Potential of Anti-Cancer Activity of Secondary Metabolic Products from Marine Fungi
Abstract
:1. Introduction
2. Systematic Literature Review Methodology
3. Bibliometric Analysis
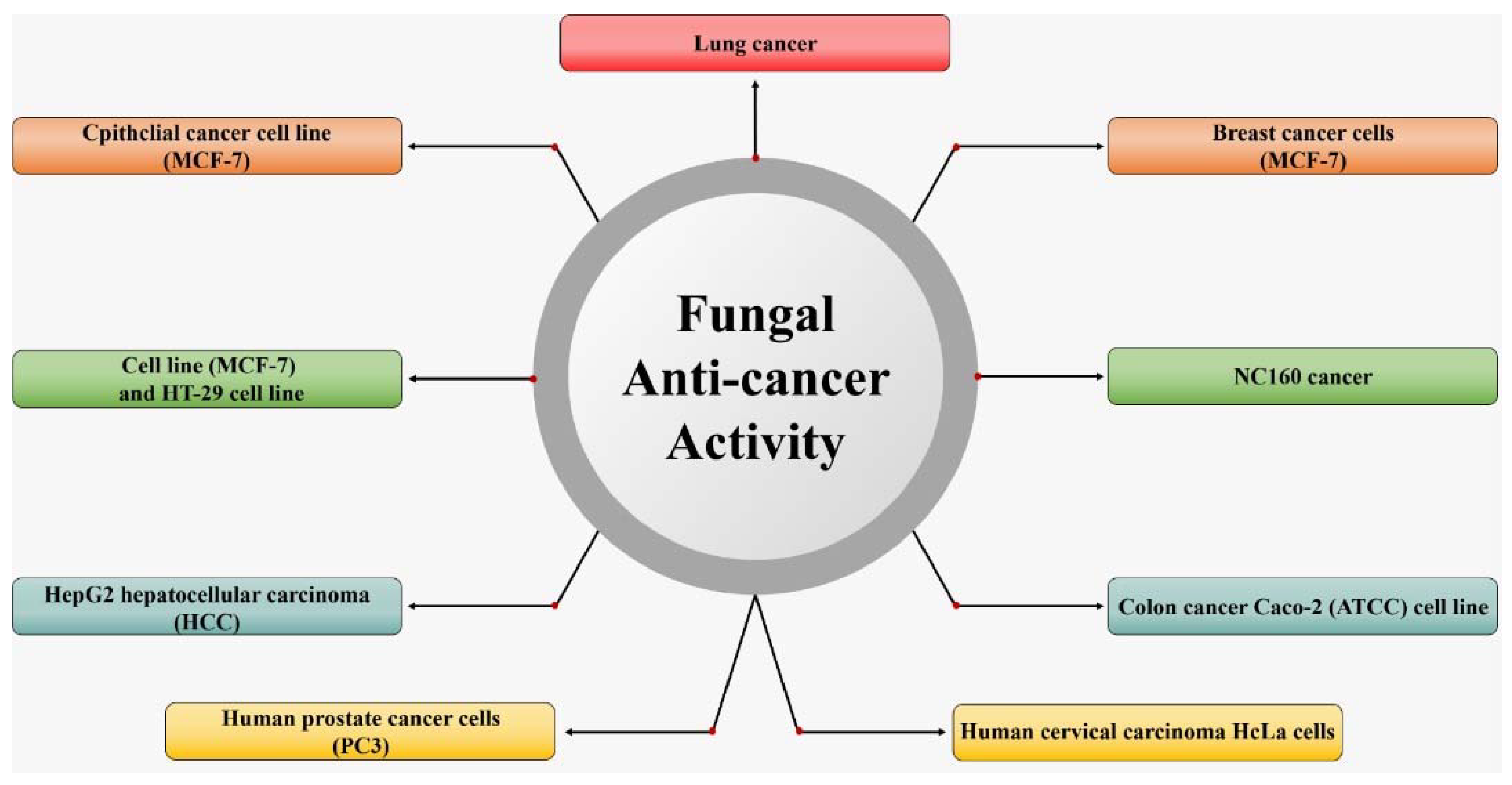
4. Potential of Secondary Metabolic Substances from Marine Fungi as Anticancer Agents
5. Application of L-Asparaginase as an Anti-Cancer Agent
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, P.N.; Yen, M.-R.; Chiang, C.-Y.; Lin, H.-C.; Chen, P.-Y. Detecting and prioritizing biosynthetic gene clusters for bioactive compounds in bacteria and fungi. Appl. Microbiol. Biotechnol. 2019, 103, 3277–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine Fungi: A Source of Potential Anticancer Compounds. Front. Microbiol. 2018, 8, 2536. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sørensen, J.L.; Hansen, F.T.; Arvas, M.; Syed, M.F.; Hassan, L.; Benz, J.P.; Record, E.; Henrissat, B.; Pöggeler, S.; et al. Genome Sequencing and analyses of Two Marine Fungi from the North Sea Unraveled a Plethora of Novel Biosynthetic Gene Clusters. Sci. Rep. 2018, 8, 10187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vala, A.K.; Sachaniya, B.; Dudhagara, D.; Panseriya, H.Z.; Gosai, H.; Rawal, R.; Dave, B.P. Characterization of L-asparaginase from marine-derived Aspergillus niger AKV-MKBU, its antiproliferative activity and bench scale production using industrial waste. Int. J. Biol. Macromol. 2018, 108, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Doriya, K.; Kumar, D.S. Solid state fermentation of mixed substrate for l-asparaginase production using tray and in-house designed rotary bioreactor. Biochem. Eng. J. 2018, 138, 188–196. [Google Scholar] [CrossRef]
- Paul, V.; Tiwary, B.N. An investigation on the acrylamide mitigation potential of l-asparaginase from Aspergillus terreus BV-C strain. Biocatal. Agric. Biotechnol. 2020, 27, 101677. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.A.; Awad, M.F.; El-Shenawy, F.S.; El-Bondkly, A.M.A. Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J. Biol. Sci. 2021, 28, 2540–2548. [Google Scholar] [CrossRef]
- Krishnapura, P.R.; Belur, P.D. Partial purification and characterization of L-asparaginase from an endophytic Talaromyces pinophilus isolated from the rhizomes of Curcuma amada. J. Mol. Catal. B Enzym. 2016, 124, 83–91. [Google Scholar] [CrossRef]
- Baskar, G.; Sree, N.S. Synthesis, characterization and anticancer activity of β-cyclodextrin-Asparaginase nanobi-ocomposite on prostate and lymphoma cancer cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101417. [Google Scholar] [CrossRef]
- Jenila, A.V.; Gnanadoss, J.J. Formulation of A Suitable Medium and its Optimization for Maximizing L-Asparaginase Production from Endophytic Fungi Fusarium sp. LCJ273. Biosci. Biotechnol. Res. Asia 2018, 15, 887–898. [Google Scholar] [CrossRef]
- Fan, B.; Dewapriya, P.; Li, F.; Grauso, L.; Blümel, M.; Mangoni, A.; Tasdemir, D. Pyrenosetin D, a New Pentacyclic Decalinoyltetramic Acid Derivative from the Algicolous Fungus Pyrenochaetopsis sp. FVE-087. Mar. Drugs 2020, 18, 281. [Google Scholar] [CrossRef]
- Ashok, A.; Doriya, K.; Rao, J.V.; Qureshi, A.; Tiwari, A.K.; Kumar, D.S. Microbes Producing L-Asparaginase free of Glutaminase and Urease isolated from Extreme Locations of Antarctic Soil and Moss. Sci. Rep. 2019, 9, 1423. [Google Scholar] [CrossRef] [Green Version]
- Elshafei, A.M.; El-Ghonemy, D.H. Screening and media optimization for enhancing L-asparaginase production, an anticancer agent, from different filamentous fungi in solid state fermentation. Biotechnol. J. Int. 2015, 1–15. [Google Scholar] [CrossRef]
- Golbabaie, A.; Nouri, H.; Moghimi, H.; Khaleghian, A. L-asparaginase production and enhancement by Sarocladium strictum: In vitro evaluation of anti-cancerous properties. J. Appl. Microbiol. 2020, 129, 356–366. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Lee, J.S.; Qian, Z.-J.; Li, Y.-X.; Kim, K.-N.; Heo, S.-J.; Jeon, Y.-J.; Park, W.S.; Choi, I.-W.; Je, J.-Y.; et al. Gliotoxin Isolated from Marine Fungus Aspergillus Sp. Induces Apoptosis of Human Cervical Cancer and Chondrosarcoma Cells. Mar. Drugs 2013, 12, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Gao, W.; Guan, D.; Wang, J.; Liu, M.; Chen, C.; Zhu, H.; Zhou, Y.; Lai, Y.; Hu, Z.; et al. Butenolides from a marine-derived fungus Aspergillus terreus with antitumor activities against pancreatic ductal adenocarcinoma cells. Bioorganic Med. Chem. 2018, 26, 5903–5910. [Google Scholar] [CrossRef]
- Malhão, F.; Ramos, A.A.; Buttachon, S.; Dethoup, T.; Kijjoa, A.; Rocha, E. Cytotoxic and Antiproliferative Effects of Preussin, a Hydroxypyrrolidine Derivative from the Marine Sponge-Associated Fungus Aspergillus candidus KUFA 0062, in a Panel of Breast Cancer Cell Lines and Using 2D and 3D Cultures. Mar. Drugs 2019, 17, 448. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-J.; Wang, S.-W.; Chiang, Y.-R.; Pang, K.-L.; Kuo, Y.-H.; Shih, T.-Y.; Lee, T.-H. Highly Oxygenated Constituents from a Marine Alga-Derived Fungus Aspergillus giganteus NTU967. Mar. Drugs 2020, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- El-Hady, F.K.A.; Shaker, K.H.; Souleman, A.M.A.; Fayad, W.; Abdel-Aziz, M.S.; Hamed, A.A.; Iodice, C.; Tommonaro, G. Comparative Correlation Between Chemical Composition and Cytotoxic Potential of the Coral-Associated Fungus Aspergillus sp. 2C1-EGY Against Human Colon Cancer Cells. Curr. Microbiol. 2017, 74, 1294–1300. [Google Scholar] [CrossRef]
- Wijesekara, I.; Zhang, C.; Van Ta, Q.; Vo, T.-S.; Li, Y.-X.; Kim, S.-K. Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma HeLa cells. Microbiol. Res. 2014, 169, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Inagaki, M.; Chai, H.-B.; Wieboldt, T.; Rapplye, C.; Rakotondraibe, L.H. Halogenated Compounds from Directed Fermentation of Penicillium concentricum, an Endophytic Fungus of the Liverwort Trichocolea tomentella. J. Nat. Prod. 2017, 80, 1397–1403. [Google Scholar] [CrossRef]
- Jones, E.G.; Pang, K.-L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J. An online resource for marine fungi. Fungal Diversity 2019, 96, 347–433. [Google Scholar] [CrossRef]
- Brereton, P.; Kitchenham, B.A.; Budgen, D.; Turner, M.; Khalil, M. Lessons from applying the systematic literature review process within the software engineering domain. J. Syst. Softw. 2007, 80, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Heitman, J.; Hom, E.F.Y.; Ianiri, G.; Jones, A.C.; et al. Fungi in the Marine Environment: Open Questions and Unsolved Problems. mBio 2019, 10, e01189-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.J. Natural Products from Marine Fungi. Mar. Drugs 2020, 18, 230. [Google Scholar] [CrossRef]
- Zin, S.R.M.; Kassim, N.M.; Alshawsh, M.A.; Hashim, N.E.; Mohamed, Z. Biological activities of Anastatica hierochuntica L.: A systematic review. Biomed. Pharmacother. 2017, 91, 611–620. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef]
- Petersen, L.-E.; Kellermann, M.Y.; Schupp, P.J. Secondary Metabolites of Marine Microbes: From Natural Products Chemistry to Chemical Ecology. In Youmares 9—The Oceans: Our Research, Our Future; Springer Science and Business Media LLC: Oldenburg, Germany; Cham, Switzerland, 2019; pp. 159–180. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S.; Ansari, M.I.; Ahmad, A.; Mishra, M. Major bioactive metabolites from marine fungi: A Review. Bioinformation 2015, 11, 176–181. [Google Scholar] [CrossRef]
- Oda, T.; Namikoshi, M.; Akano, K.; Kobayashi, H.; Honma, Y.; Kasahara, T. Verrucarin A Inhibition of MAP Kinase Activation in a PMA-stimulated Promyelocytic Leukemia Cell Line. Mar. Drugs 2005, 3, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Zabielska, J.; Sledzinski, T.; Stelmanska, E. Acyl-Coenzyme A: Cholesterol Acyltransferase Inhibition in Cancer Treatment. Anticancer. Res. 2019, 39, 3385–3394. [Google Scholar] [CrossRef] [PubMed]
- Grinde, M.T.; Hilmarsdottir, B.; Tunset, H.M.; Henriksen, I.M.; Kim, J.; Haugen, M.H.; Rye, M.B.; Mælandsmo, G.M.; Moestue, S.A. Glutamine to proline conversion is associated with response to glutaminase inhibition in breast cancer. Breast Cancer Res. 2019, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Li, Q.X.; Liu, R.J.; Shen, X.Q. Chinese medicine Amygdalin and β-glucosidase combined with antibody en-zymatic prodrug system as a feasible antitumor therapy. Chin. J. Integr. Med. 2018, 24, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef] [Green Version]
- Guest, T.C.; Rashid, S. Anticancer laccases: A review. J. Clin. Exp. Oncol. 5 2016, 1, 2. [Google Scholar]
- Su, Y.-C.; Cheng, T.-C.; Leu, Y.-L.; Roffler, S.R.; Wang, J.-Y.; Chuang, C.-H.; Kao, C.-H.; Chen, K.-C.; Wang, H.-E.; Cheng, T.-L. PET Imaging of β-Glucuronidase Activity by an Activity-Based 124I-Trapping Probe for the Personalized Glucuronide Prodrug Targeted Therapy. Mol. Cancer Ther. 2014, 13, 2852–2863. [Google Scholar] [CrossRef] [Green Version]
- Fedrowitz, M.; Hass, R.; Bertram, C.; Löscher, W. Salivary α-amylase exhibits antiproliferative effects in primary cell cultures of rat mammary epithelial cells and human breast cancer cells. J. Exp. Clin. Cancer Res. 2011, 30, 102. [Google Scholar] [CrossRef] [Green Version]
- Mook, O.R.; Frederiks, W.M.; Van Noorden, C.J. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta 2004, 1705, 69–89. [Google Scholar] [CrossRef]
- Saber-Moghaddam, N.; Nomani, H.; Sahebkar, A.; Johnston, T.P.; Mohammadpour, A.H. The change of immunosuppressive regimen from calcineurin inhibitors to mammalian target of rapamycin (mTOR) inhibitors and its effect on malignancy following heart transplantation. Int Immunopharmacol. 2019, 69, 150–158. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhou, T.; Zhao, Y.Y.; Chen, L.; Gong, W.M.; Xia, W.Q.; Ying, G.M.; Zheng, H.Q.; Zhang, Q.Q. Antitumor effects and related mechanisms of penicitrinine A, a novel alkaloid with a unique spiro skeleton from the marine fungus Penicillium citrinum. Mar. Drugs 2015, 13, 4733–4753. [Google Scholar] [CrossRef]
- Law, B.K. Rapamycin: An anti-cancer immunosuppressant? Crit. Rev. Oncol. 2005, 56, 47–60. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Rapamycin for longevity: Opinion article. Aging 2019, 11, 8048–8067. [Google Scholar] [CrossRef]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
- Wakharde, A.A.; Awad, A.H.; Bhagat, A.; Karuppayil, S.M. Synergistic Activation of Doxorubicin against Cancer: A Review. Am. J. Clin. Microbiol. Antimicrob. 2018, 1, 1009. [Google Scholar]
- Falini, B.; Brunetti, L.; Martelli, M.P. Dactinomycin in NPM1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Líšková, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, R.-G.; Zhen, Y.-S. Enediyne anticancer antibiotic lidamycin: Chemistry, biology and pharmacology. Ant Cancer Agents Med. Chem. 2008, 8, 123–131. [Google Scholar] [CrossRef]
- Gederaas, O.A.; Søgaard, C.; Viset, T.; Bachke, S.; Bruheim, P.; Arum, C.-J.; Otterlei, M. Increased Anticancer Efficacy of Intravesical Mitomycin C Therapy when Combined with a PCNA Targeting Peptide. Transl. Oncol. 2014, 7, 812–823. [Google Scholar] [CrossRef] [Green Version]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Yan, B.; Gao, L.; Dong, C.; Zhong, J.; D’Ortenzio, M.; Nguyen, B.; Lee, S.S.; Hu, X.; Liang, F. Targeted Delivery of Bleomycin: A Comprehensive Anticancer Review. Curr. Cancer Drug Targets 2016, 16, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jin, H.; Song, B.; Zhu, X.; Zhao, H.; Cai, J.; Lu, Y.; Chen, B.; Lin, Y. The cytotoxicity and anticancer mechanisms of alterporriol L, a marine bianthraquinone, against MCF-7 human breast cancer cells. Appl. Microbiol. Biotechnol. 2012, 93, 777–785. [Google Scholar] [CrossRef]
- Pang, X.; Lin, X.; Wang, P.; Zhou, X.; Yang, B.; Wang, J.; Liu, Y. Perylenequione Derivatives with Anticancer Activities Isolated from the Marine Sponge-Derived Fungus, Alternaria sp. SCSIO41014. Mar. Drugs 2018, 16, 280. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.A.; Castro-Carvalho, B.; Prata-Sena, M.; Malhão, F.; Buttachon, S.; Dethoup, T.; Kijjoa, A.; Rocha, E. Can marine-derived fungus Neosartorya siamensis KUFA 0017 extract and its secondary metabolites enhance antitumor activity of doxorubicin? An in vitro survey unveils interactions against lung cancer cells. Environ. Toxicol. 2020, 35, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Mathan, S.; Smith, A.A.; Kumaran, J.; Prakash, S. Anticancer and Antimicrobial Activity of Aspergillus protuberus SP1 Isolated from Marine Sediments of South Indian Coast. Chin. J. Nat. Med. 2011, 9, 286–292. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Kim, W.-S.; Park, S.J.; Kim, S.-K. Apoptotic effect of physcion isolated from marine fungus Microsporum sp. in PC3 human prostate cancer cells. Fish. Aquat. Sci. 2018, 21, 22. [Google Scholar] [CrossRef]
- Eamvijarn, A.; Kijjoa, A.; Bruyère, C.; Mathieu, V.; Manoch, L.; Lefranc, F.; Silva, A.; Kiss, R.; Herz, W. Secondary Metabolites from a Culture of the Fungus Neosartorya pseudofischeri and Their In Vitro Cytostatic Activity in Human Cancer Cells. Planta Medica 2012, 78, 1767–1776. [Google Scholar] [CrossRef] [Green Version]
- Dezaire, A.; Marchand, C.H.; Vallet, M.; Ferrand, N.; Chaouch, S.; Mouray, E.; Larsen, A.K.; Sabbah, M.; Lemaire, S.D.; Prado, S.; et al. Secondary Metabolites from the Culture of the Marine-derived Fungus Paradendryphiella salina PC 362H and Evaluation of the Anticancer Activity of Its Metabolite Hyalodendrin. Mar. Drugs 2020, 18, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farha, A.K.; Hatha, A.M. Bioprospecting potential and secondary metabolite profile of a novel sediment-derived fungus Penicillium sp. ArCSPf from continental slope of Eastern Arabian Sea. Mycology 2019, 10, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parthasarathy, R.; Chandrika, M.; Rao, H.Y.; Kamalraj, S.; Jayabaskaran, C.; Pugazhendhi, A. Molecular profiling of marine endophytic fungi from green algae: Assessment of antibacterial and anticancer activities. Process. Biochem. 2020, 96, 11–20. [Google Scholar] [CrossRef]
- Oppong-Danquah, E.; Passaretti, C.; Chianese, O.; Blümel, M.; Tasdemir, D. Mining the Metabolome and the Agricultural and Pharmaceutical Potential of Sea Foam-Derived Fungi. Mar. Drugs 2020, 18, 128. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, I.E.; Gross, H.; Pontius, A.; Kehraus, S.; Krick, A.; Kelter, G.; Maier, A.; Fiebig, H.-H.; Koenig, G.M. ChemInform Abstract: Epoxyphomalin A (I) and B (II), Prenylated Polyketides with Potent Cytotoxicity from the Marine-Derived Fungus Phoma sp. Org. Lett. 2010, 41, 5014–5017. [Google Scholar] [CrossRef]
- Anand, B.G.; Thomas, C.N.; Prakash, S. In vitro cytotoxicity and antimicrobial activity of Talaromyces flavus SP5 in-habited in the marine sediment of Southern Coast of India. Chin. J. Nat. Med. 2016, 14, 913–921. [Google Scholar] [PubMed]
- Kebede, B.; Wrigley, S.K.; Prashar, A.; Rahlff, J.; Wolf, M.; Reinshagen, J.; Gribbon, P.; Imhoff, J.F.; Silber, J.; Labes, A.; et al. Establishing the Secondary Metabolite Profile of the Marine Fungus: Tolypocladium geodes sp. MF458 and Subsequent Optimisation of Bioactive Secondary Metabolite Production. Mar. Drugs 2017, 15, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedaiwy, M.Y.; Awadalla, O.A.; Abou-Zeid, A.M.; Hamada, H.T. Optimal conditions for production of L-asparaginase from Aspergillus tamarii. Egypt. J. Exp. Biol. 2016, 12, 229–237. [Google Scholar]
- Da Rocha, W.R.V.; Costa-Silva, T.A.; Agamez-Montalvo, G.S.; Feitosa, V.A.; Machado, S.E.F.; de Souza Lima, G.M.; Pessoa, A., Jr.; Alves, H.S. Screening and optimizing fermentation production of l-asparaginase by Aspergillus terreus strain S-18 isolated from the Brazilian Caatinga Biome. J. Appl. Microbiol. 2019, 126, 1426–1437. [Google Scholar] [CrossRef]
- Da Cunha, K.C.; Sutton, D.A.; Fothergill, A.W.; Gené, J.; Cano, J.; Madrid, H.; de Hoog, S.; Crous, P.W.; Guarro, J. In vitro antifungal susceptibility and molecular identity of 99 clinical isolates of the opportunistic fungal genus Curvular-ia. Diagn. Microbiol. Infect. Dis. 2013, 76, 168–174. [Google Scholar] [CrossRef]
- El-Said, A.H.; Shebany, Y.M.; Hussein, M.A.; El-Dawy, E.G. Antimicrobial and L-asparaginase activities of endophytic fungi isolated from Datura innoxia and Hyoscyamus muticus medicinal plants. Eur. J. Biol. Res. 2016, 6, 135–144. [Google Scholar]
- Gonçalves, A.B.; Maia, A.C.F.; Rueda, J.A.; Vanzela, A.P.D.F.C. <b> Fungal production of the anti-leukemic enzyme L-asparaginase: From screening to medium development. Acta Sci. Biol. Sci. 2016, 38, 387–394. [Google Scholar]
- Cardoso, S.L.; De Freitas, M.M.; De Souza, P.M.; Homem-De-Mello, M.; Silveira, D.; Fonseca-Bazzo, Y.M.; Filho, E.X.; Junior, A.P.; Magalhães, P.O. Optimization of aqueous two-phase micellar system for partial purification of L-asparaginase from Penicillium sp. grown in wheat bran as agro-industrial residue. Braz. J. Microbiol. 2020, 51, 1–10. [Google Scholar] [CrossRef]
- Vieira, W.F.; Correa, H.T.; Campos, E.S.; Sette, L.D.; Pessoa Jr, A.; Cardoso, V.L.; Coutinho Filho, U. A novel multiple reactor system for the long-term production of L-asparaginase by Penicillium sp. LAMAI 505. Process Biochem. 2020, 90, 23–31. [Google Scholar] [CrossRef]



| (a) | ||
|---|---|---|
| No. | Objectives | Questions |
| 1 | To identify the potential application of secondary metabolic substances as anti-cancer against | How do secondary metabolic substances affect cancer cells? |
| 2 | To verify the effectiveness of the asparaginase enzyme against cancer cells | How does the asparaginase work to inhibit the cancer cells? |
| (b) | ||
| Categories | Description | |
| Context | This SLR presents the potential of secondary metabolic substances as anti-cancer. | |
| Objectives | The objective of the systematic review is to answer the questions regarding the application of secondary metabolic substances for cancer treatment. | |
| Method | The method used in this SLR process is data collection, screening, data verification, data analysis, and discussion. | |
| Result | The result shows the performance of each advanced technology, the advantages, and disadvantages of each method. | |
| Conclusion | The discussion regarding the objectives and questions is successfully achieved with clear discussion. | |
| Year | Initial | R | Included | Excluded |
|---|---|---|---|---|
| 2020 | 22 | 0.0182 | 4 | 18 |
| 2019 | 36 | 0.0111 | 5 | 31 |
| 2018 | 29 | 0.0207 | 3 | 26 |
| 2017 | 28 | 0.0143 | 4 | 24 |
| 2016 | 22 | 0.0364 | 5 | 17 |
| 2015 | 24 | 0.0167 | 3 | 21 |
| 2014 | 27 | 0.0222 | 7 | 20 |
| 2013 | 17 | 0.0471 | 6 | 11 |
| 2012 | 20 | 0.0500 | 4 | 16 |
| 2011 | 13 | 0.0462 | 3 | 10 |
| 2010 | 16 | 0.0625 | 6 | 10 |
| Total | 254 | 0.3452 | 50 | 204 |
| Enzyme | Molecular Structure | Gene Location (Human) | Role | References |
|---|---|---|---|---|
| Sterol O-acyltransferase (ACAT1) |  |  | The enzyme is involved in a variety of cancer types because of its association with increasing cholesteryl ester levels. The anti-cancer drug acts by inhibiting the enzyme leading to suppression of proliferation in a range of cancer cell types. | [29] |
| Glutaminase |  |  | The enzyme acts by inhibiting the target breast cancer cells by blocking the conversion of glutamine to glutamate. Therefore, some studies indicated that the enzyme is thereby potentially interfering with anaplerosis and the synthesis of amino acids and glutathione. | [30] |
| β-Glucosidase | 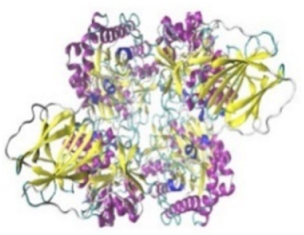 |  | β-Glucosidase plays an essential role in the inhibition of cancer cells by combing with cancer-cell-surface antigens leading to converting amarogentin to an active drug that acts on cancer cells and the surrounding antibodies to achieve a killing effect. | [31] |
| Lactate dehydrogenase B (LDHB) | 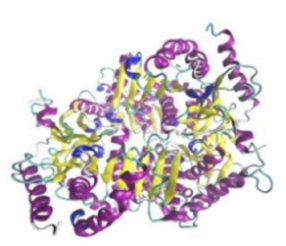 |  | The enzyme is an intracellular enzyme and is released into the bloodstream when the cells are damaged due to tissue destruction caused by tumor growth. Therefore, the increase of enzyme levels in the blood is usually used as indicators of tissue damage. | [32] |
| Laccase | 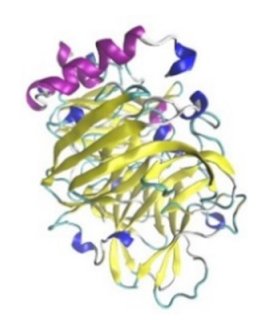 | 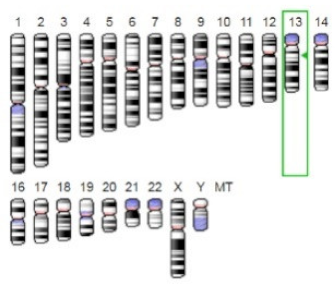 | Laccases from basidiomycetes fungi exhibited high potential as anti-cancer as well as having anti-proliferative activities primarily against breast cancer and liver carcinoma cell lines. | [33] |
| β-Glucuronidase |  | 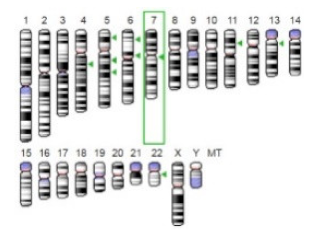 | Beta-glucuronidase (βG) is a biomarker for the diagnosis of cancer and prodrug therapy. Therefore, the image βG activity in patients is associated with the personalized glucuronide prodrug cancer therapy. | [34] |
| α-amylase |  |  | The experiments conducted on the primary cell cultures of human breast cancer cells exhibited an anti-proliferative effect for salivary α-amylase. | [35] |
| Matrix metallopeptidase 9 (MMP-9) |  |  | The modulation of gelatinase expression in the host cells is associated with the interactions between cancer cells and host tissues. Therefore, the inhibition of gelatinases by synthetic MMP inhibitors is an attractive approach to block cancer progression. | [36] |
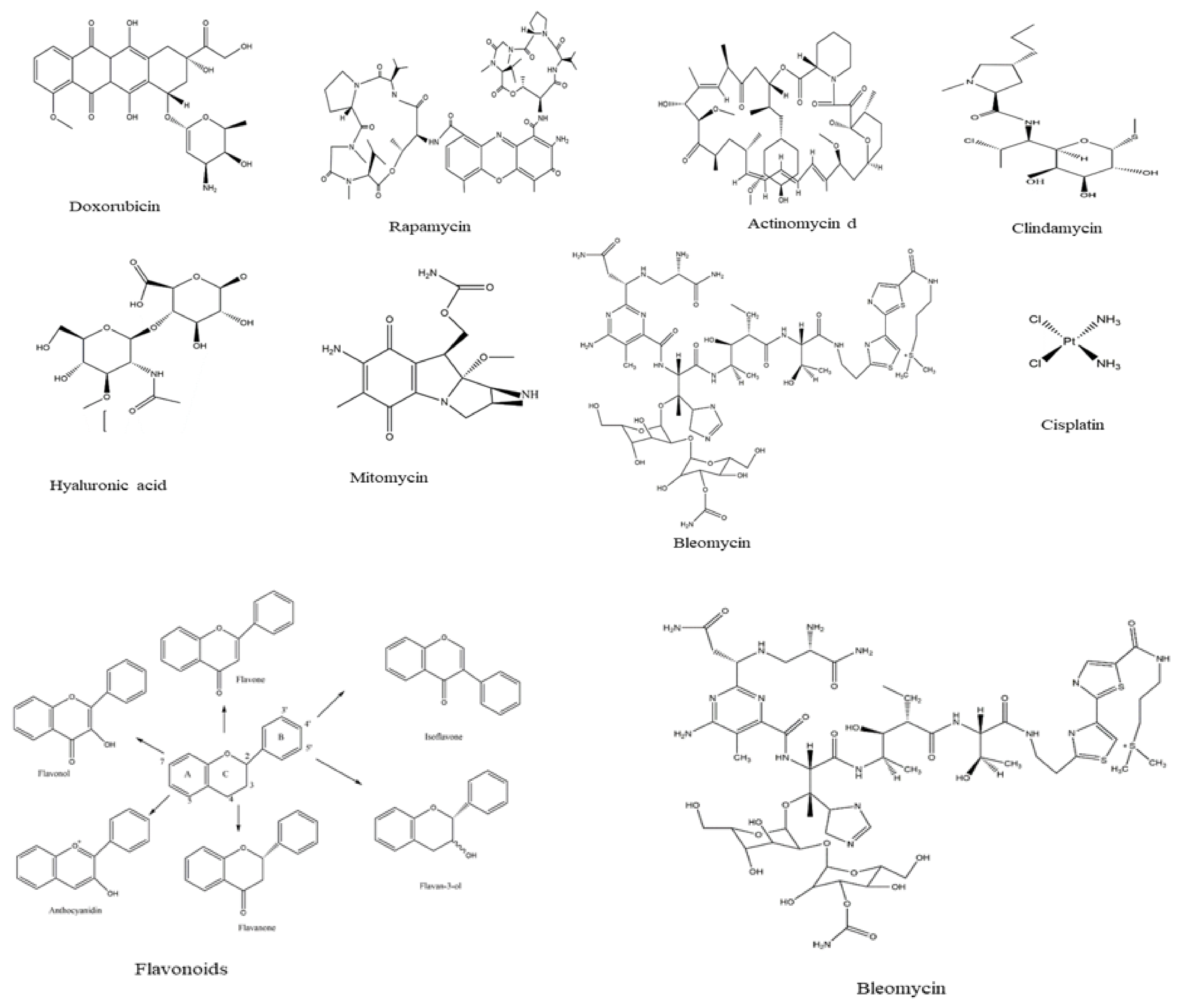
| Drug | Formula | Utilization | Side Effects | References |
|---|---|---|---|---|
| Doxorubicin | C17H30ClNO11 | The drug is used as anti-cancer for a wide range of cancers such as hematological malignancies, soft tissue sarcomas, and acute lymphoblastic leukemia | Vomiting, nausea, loss of appetite, diarrhea, darkening of skin or nails, missed menstrual periods, tiredness, weakness, puffy eyelids, eye redness, as well as the appearance of a reddish color to urine, tears, and sweat. | [42] |
| Actinomycin d | C62H86N12O16 | The drug has anti-cancer properties against Wilms tumor, Ewing′s sarcoma, rhabdomyosarcoma, testicular cancer, trophoblastic neoplasia, and ovarian cancer. | The drug is associated with low red and white blood cell levels, decrease in the low platelet levels leading to increased risk of infection, anemia, and bleeding. Nausea and vomiting, hair loss, sores in the mouth, skin reactions, diarrhea, acne, peeling, skin eruptions, and sensitivity to sunlight. | [43] |
| Flavonoids | The flavonoids have high potential as anti-cancer agents and exhibited great potential against cancer cells. | The side effects have been reported for the flavonoids. However, some reports indicate the presence of anemia, fever, and hives which have been disappeared when treatment was discontinued. | [44] | |
| Rapamycin | C51H79NO13 | The drug acts by inhibiting the tumor growth leading to halting tumor cell proliferation, and tumor cell apoptosis, and then suppressing tumor angiogenesis. | The side effects of the drug include stomatitis and mycositis which are associated with high doses or long term used. Moreover, some studies reported non-infectious interstitial pneumonitis and hyperglycemia. | [40] |
| Clindamycin | C18H33ClN2O5S | Clidamycin which is classified as a member of the enediyne anti-cancer antibiotic family exhibited cytotoxicities against cancers in vitro and in vivo. | Nausea, diarrhea, and vomiting, heartburn, metallic taste in the mouth, abdominal and joint pain, skin rash, redness, itching, vaginal itching, and burning. | [45] |
| Hyaluronic acid | (C14H21NO11)n | The drug is common because it is biocompatible, non-toxic, biodegradable, and non-immunogenic, as well as HA receptors are overexpressed on many tumor cells | Pain, redness, bruising, swelling, and itching. | [41] |
| Mitomycin | C15H18N4O5 | Mitomycin is anti-cancer produced by Streptomyces caespitosus and exhibited high efficiency against a wide variety of cancer types | Anorexia, fever, vomiting, and nausea, as well as a blurring of vision, headache, drowsiness, confusion, fatigue, syncope, thrombophlebitis, anemia, diarrhea, hematemesis, and pain | [46] |
| Cisplatin | [Pt(NH3)2Cl2] | The drug is among the most effective anti-cancer against solid tumors and acts by damaging DNA and inhibiting DNA synthesis. | Nausea, low blood counts, vomiting, ototoxicity hearing loss, ringing in the ears, kidney toxicity, blood test abnormalities | [47] |
| Bleomycin | C55H84N17O21S3 | The drug is used in combination with surgery or radiotherapy against squamous cell cancers, sarcoma, melanoma, both Hodgkin′s and non-Hodgkin′s lymphoma as well as testicular cancer | Fever, chills, redness, stretch marks, and darkening of the skin, peeling and thickening, nail thickening and banding as well as hair loss. | [48] |
| Doxorubicin | C17H30ClNO11 | The drug is used as anti-cancer for a wide range of cancers such as hematological malignancies, soft tissue sarcomas, and acute lymphoblastic leukemia | Vomiting, nausea, loss of appetite, diarrhea, darkening of skin or nails, missed menstrual periods, tiredness, weakness, puffy eyelids, eye redness, as well as the appearance of a reddish color to urine, tears, and sweat | [42] |
| Actinomycin d | C62H86N12O16 | The drug has anti-cancer properties against Wilms tumor, Ewing′s sarcoma, rhabdomyosarcoma, testicular cancer, trophoblastic neoplasia, and ovarian cancer. | The drug is associated with low red and white blood cell levels, decrease in the low platelet levels leading to increased risk of infection, anemia, and bleeding. Nausea and vomiting, hair loss, sores in the mouth, skin reactions, diarrhea, acne, peeling, skin eruptions, and sensitivity to sunlight | [43] |
| Flavonoids | The flavonoids have high potential as anti-cancer agents and exhibited great potential against cancer cells. | Side effects reported for the flavonoids indicate the presence of anaemia, fever, and hives which have been disappeared when treatment was discontinued | [44] | |
| Rapamycin | C51H79NO13 | The drug acts by inhibiting the tumor growth leading to halting tumor cell proliferation, and tumor cell apoptosis, and then suppressing tumor angiogenesis. | The side effect of the drug includes stomatitis and mycositis which is associated with high doses or chronically used. Moreover, some studies reported non-Infectious interstitial pneumonitis and hyperglycemia | [40] |
| Clindamycin | C18H33ClN2O5S | Clidamycin which is classified as a member of the enediyne anti-cancer antibiotic family exhibited cytotoxicities against cancers in vitro and in vivo. | Nausea, diarrhea, and vomiting, heartburn, metallic taste in the mouth, abdominal and joint pain, skin rash, redness, itching, vaginal itching and burning. | [45] |
| Hyaluronic acid | (C14H21NO11)n | The drug is common because it is biocompatible, non-toxic, biodegradable, and non-immunogenic, as well as the HA receptors are overexpressed on many tumour cells | Pain, redness, bruising, swelling, and itching | [41] |
| Mitomycin | C15H18N4O5 | Mitomycin is anti-cancer produced by Streptomyces caespitosus and exhibited high efficiency against a wide variety of cancer types | Anorexia, fever, vomiting, and nausea, as well as a blurring of vision, headache, drowsiness, confusion, fatigue, syncope, thrombophlebitis, anemia, diarrhea, hematemesis, and pain | [46] |
| Cisplatin | [Pt(NH3)2Cl2] | The drug is among the most effective anti-cancer against solid tumours and acts by damaging DNA and inhibiting DNA synthesis. | Nausea, low blood counts, vomiting, ototoxicity hearing loss, ringing in the ears, kidney toxicity, blood test abnormalities | [47] |
| Fungal Genus | Fungal Species | Active Substances | Effectiveness against Cancer | References |
|---|---|---|---|---|
| Alternaria | Alternaria sp. obtained from the fruit of a mangrove tree Aegiceras corniculatum | Alterporriol L (7) | Alterporriol L changed the cancer cell morphology and exhibited a significant inhibition of cell growth, as well as inducing cancer cell apoptosis or necrosis in breast cancer cells lines. | [49] |
| Alternaria sp. from a Callyspongia sp. sponge | Perylenequinone derivatives An altenusin derivative Phthalide racemates (9), Phenol derivatives. | These compounds exhibited cytotoxic activities against human erythroleukemia, human gastric carcinoma cells, and hepatocellular carcinoma cells. | [50] | |
| Aspergillus | Aspergillus sp., from marine brown algae | Gliotoxin (10) | Anti-cancer activity and apoptosis of cancer cells and DNA fragmentation, as well as induced activation of caspase-3, 8 and 9, down-regulation of Bcl-2, up-regulation of Bax in human cervical cancer (Hela) and human chondrosarcoma cells. | [12] |
| A. candidus associated with the marine sponge Epipolasis sp. | Preussin (11) (10 µM) | Preussin exhibited an ability to cause cell death as confirmed by caspase-3 immunostaining of breast cancer cells. | [14] | |
| Aspergillus giganteus isolated from Ulva lactuca | Aspergilsmins A–G, Patulin (8), Deoxytryptoquivaline (12), tryptoquivaline Quinadoline B. IC50 values between 2.7–7.3 µM | The compounds have exhibited anti-cancer activity against human hepatocellular carcinoma cells and prostate cancer cells. | [15] | |
| Aspergillus sp. | Hexadecanoic, Octadecanoic (13), Octadecenoic acids. | The compounds had significantly high cytotoxic activity against colorectal cancer cells. | [16] | |
| A.Protuberus isolated from marine sediments | n-butanol extract of mycelium | The extract exhibited anti-cancer activity against the Hep 2 cell line. | [52] | |
| A. terreus from sea deposit | Butenolide derivatives, Asperlides A–C Butenolides (+)-3′,3′-di-(dimethylallyl)-butyrolactone II, Versicolactone B (14) | The compounds exhibited anti-cancer activity against hepatocellular carcinoma, hepatocellular carcinoma, and pancreatic duct cancer. | [13] | |
| Aspergillus Neosartorya Talaromyces | A. similanensis, N. paulistensis, and T. trachyspermus | Crude ethyl acetate extracts | The extract exhibited anti-cancer activity against HepG2, HCT116, and A375. | [51] |
| Microsporum | Microsporum sp. isolated from the surface of a marine red alga Lomentaria catenata | Physcion (11.8 mg) | The compound induces cell apoptosis through down-regulating of Bcl-2 expression, up-regulating of Bax expression, as well as induced the formation of reactive oxygen species in HeLa cells. | [17] |
| Microsporum sp. isolated from the surface of marine red algae, Lomentaria catenata, | Physcion physcion activated caspase-3,8, 9, Ras, Bcl-xL, and Bcl-2 Bax (0–50 µM) | Physcion decreases cell proliferation and induces cell apoptosis in human prostate cancer cells. | [53] | |
| Neosartorya | N. pseudofischeri | 1,4-diacetyl-2,5-dibenzylpiperazine (16), Derivative, A quinazolinone-containing indole derivative, A new ester of 2,4-dihydroxy-6-methylbenzoic acid. | The compounds exhibited anti-cancer activity against human glioblastoma and non-small cell lung cancer Apoptosis-resistant cells, and distinct cancer cell lines. | [54] |
| N. siamensis isolated from Rumphella sp. sea fan | 2,4-dihydroxy-3-methylacetophenone (1), Nortryptoquivaline (2) Chevalone C (3), tryptoquivaline H (4), Epifiscalin-C (5) (0.54 to 100 µg/mL) | Effects on DNA damage, ultrastructural modifications, and intracellular accumulation in lung cancer cells. NS extract has cytotoxicity by inhibiting cell proliferation, increasing intracellular accumulation of Dox, and inducing cell death in lung cancer | [51] | |
| Paradendryphiella | P. salina (from the brown alga Pelvetia caniculata) | Hyalodendrin (251.53 mg) | The compound induces the changes in the phosphorylation status of p53 and altered expression of epithelial cancer cell line | [55] |
| Penicillium | P. concentricum isolated from the healthy liverwort Trichocolea tomentella | 2-Bromogentisyl alcohol (6), 3-hydroxy, benzenemethanol, Epoxydon 6-dehydroxy-6-bromogabosine C, 2-chlorogentisyl alcohol, gentisyl alcohol Griseofulvin (IC50 values of 8.4, 9.7, and 5.7 μM) | The compounds exhibited anti-cancer activity against the human caucasian colon adenocarcinoma cells line | [18] |
| Penicillium sp. isolated from marine sediments | (Z)-Octadec-9-enamide (oleamide) IC50 = 22.79 μg/mL | Anti-cancer activity against breast cancer cells. | [56] | |
| P. citrinum from marine sediments | Penicitrinine A (12–100 µM) | The compound induces A-375 cell apoptosis by decreasing the expression of Bcl-2 and increasing the expression of Bax in multiple tumor types | [38] | |
| P. oxalicum (from marine algae Chaetomorpho anteria) | Anthraquinone Cinnamic acid. (20–100 µg/mL) | The extract enhanced the membrane damage and apoptosis in breast cancer cells. The extract inhibited anti-cancer activity against human breast cancer and HeLa cells. | [57] | |
| Penicillium, Cladosporium, Emericellopsis and Plectosphaerella | Penicillium, Cladosporium, Emericellopsis and Plectosphaerella | Crude extracts | The extracts exhibited inhibitory potential anti-cancer against A-549 lung carcinoma cells, breast cancer, and human keratinocytes. | [58] |
| Phoma | Phoma sp. (obtained from the marine sponge Ectyplasia perox) | Epoxyphomalin A and B (IC50, 0.17–0.33 μg/mL] | The compound has strong cytotoxic properties in different cancer cell lines. | [59] |
| Pyrenochaetopsis | Pyrenochaetopsis sp. -from Fucus vesiculosus | Pentacyclic decalinoylspirotetramic acid, Pyrenosetin D (IC50 values of 77.5 and 39.3 µM) | Pyrenosetin D showed anti-cancer activity against the melanoma cell line and noncancerous keratinocyte cell line. | [7] |
| Talaromyces | T. flavus SP5 from marine sediment | Gusation A, 2-amino-1,3,4-trihydroxy-8-octadecene (18), Vitamin E, Tetradecanoic acid, 12-dimethyl-methyl ester, 2-O-benzyl-3,4-O isopropylidene-D-rythrosedi-ethyldithioacetal, methyl, acetate, 2,5-bis (4-methoxyphenyl)thiophene, and chalcone(LC50 value of 25.7 μg/mL) | The compounds exhibited anti-cancer activity against the HEp2 carcinoma cell line. | [60] |
| Tolypocladium | T. geodes isolated from a sponge sample. | Cyclosporin A (19), Efrapeptin D (17), Pyridoxatin, Terricolin A, Malettinins B and E, Tolypocladenols (different IC50) | Anti-tumor effects against cancer cell line panel. | [61] |
| Fungal Genus | Fungal Species | Production/Medium/ and Substrate | Factors Investigated | Characteristics | References |
|---|---|---|---|---|---|
| Aspergillus | A. niger AKV-MKBU | MCD | Tween 80 and Triton X-100, pH 4–10 | Stable at pH 4–10, at 20–400 °C. is 0.8141 mM and Vmax, 6.228 μM/mg/min. | [4] |
| A. tubingensis IBBL1 | SSF (Tray bioreactor) Cottonseed cake, wheat bran, and red gram husk consecutive | pH-8, 2%(w/w) inoculum, room temperature | Maximum activity was 20.58 U/gds after 120 h | [5] | |
| A. terreus | SmF and SSF, flaxseed oil cake, mustard oil cake, sugarcane bagasse | Temperature (25–45 °C), time (96–192 h), agitation (0–250 rpm) and inoculum (1–5 mL/100 mL media), l-asparagine and dextrose, pH 5.5. | The highest activity (84.3 U) with flaxseed oil cake as the substrate for the production of a purified enzyme with 6.39- fold purity. | [6] | |
| A. terreus | MCD | pH 6.2, 120 rpm, 32 °C, 72 h | The activity was 47.29 U/ mL. | [6] | |
| A. niger LBA 02 | SSF wheat bran, soybean meal, rice meal, chicken feather meal, chicken viscera meal, passion fruit peel flour | Temperature (25–35 °C), initial moisture content (40–60%) and inoculum concentration (2.1–7.99 × 106 spores/g). | The highest activity recorded with passion fruit peel flour (2380.11 U/gds) after 48 h at 30 °C, 3746.78 U/gds with 60% of moisture and (2.1 × 106) spores/g after 24 h at 25 °C. | [64] | |
| A. tamarii | SmF MCD | Incubation periods (1, 2, 3, 4, 5, 6, 7, 8, and 9 days), temperatures (25, 30, 35, and 40 °C). pH (2, 3, 4, 5, 6, 7), carbon source, sucrose, glucose, maltose, and lactose nitrogen source asparagine, yeast extract, peptone, and glutamine. | The highest activity (11.01 u/mL) at 30 °C for 7days of incubation pH 7.0, sucrose is the most effective carbon source, with L-asparagine as sole nitrogen source | [59] | |
| A. niger SVUAn1 | MCD | 30 °C and incubation periods (24–144 h), pH 6.2, 0.2% of carboxy-methylcellulose, fructose, maltose, starch, sucrose, and yeast extract. | Maximum production achieved at 96 h (4 days) with the incorporation of glucose as a carbon source in the culture medium | [65] | |
| A. terreus | Fermentation in a 5-l bioreactor system with MCD medium | 30 °C, 120 rpm, 96 h, glucose, sucrose, lactose or fructose, arginine, glutamine, asparagine, tyrosine, leucine, tryptophan, or histidine), urea, ammonium nitrate, sulfate. | Maximum production is of 108 U with glucose, proline, and asparagine. | [63] | |
| Fusarium | Fusarium sp. LCJ273 | SmF MCD Broth and wheat bran | Dextrose, ammonium sulfate Production at 120 rpm for 5–8 days. | Activity was recorded 9.18 ± 0.9 U/mL, at 3 g/L Dextrose, 20 g/L ammonium sulphate and 13.69 ± 0.4 U/mL at 2.5 g/L wheat bran after 5 d. | [7] |
| Penicillium sp. Fusarium sp. | Penicillium sp. T6.2) (Fusarium sp.) | stationary liquid state bioprocesses Medium Bacelar-1 Medium | Glycerol; L-asparagine; 105 mL−1 of inoculum, incubated for 72 h at 30 °C. | The enzyme activity was 8.3 U min/ mL from Penicillium sp.) and 11.4 U min/ mL from Fusarium sp. after 72 h in Bacelar-1 medium. | [66] |
| Penicillium | Penicillium sp. | the fermentation process in potato dextrose broth wheat bran | Temperature (25–35 °C), the incubation period (48–96 h), and initial pH (5–9), L-proline, L-asparagine, ammonium sulfate, yeast extract, sucrose, and glucose. | Maximum activity (2.33 IU/mL) detected at 2.8% L-asparagine 4.0% L-proline, 0.75% Potato dextrose broth, and 0.1% Sucrose. | [67] |
| Penicillium sp. LAMAI 505 | SSF multiple reactors with immobilized cells | pH level (pH), residence time (RT), the time between cycles (TC), and concentration of glucose and L-asparagine. | L-asparaginase activity was 13.7 U/gds was achieved at a residence time of 33.5 min, pH of 5.1, and concentrations of L-asparagine and glucose of 1.2 and 3.0 g/L. | [68] | |
| Talaromyces | T. pinophilus | MCD | Agitation rate (100–150 rpm), pH (4.0–9.5), temperature (15–40 °C), and (7–29 days), glucose (2–15), starch (2.5–15), yeast extract (2.5–15), and L-asparagine (5–15). | The enzyme activity of 108.95 U/mL was recorded at 120 rpm after at 120 h with L-asparagine, and starch as the carbon source than glucose. | [69] |
| Trichosporon | T. asahii IBBLA1 | NA | Temperature, pH, L-Asparagine concentration and glucose concentration. | Optimum enzyme activity of 20.57 U mL−1 was obtained at 30 °C and pH of 7.0 after 60 h | [9] |
| Trichoderma | T. viride F2 | SSF maize, rice bran, rice husk, wheat bran, wheat germ, rice straw, cottonseed wastes | pH value (3.0–8.0), 50 to 86% of moisture content, incubation temperature (25, 28, 35, and 40 °C). Inoculum size (1 × 104–1 × 109), surfactants (Tween 20,60 and 80 and Triton X-100 at 0.1% w/v). Carbon source (Glucose, sucrose, maltose, fructose, xylose, galactose, arabinose, soluble starch, and raffinose at 1.0% w/v), urea, yeast extract, casein, malt extract, proline, and peptone at 0.5%. | Maximal production 113.43 ± 5.11 U/g-ds with 75% of moisture content of 75%, 1 × 108 spores/mL, pH 5.0, at 28 °C for 4 days. Tween 20 enhanced the production by 1.19 folds. Glucose was the best carbon. | [10] |
| Sarocladium | S. strictum | MCD L-asparagine | pH 6.8, the incubation period was 2–3 days, carbon sources were D-glucose, starch and molasses, glycerol, ammonium sulphate as a mineral nitrogen source, and soybean powder and yeast extract | 1.84-fold increase in enzyme production, and Vmax was 9.74 m/mol and 8.19 mol/ min | [11] |
| Cancer Type | Preparation | Activity | References |
|---|---|---|---|
| Prostate cancer cell lines lymphoma cancer cells | L-Asparaginase was incorporated into nano biocomposites synthesized using β-cyclodextrin and chitosan. | The mixture exhibited high activity at the concentration of 125 μg/mL and against lymphoma cancer cells (U937) with IC50 value at 500 μg/mL of β-cyclodextrin-Asparaginase nanobiocomposite. | [6] |
| Cervical and brain cancer cell lines | The enzyme was immobilized onto a nanobiocomposite consisting of β-cyclodextrin and Gelatin. | The anticancer activity was 42.13% at 500 μg/mL and 48.60% at 62.5 μg/mL respectively. | [6] |
| K562 and HL60 cancer cell lines lymphoblastic leukemia | A crude enzyme was mixed with cell viability and then incubated for 24 h at 37 °C inside a CO2 incubator, thereafter, ten μL of 10% MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was added and incubated for 3 h, 100 μL of DMSO was added to the mixture, the absorbance was measured at A570 nm. | The toxicity of L-asparaginase against K562 and HL60 cancer cell lines and L6 as normal cells was determined with IC50 values were calculated as 0.4 and 0.5 IU/mL for K562 and HL60 respectively | [11] |
| Childhood leukemia | L-Asparaginase with a dose of ≥ 6000 IU/sq m three times weekly. | L-Asparaginase was effective in re-inducing remissions at 9.5% for 300 IU/sq m; 35.1% for 3000 IU/sq m; 53.5% for 6000 IU/sq m; and 62.5% for 12,000 IU/sq m. | [33] |
| Acute lymphoblastic leukemia | L-Asparaginase in doses from 10 to 1000 international units/kg body weight per day for 2 to 20 days. | 66% response rate for acute lymphoblastic leukemia and an approximately 12% response rate for nonlymphocytic leukemia. | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noman, E.; Al-Shaibani, M.M.; Bakhrebah, M.A.; Almoheer, R.; Al-Sahari, M.; Al-Gheethi, A.; Radin Mohamed, R.M.S.; Almulaiky, Y.Q.; Abdulaal, W.H. Potential of Anti-Cancer Activity of Secondary Metabolic Products from Marine Fungi. J. Fungi 2021, 7, 436. https://doi.org/10.3390/jof7060436
Noman E, Al-Shaibani MM, Bakhrebah MA, Almoheer R, Al-Sahari M, Al-Gheethi A, Radin Mohamed RMS, Almulaiky YQ, Abdulaal WH. Potential of Anti-Cancer Activity of Secondary Metabolic Products from Marine Fungi. Journal of Fungi. 2021; 7(6):436. https://doi.org/10.3390/jof7060436
Chicago/Turabian StyleNoman, Efaq, Muhanna Mohammed Al-Shaibani, Muhammed Adnan Bakhrebah, Reyad Almoheer, Mohammed Al-Sahari, Adel Al-Gheethi, Radin Maya Saphira Radin Mohamed, Yaaser Qaeed Almulaiky, and Wesam Hussain Abdulaal. 2021. "Potential of Anti-Cancer Activity of Secondary Metabolic Products from Marine Fungi" Journal of Fungi 7, no. 6: 436. https://doi.org/10.3390/jof7060436








