. J. Clin. Microbiol. 2007, 45, 3086–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogliati, M.; Medica, L.M.; Biomediche, S.; Pascal, V. Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An atlas of molecular types. Hindawi Publ. Corp. Sci. 2013, 2013, 1–23. [Google Scholar]
- Kidd, S.E.; Hagen, F.; Tscharke, R.L.; Huynh, M.; Bartlett, K.H.; Fyfe, M.; Macdougall, L.; Boekhout, T.; Kwon-Chung, K.J.; Meyer, W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Co-lumbia, Canada). Proc. Natl. Acad. Sci. USA 2004, 101, 17258–17263. [Google Scholar] [CrossRef] [Green Version]
- Escandón, P.; Lizarazo, J.; Agudelo, C.I.; Castañeda, E. Cryptococcosis in Colombia: Compilation and analysis of data from laboratory based surveillance. J. Fungi (Basel) 2018, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Granados, D.P.; Castañeda, E. Isolation and characterization of Cryptococcus neoformans varieties recovered from natu-ral sources in Bogotá, Colombia, and study of ecological conditions in the área. Microb. Ecol. 2005, 49, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Valiente, M.F.; Alberdi, M.; Meseguer, I.; Torres Rodriguez, J.M. Isolation of Cryptococcus neoformans in environmental samples from Alicante. [Aislamiento de Cryptococcus neoformans en muestras de medio ambiente de Alicante]. Rev. Iberoam. Micol. 1997, 14, 63–64. [Google Scholar]
- Noguera, M.C.; Escandón, P.; Castañeda, E. Cryptococcosis in Atlántico, Colombia: An approximation of the preva-lence of this mycosis and the distribution of the etiological agent in the environment. Rev. Soc. Bras. Med. Trop. 2015, 48, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Instituto de Hidrología Meteorología y Estudios Ambientales—IDEAM. Study of the Climatic Characterization of Bogotá and the Upper Tunjuelo River Basin; Instituto de Hidrología Meteorología y Estudios Ambientales: Bogotá, Colombia, 1999; pp. 19–21. [Google Scholar]
- Gonzalo Hurtado, M. Analysis of the Average Behavior and Long-Term Trends of the Mean Maximum Temperature for the Hydroclimatic Regions of Colombia; Instituto de Hidrología Meteorología y Estudios Ambientales: Bogotá, Colombia, 2012; p. 61. [Google Scholar]
- IDEAM. Map: Multi-Year Average Annual Total Mean Precipitation 1981–2010; Instituto de Hidrología Meteorología y Estudios Ambientales: Bogotá, Colombia, 2014; p. 1. [Google Scholar]
- Bennett, J.; Kwon-Chung, K.; Theodore, T.Y. Biochemical differences between Cryptococcus neoformans serotypes. [Diferencias bioquímicas entre los serotipos de Cryptococcus neoformans]. Med. Mycol. 1978, 16, 167–174. [Google Scholar] [CrossRef]
- Cadena, M.; López, N.; Carlos, R.; Vega, A.; González, O.; Cubillos, M.I. Climatological Yearbook 2016; Instituto de Hidrología Meteorología y Estudios Ambientales: Bogotá, Colombia, 2016; p. 355. [Google Scholar]
- Mak, S.; Vélez, N.; Castañeda, E.; Escandón, P.; Colombian Group. The Fungus among Us: Cryptococcus neoformans and Cryptococcus gattii Ecological Modeling for Colombia. J. Fungi 2015, 1, 332–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granados, D.P.; Castañeda, E. Influence of climatic conditions on the isolation of members of the Cryptococcus neoformans species complex from trees in Colombia from 1992–2004. FEMS Yeast Res. 2006, 6, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Watkins, R.; King, J.; Johnston, S. Nutritional Requirements and Their Importance for Virulence of Pathogenic Crypto-coccus Species. Microorganisms 2017, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Mazuelos, E.M.; Ana Isabel, A.G. Microbiological aspects of cryptococcosis in the post-HAART era. [Aspectos microbi-ológicos de la criptococosis en la era post-TARGA]. Enferm. Infecc. Microbiol. Clin. 2010, 28, 40–45. [Google Scholar] [CrossRef]
- Cogliati, M.; Puccianti, E.; Montagna, M.T.; De Donno, A.; Susever, S.; Ergin, C.; Velegraki, A.; Ellabib, M.S.; Nardoni, S.; Macci, C.; et al. Fundamental niche prediction of the pathogenic yeasts Cryptococcus neoformans and Cryptococcus gattii in Europe. Environ. Microbiol. 2017, 19, 4318–4325. [Google Scholar] [CrossRef]
- Sorrell, T.C.; David, E.H. Ecology of Cryptococcus neoformans in central Africa. Rev. Iberoam. Micol. 1997, 14, 42–43. [Google Scholar] [PubMed]
- Wang, Y.; Aisen, P.; Casadevall, A. Cryptococcus neoformans melanin and virulence: Mechanism of action. Infect. Immun. 1995, 63, 3131–3136. [Google Scholar] [CrossRef] [Green Version]
- Lazéra, M.S.; Pires, F.D.; Camillo-Coura, L.; Nishikawa, M.M.; Bezerra, C.C.; Trilles, L.; Wanke, B. Natural habitat of Cryptococcus neoformans var. neoformans in decaying wood forming hollows in living trees. J. Med. Vet. Mycol. 1996, 34, 127–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escandón, P.; Huérfano, S.; Castañeda, E. Experimental inoculation of Terminalia catappa with an environmental isolate of Cryptococcus neoformans var. gattii serotype C. Inoculación experimental de Terminalia catappa con un aislamiento ambien-tal de Cryptococcus neoformans var. gattii serotipo C]. Biomédica 2002, 22, 524. [Google Scholar] [CrossRef] [Green Version]
- Randhawa, H.S.; Kowshik, T.; Khan, Z.U. Decayed wood of Syzygium cumini and Ficus religiosa living trees in Del-hi/New Delhi metropolitan area as natural habitat of Cryptococcus neoformans. Med. Mycol. 2003, 41, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitz, S.M. The Ecology of Cryptococcus neoformans and the Epidemiology of Cryptococcosis. Clin. Infect. Dis. 1991, 13, 1163–1169. [Google Scholar] [CrossRef]
- Ruiz, A.; Fromtling, R.A.; Bulmer, G.S. Distribution of Cryptococcus neoformans in a natural site. Infect. Immun. 1981, 31, 560–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordóñéz, N.; Castaneda, E. Serotipificación de aislamientos clínicos y del medio ambiente de Cryptococcus neoformans en Colombia. Biomédica 1994, 14, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Litvintseva, A.P.; Thakur, R.; Reller, L.B.; Mitchell, T.G. Prevalence of Clinical Isolates of Cryptococcus gattii Serotype C among Patients with AIDS in Sub-Saharan Africa. J. Infect. Dis. 2005, 192, 888–892. [Google Scholar] [CrossRef] [Green Version]
- Escandón, P.; Sánchez, A.; Martínez, M.; Meyer, W.; Castañeda, E. Molecular epidemiology of clinical and environmental isolates of the Cryptococcus neoformans species complex reveals a high genetic diversity and the presence of the molecular type VGII mating type a in Colombia. FEMS Yeast Res. 2006, 6, 625–635. [Google Scholar] [CrossRef] [Green Version]
| Reference (Number) | City | Sampling Period | Total of Samples | Climatic Conditions | Isolated Species | Type of Samples | |||
|---|---|---|---|---|---|---|---|---|---|
| Number | Positive Environmental Isolated (%) | Species | n | Samples | Samples Species | ||||
| [83] | Barranquilla | September 2012–November 2014 | 2068 | 0.4 | T (°C): 28 P (mm): 500–1500 | C. neoformans | 8 | Leaves, flower, land, cortex, dendrites | Almendro (Terminalia catappa), Tabebuia rosea |
| [20] | Bogotá | 1997–1999 | 426 | 3.99 | T (°C): 13.4 * | C. laurentii | 14 | Bark | Eucalyptus camaldulensis |
| C. neoformans var. neoformans, serotype A | 2 | ||||||||
| C. albidus | 1 | ||||||||
| [21] | Bogotá | 1994 (August and November) | 562 | 4.72 | T (°C): 16 RH (%): 62.5 | C. laurentii | 8 | Flower (2), seeds (2), leaves (1), stems (1), debris (2) | Eucalyptus sp. |
| C. macerans | 5 | Flower (2), leaves (2), stems (1) | |||||||
| C. gattii serotype C | 4 | Seeds (1), leaves (2), stems (1) | |||||||
| [81] | Bogotá | 2003 (January–May) | 480 | 7.92 | T(°C):19.124 500 and 1000 mm * | C. neoformans var. gattii | 33 | Bark (23), soil (12), and hollow (3) | Ficus soatensis (11), Ficus tequendamae (2), Croton bogotanus (1), Croton funckianus (1), Coussapoa sp. (1), Eucalyptus camaldulensis (7), Eucalyptus golobulus (3), (2), Cupressus lusitanica (1), Acacia decurriiiiens (3) |
| C. neoformans var. grubii | 5 | ||||||||
| [59] | Bogotá | 2007 (February and August) | 128 | 20 | T(°C): 19.124 500 and 1000 mm * | C. gattii | 15 | Bark (28), soil (37), debris (52), seeds (4), and flowers (7). | Corymbia ficifolia |
| [28] | Santiago de Cali | - | 119 | 49.58 | T (°C): 28.55 * | C. neoformans var. neoformans | 59 | Pigeon feces | Columba livia |
| [29] | Santiago de Cali | 1994–1995 | 380 | 1.6 | pH 6.0 | C. neoformans var. neoformans | 1 | Bird droppings | Polyborus plancus |
| [25] | Santiago de Cúcuta | 1997 May–September | 157 | 12.546 | P (mm): 763 | C. gattii serotype C | 4 | Debris | Polyborus plancus Terminalia catappa |
| [24] | Cúcuta | 1997–1999 | 370 | 19.38 | T (°C): 28.83 RH (%): 69 P (mm): 595–1341 | C. gattii, serotype C | 31 | Debris | Terminalia catappa (almond-tree) |
| [26] | Cúcuta | 2008 (January, February and August) | 4389 | 0.14 | T (°C): 28.8 (RH) (%): 68 | C. gattii (serotype C) | 2 | Soil around the trees | Ficus sp. |
| C. gattii (serotype B) | 1 | ||||||||
| C. neoformans (Serotype A) | 3 | Soil around the trees | Ficus sp. | ||||||
| [27] | Cúcuta | October 2016–April 2017 | 1300 | 4.3 | T (°C): 24.95 P (mm): 70–64 * - | C. neoformans var, grubii | 19 | Soil, Bark, nuts and leaves. | Mango (Mangifera indica), Oití (Licania tomentosa), Samán (Samanea saman), Tamarindo (Tamarindus indica), Totumo (Crescentia cujete), Chiminango (Pithecellobium dulce) Limón swingle (Swinglea glutinosa), Mamoncillo (Melicoccus bijugatus) y Almendro (Terminalia catappa) |
| [56] | Cundinamarca | - | 765 | 13.59 | T (°C): 12–18 | C. neoformans, serotype A | 32 | Pigeon feces (31), debris (1) | Columba livia, Eucalyptus spp. |
| C. gatti serotype B | 62 | Debris | Eucalyptus spp. | ||||||
| [60] | La Calera | 2003 (February and March) | 167 | 27.54 | T (°C): 13 | C. gattii serotype B | 46 | Debris | Eucalyptus spp. |
| [22] | Neiva | 2018 | 118 | 6.8 | T(°C):27.7 442 masl | C. neoformans var. grubii | 8 | Pigeon feces (8) Fruits, leaves, and debris of almond trees (0) | Columba livia Terminalia catappa |
| [19] | Montería | 2008–2009 | 2445 | 8.88 | T (°C): 27 | C. gattii | 217 | Flowers, bark and soil | Terminalia catappa |
| [23] | Pasto | - | 128 | 26.56 | Considerable humidity and sunshine conditions | C. neoformans | 34 | Pigeon feces | Columba livia |
| [18] | Popayán | 2012–2013 (September–June) | 303 | 38.94 | T(°C):18–191735 masl | C. neoformans var. grubii | 118 | Bird droppings | Columba livia, Bubulcus ibis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna-Espinosa, B.-N.; Guzmán-Sanabria, D.; Forero-Castro, M.; Escandón, P.; Sánchez-Quitian, Z.A. Environmental Status of Cryptococcus neoformans and Cryptococcus gattii in Colombia. J. Fungi 2021, 7, 410. https://doi.org/10.3390/jof7060410
Serna-Espinosa B-N, Guzmán-Sanabria D, Forero-Castro M, Escandón P, Sánchez-Quitian ZA. Environmental Status of Cryptococcus neoformans and Cryptococcus gattii in Colombia. Journal of Fungi. 2021; 7(6):410. https://doi.org/10.3390/jof7060410
Chicago/Turabian StyleSerna-Espinosa, Briggith-Nathalia, Diomedes Guzmán-Sanabria, Maribel Forero-Castro, Patricia Escandón, and Zilpa Adriana Sánchez-Quitian. 2021. "Environmental Status of Cryptococcus neoformans and Cryptococcus gattii in Colombia" Journal of Fungi 7, no. 6: 410. https://doi.org/10.3390/jof7060410






