Inhibition of Mycotoxigenic Fungi in Different Vegetable Matrices by Extracts of Trichoderma Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Fungal Strains and Culture Conditions
2.3. Liquid Culture, Production and Extraction of Metabolites
2.4. Inoculum Preparation
2.5. Study of the Extracted Metabolites in Different Matrices
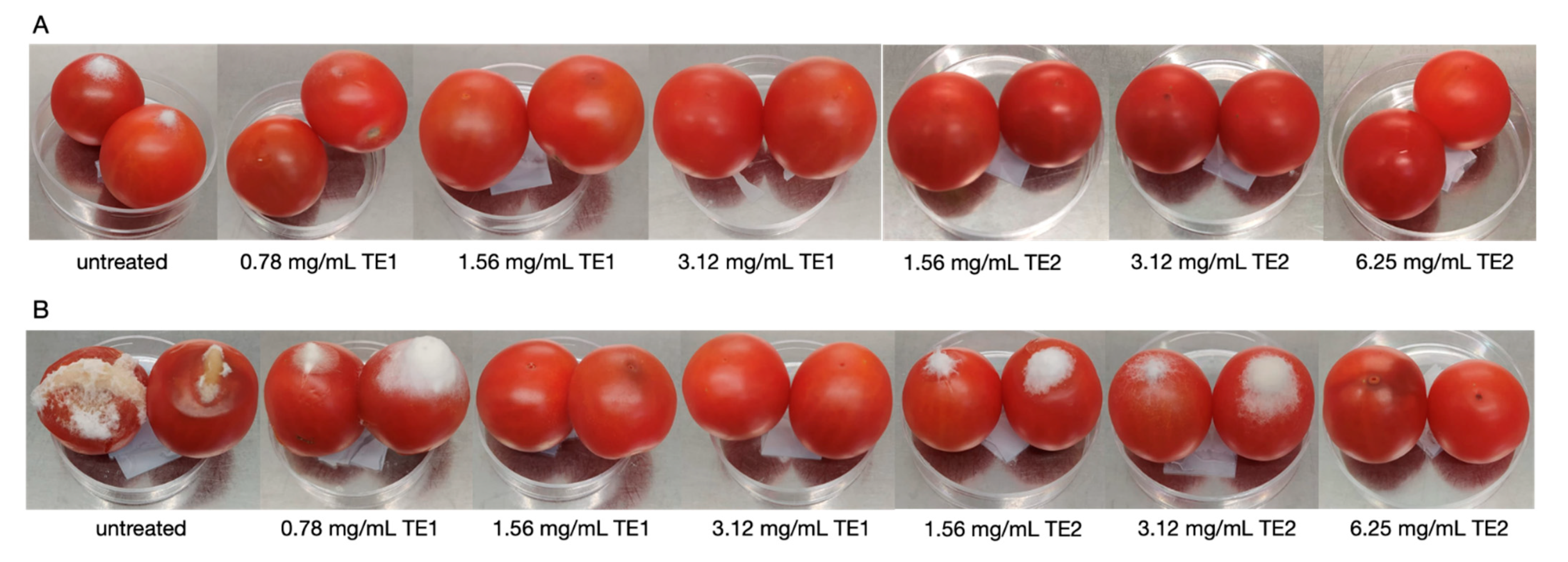
2.5.1. Tomato Fruits
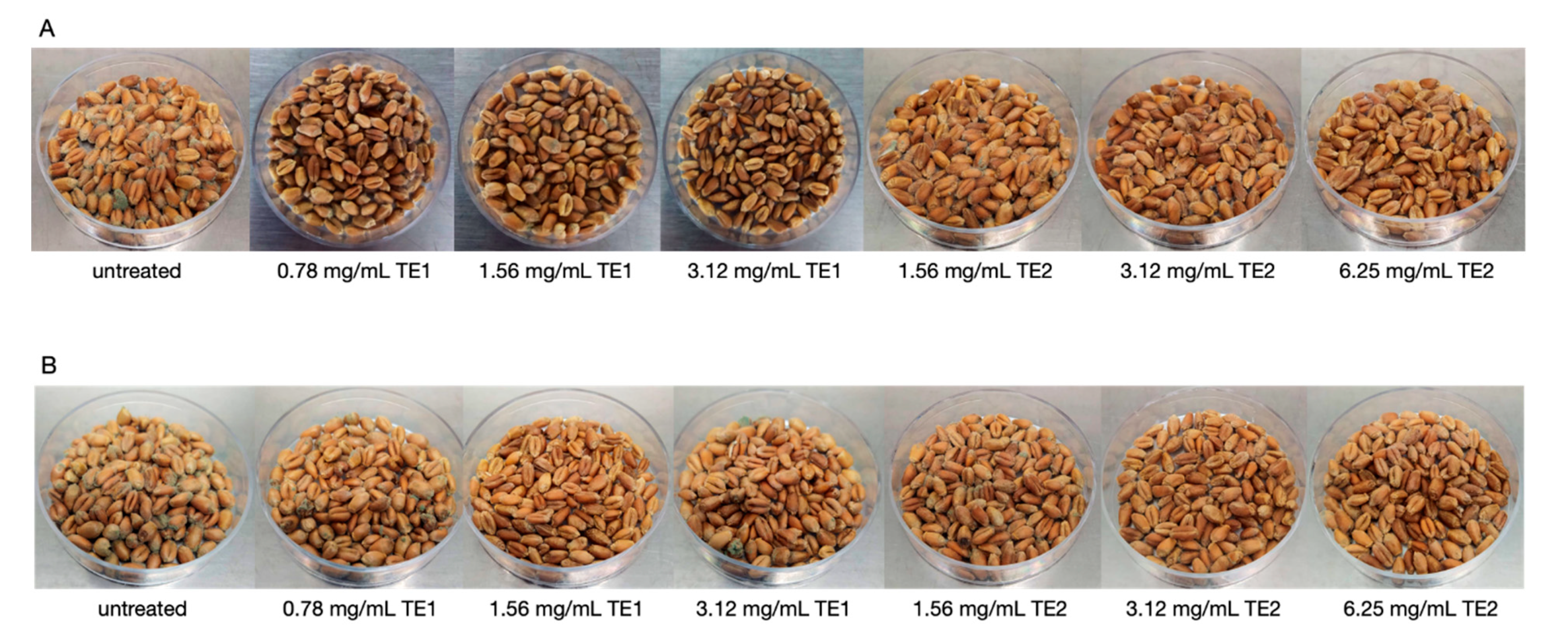
2.5.2. Wheat and Maize
2.6. Viable Microbial Counting
2.7. Mycotoxin Analysis
2.8. Statistical Analysis
3. Results and Discussion
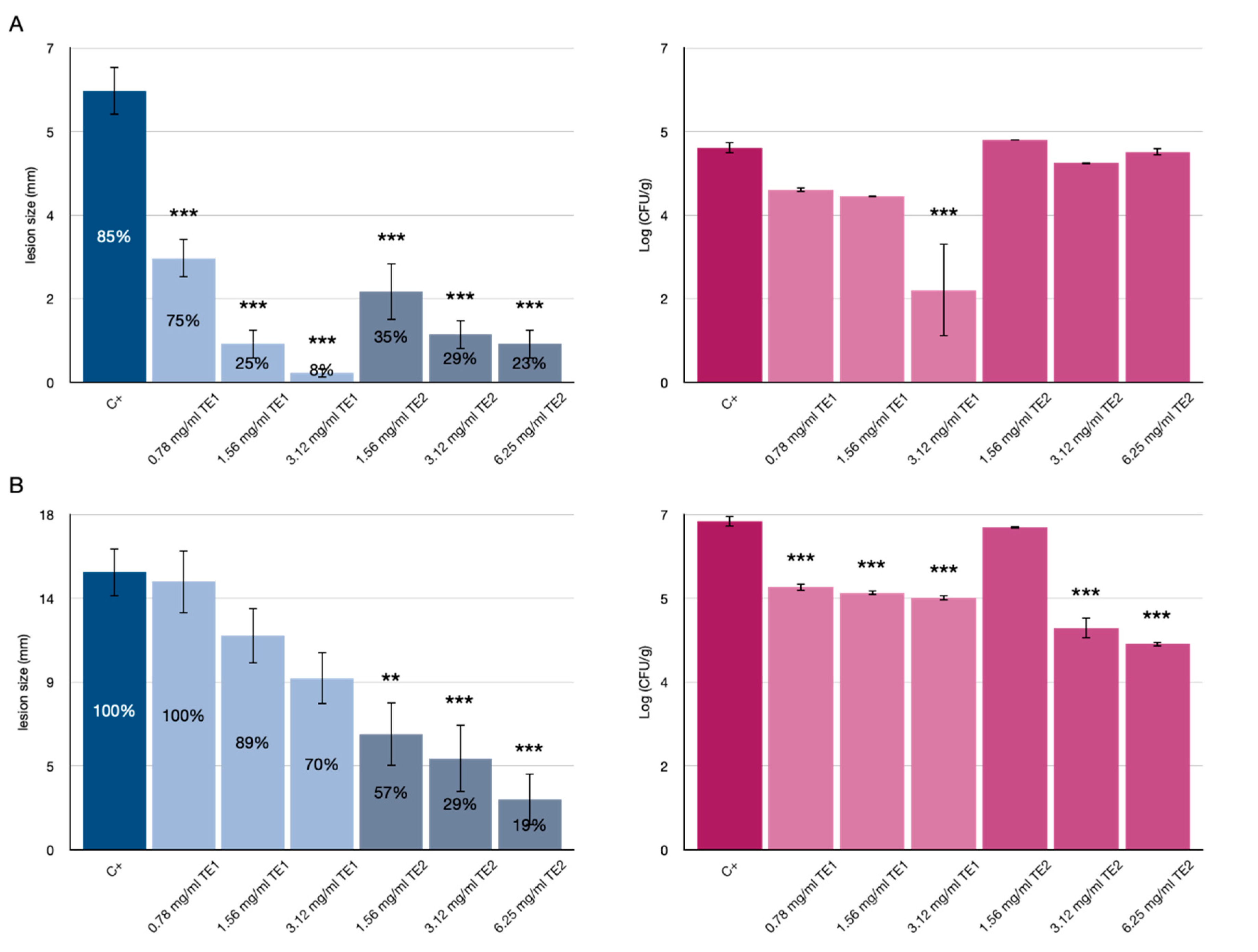
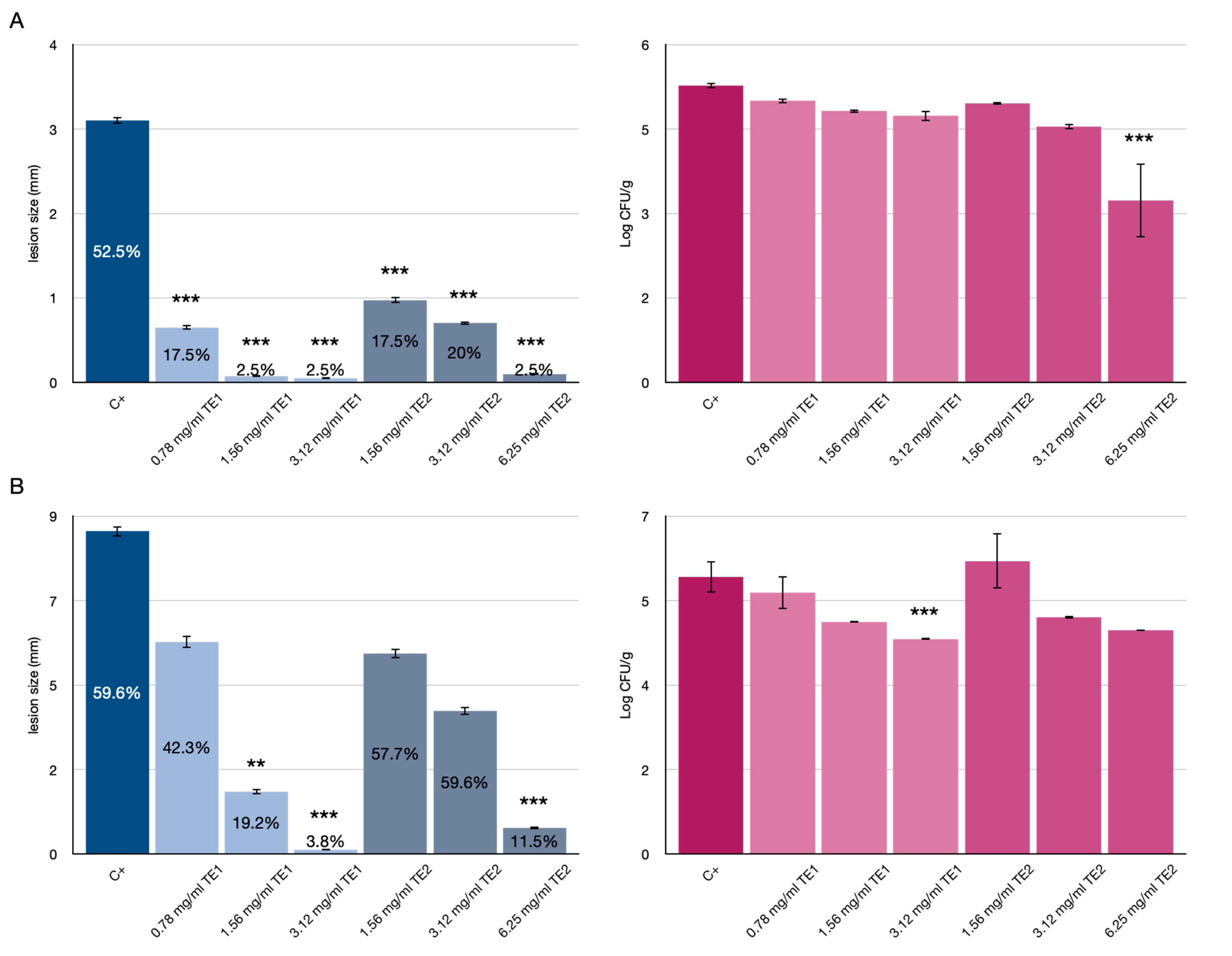
3.1. Tomato Fruits Bio-Preservation
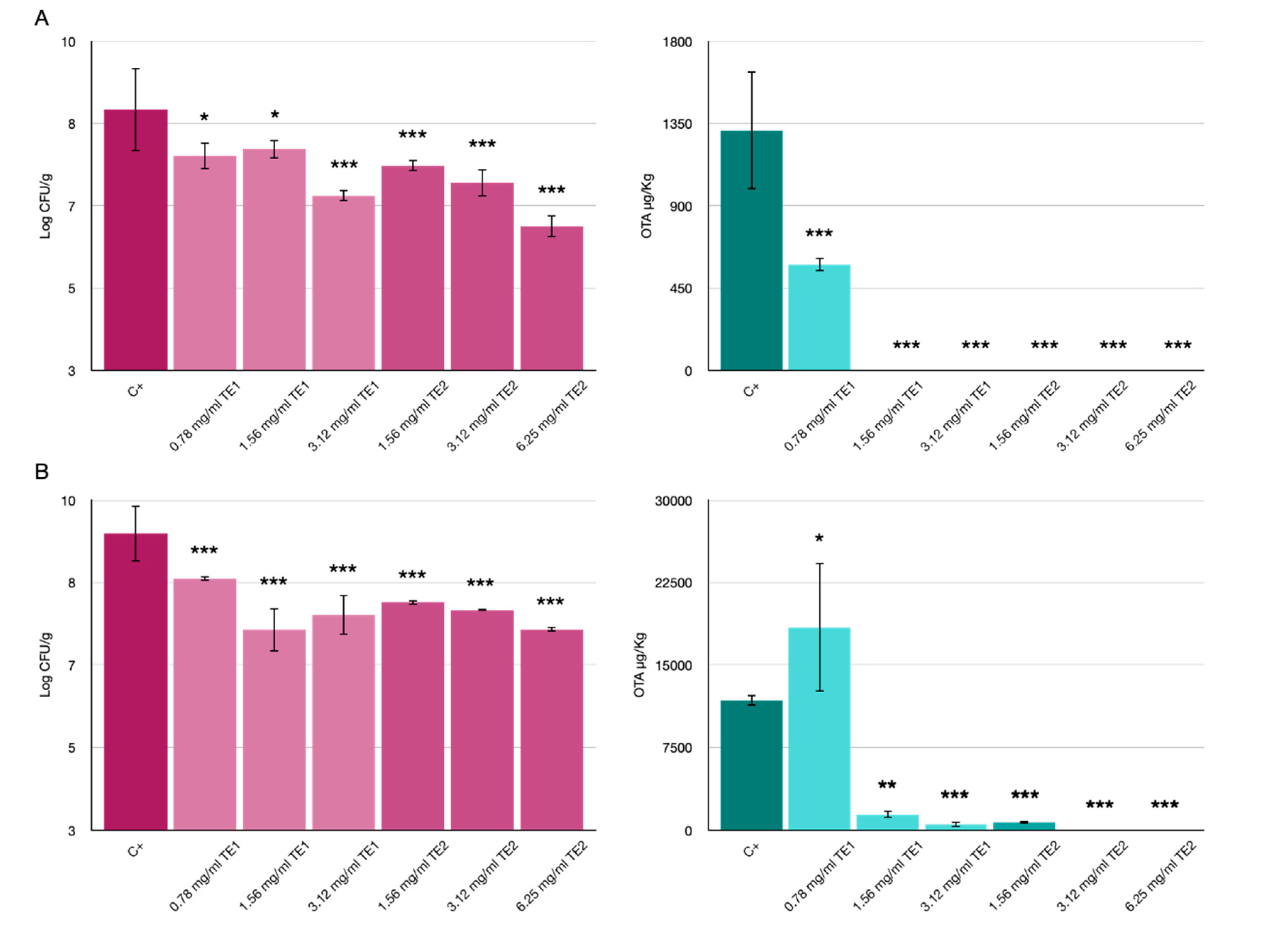
3.2. Bio-Preservation of Wheat
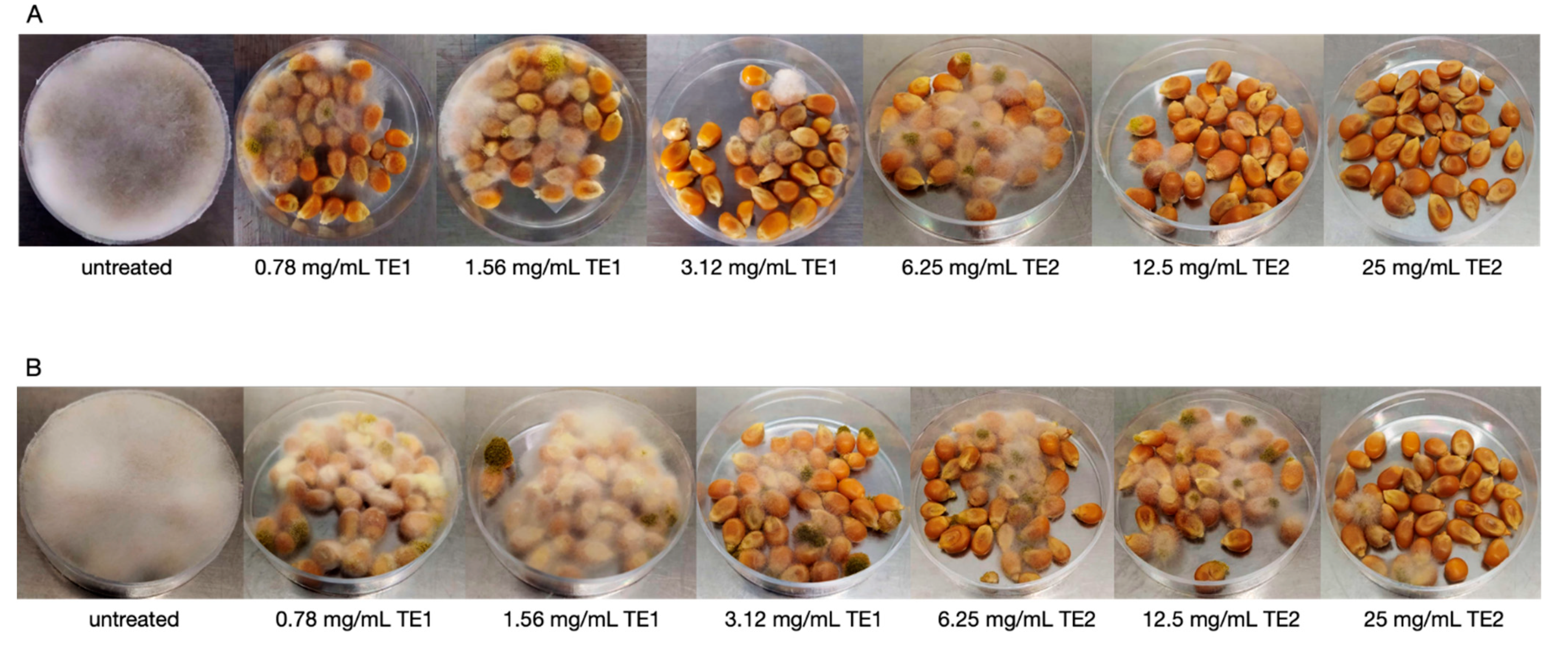
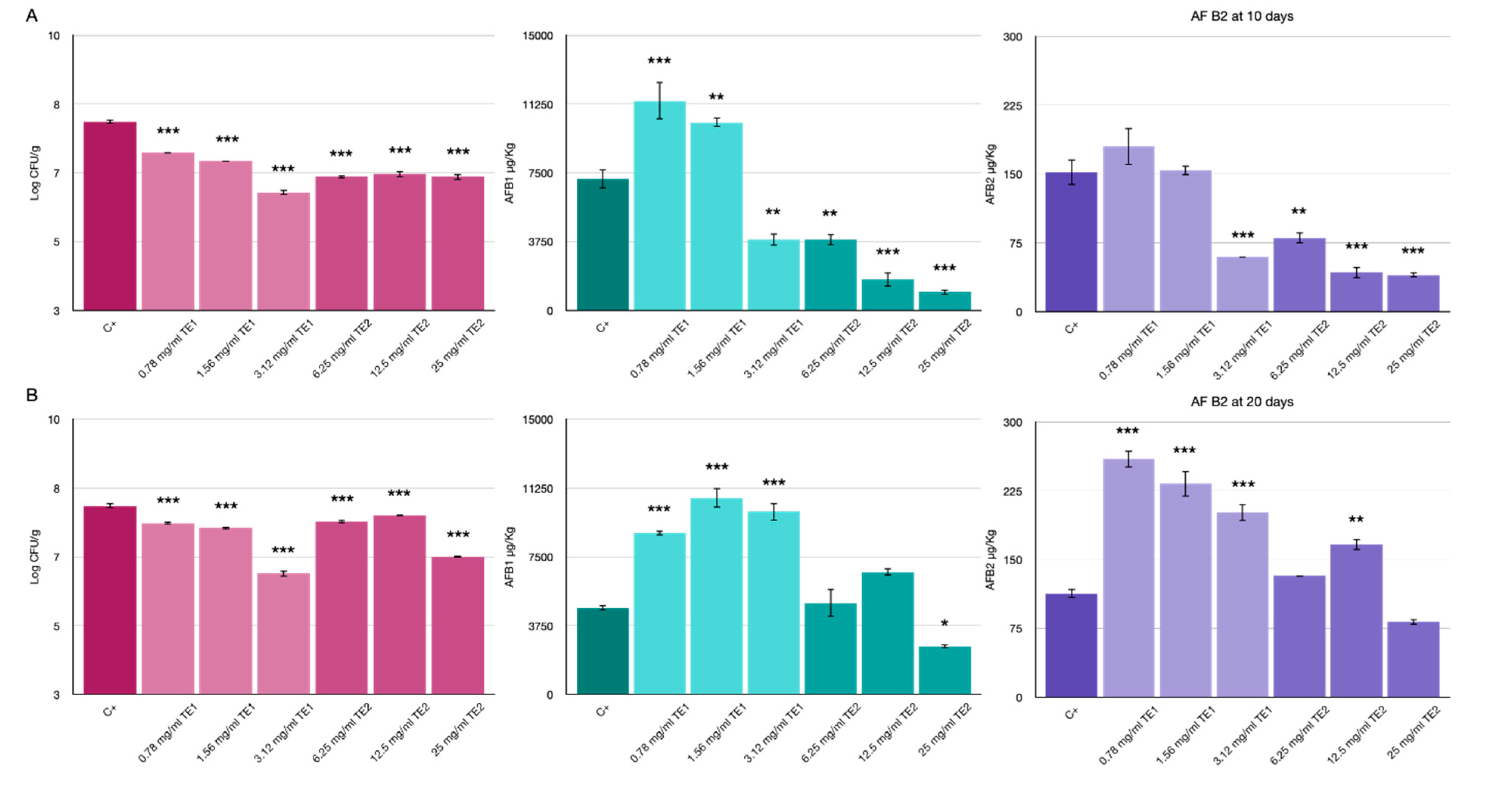
3.3. Bio-Preservation of Maize
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutot, M.; Nelson, L.M.; Tyson, R.C. Predicting the Spread of Postharvest Disease in Stored Fruit, with Application to Apples. Postharvest Biol. Technol. 2013, 85, 45–56. [Google Scholar] [CrossRef]
- Stankovic, S.; Levic, J.; Petrovic, T.; Logrieco, A.; Moretti, A. Pathogenicity and Mycotoxin Production by Fusarium Proliferatum Isolated from Onion and Garlic in Serbia. Eur. J. Plant Pathol. 2007, 118, 165–172. [Google Scholar] [CrossRef]
- Somma, S.; Petruzzella, A.L.; Logrieco, A.F.; Meca, G.; Cacciola, O.S.; Moretti, A. Phylogenetic Analyses of Fusarium Graminearum Strains from Cereals in Italy, and Characterisation of Their Molecular and Chemical Chemotypes. Crop Pasture Sci. 2014, 65, 52–60. [Google Scholar] [CrossRef]
- Aloi, F.; Riolo, M.; Sanzani, S.M.; Mincuzzi, A.; Ippolito, A.; Siciliano, I.; Pane, A.; Gullino, M.L.; Cacciola, S.O. Characterization of Alternaria Species Associated with Heart Rot of Pomegranate Fruit. J. Fungi 2021, 7, 172. [Google Scholar] [CrossRef]
- Masi, M.; Aloi, F.; Nocera, P.; Cacciola, S.O.; Surico, G.; Evidente, A. Phytotoxic Metabolites Isolated from Neufusicoccum Batangarum, the Causal Agent of the Scabby Canker of Cactus Pear (Opuntia ficus-indica L.). Toxins 2020, 12, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentivenga, G.; Spina, A.; Ammar, K.; Allegra, M.; Cacciola, S.O. Screening of Durum Wheat (Triticum turgidum L. subsp. durum (Desf.) Husn.) Italian Cultivars for Susceptibility to Fusarium Head Blight Incited by Fusarium graminearum. Plants 2021, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Toman, J.; Bazin, I.; Roubal, T. Transfer of Ochratoxin A into Tea and Coffee Beverages. Toxins 2014, 6, 3438–3453. [Google Scholar] [CrossRef] [PubMed]
- Drusch, S.; Ragab, W. Mycotoxins in Fruits, Fruit Juices, and Dried Fruits. J. Food Prot. 2003, 66, 1514–1527. [Google Scholar] [CrossRef]
- Chu, F.S. Mycotoxins: Food Contamination, Mechanism, Carcinogenic Potential and Preventive Measures. Mutat. Res. Genet. Toxicol. 1991, 259, 291–306. [Google Scholar] [CrossRef]
- Fung, F.; Clark, R.F. Health Effects of Mycotoxins: A Toxicological Overview. J. Toxicol. Clin. Toxicol. 2004, 42, 217–234. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxins in the Food Chain: Human Health Implications. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 95–101. [Google Scholar]
- Bullerman, L.B.; Bianchini, A. Stability of Mycotoxins during Food Processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef]
- Amiri, A.; Dugas, R.; Pichot, A.L.; Bompeix, G. In vitro and in vitro activity of eugenol oil (Eugenia caryophylata) against four important postharvest apple pathogens. Int. J. Food Microbiol. 2008, 126, 13–19. [Google Scholar] [CrossRef]
- Merrington, G.; Nfa, L.W.; Parkinson, R.; Redman, M.; Winder, L. Agricultural Pollution: Environmental Problems and Practical Solutions; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Valenzuela, N.L.; Angel, D.N.; Ortiz, D.T.; Rosas, R.A.; García, C.F.O.; Santos, M.O. Biological Control of Anthracnose by Postharvest Application of Trichoderma Spp. on Maradol Papaya Fruit. Biol. Control 2015, 91, 88–93. [Google Scholar] [CrossRef]
- Dal Bello, G.; Lampugnani, G.; Abramoff, C.; Fusé, C.; Perelló, A. Postharvest Control of Botrytis Gray Mould in Tomato by Antagonists and Biorational Compounds. IOBC-WPRS Bull. 2015, 111, 417–425. [Google Scholar]
- Quaglia, M.; Ederli, L.; Pasqualini, S.; Zazzerini, A. Biological Control Agents and Chemical Inducers of Resistance for Postharvest Control of Penicillium Expansum Link. on Apple Fruit. Postharvest Biol. Technol. 2011, 59, 307–315. [Google Scholar] [CrossRef]
- Batta, Y.A. Effect of Treatment with Trichoderma Harzianum Rifai Formulated in Invert Emulsion on Postharvest Decay of Apple Blue Mold. Int. J. Food Microbiol. 2004, 96, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Mortuza, M.G.; Ilag, L.L. Potential for Biocontrol of Lasiodiplodia Theobromae (Pat.) Griff. & Maubl. in Banana Fruits by Trichoderma Species. Biol. Control 1999, 15, 235–240. [Google Scholar]
- Sangeetha, G.; Usharani, S.; Muthukumar, A. Biocontrol with Trichoderma Species for the Management of Postharvest Crown Rot of Banana. Phytopathol. Mediterr. 2009, 48, 214–225. [Google Scholar]
- Dania, V.O. Bioefficacy of Trichoderma Species against Important Fungal Pathogens Causing Post-Harvest Rot in Sweet Potato (Ipomoea batatas (L.) Lam). J. Bangladesh Agric. Univ. 2019, 17, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Prabakar, K.; Raguchander, T.; Saravanakumar, D.; Muthulakshmi, P.; Parthiban, V.K.; Prakasam, V. Management of Postharvest Disease of Mango Anthracnose Incited by Colletotrichum Gleosporioides. Arch. Phytopathol. Plant Prot. 2008, 41, 333–339. [Google Scholar] [CrossRef]
- Nallathambi, P.; Umamaheswari, C.; Thakore, B.B.L.; More, T.A. Post-Harvest Management of Ber (Ziziphus mauritiana Lamk) Fruit Rot (Alternaria alternata Fr. Keissler) Using Trichoderma Species, Fungicides and Their Combinations. Crop Prot. 2009, 28, 525–532. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Sivasithamparam, K. Antifungal Antibiotics Produced by Trichoderma spp. Soil Biol. Biochem. 1991, 23, 1011–1020. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Stracquadanio, C.; Quiles, J.M.; Meca, G.; Cacciola, S.O. Antifungal Activity of Bioactive Metabolites Produced by Trichoderma Asperellum and Trichoderma Atroviride in Liquid Medium. J. Fungi 2020, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma Counteracts the Challenge of Phytophthora Nicotianae Infections on Tomato by Modulating Plant Defense Mechanisms and the Expression of Crinkler, Necrosis-Inducing Phytophthora Protein 1, and Cellulose-Binding Elicitor Lectin Pathogenic Effectors. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Petrikkou, E.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M.; Gomez, A.; Molleja, A.; Mellado, E. Inoculum Standardization for Antifungal Susceptibility Testing of Filamentous Fungi Pathogenic for Humans. J. Clin. Microbiol. 2001, 39, 1345–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Statistical Aspects of Microbiological Criteria Related to Foods: A Risk Manager’s Guide; Food & Agriculture Organization : Rome, Italy, 2019; Volume 24. [Google Scholar]
- Quiles, J.M.; Nazareth, T.D.M.; Luz, C.; Luciano, F.B.; Mañes, J.; Meca, G. Development of an Antifungal and Antimycotoxigenic Device Containing Allyl Isothiocyanate for Silo Fumigation. Toxins 2019, 11, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adss, I.; Hamza, H.; Hafez, E.; Heikal, H. Enhancing Tomato Fruits Post-Harvest Resistance by Salicylic Acid and Hydrogen Peroxide Elicitors against Rot Caused by Alternaria Solani. J. Agric. Chem. Biotechnol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Yao, H.; Tian, S. Effects of Pre-and Post-Harvest Application of Salicylic Acid or Methyl Jasmonate on Inducing Disease Resistance of Sweet Cherry Fruit in Storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Bakar, A.A.; Izzati, M.N.A.; Umi Kalsom, Y. Diversity of Fusarium Species Associated with Post-Harvest Fruit Rot Disease of Tomato. Sains Malays. 2013, 42, 911–920. [Google Scholar]
- Haidukowski, M.; Pascale, M.; Perrone, G.; Pancaldi, D.; Campagna, C.; Visconti, A. Effect of Fungicides on the Development of Fusarium Head Blight, Yield and Deoxynivalenol Accumulation in Wheat Inoculated under Field Conditions with Fusarium Graminearum and Fusarium Culmorum. J. Sci. Food Agric. 2005, 85, 191–198. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium Species and Their Associated Mycotoxins. In Mycotoxigenic Fungi; Springer: Berlin/Heidelberg, Germany, 2017; pp. 51–106. [Google Scholar]
- Simpson, D.R.; Weston, G.E.; Turner, J.A.; Jennings, P.; Nicholson, P. Differential Control of Head Blight Pathogens of Wheat by Fungicides and Consequences for Mycotoxin Contamination of Grain. Eur. J. Plant Pathol. 2001, 107, 421–431. [Google Scholar] [CrossRef]
- dos Santos Oliveira, M.; Furlong, E.B. Screening of Antifungal and Antimycotoxigenic Activity of Plant Phenolic Extracts. World Mycotoxin J. 2008, 1, 139–146. [Google Scholar] [CrossRef]
- Prakash, B.; Shukla, R.; Singh, P.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of Chemically Characterized Piper Betle L. Essential Oil against Fungal and Aflatoxin Contamination of Some Edible Commodities and Its Antioxidant Activity. Int. J. Food Microbiol. 2010, 142, 114–119. [Google Scholar] [CrossRef]
- da Cruz Cabral, L.; Pinto, V.F.; Patriarca, A. Application of Plant Derived Compounds to Control Fungal Spoilage and Mycotoxin Production in Foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Dubey, N.K. Exploitation of Natural Products as an Alternative Strategy to Control Postharvest Fungal Rotting of Fruit and Vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Esserti, S.; Smaili, A.; Rifai, L.A.; Koussa, T.; Makroum, K.; Belfaiza, M.; Faize, L.; Burgos, L.; Alburquerque, N.; Faize, M. Protective Effect of Three Brown Seaweed Extracts against Fungal and Bacterial Diseases of Tomato. J. Appl. Phycol. 2017, 29, 1081–1093. [Google Scholar] [CrossRef]
- Jayaraj, J.; Wan, A.; Rahman, M.; Punja, Z.K. Seaweed Extract Reduces Foliar Fungal Diseases on Carrot. Crop Prot. 2008, 27, 1360–1366. [Google Scholar] [CrossRef]
- Pangallo, S.; Nicosia, M.G.L.D.; Agosteo, G.E.; Abdelfattah, A.; Romeo, F.V.; Cacciola, S.O.; Rapisarda, P.; Schena, L. Evaluation of a Pomegranate Peel Extract as an Alternative Means to Control Olive Anthracnose. Phytopathology 2017, 107, 1462–1467. [Google Scholar] [CrossRef] [Green Version]
- La Spada, F.; Aloi, A.; Coniglione, M.; Pane, A.; Cacciola, S.O. Natural Biostimulants Elicit Plant Immune System in an Integrated Management Strategy of the Postharvest Green Mold of Orange Fruits Incited by Penicillium digitatum. Front. Plant Sci. 2021, 12, 684722. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–Plant–Pathogen Interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Lorito, M.; Sivasithamparam, K. Major Secondary Metabolites Produced by Two Commercial Trichoderma Strains Active against Different Phytopathogens. Lett. Appl. Microbiol. 2006, 43, 143–148. [Google Scholar] [CrossRef] [PubMed]
- El-Katatny, M.H.; Emam, A.S. Control of Postharvest Tomato Rot by Spore Suspension and Antifungal Metabolites of Trichoderma Harzianum. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 1505–1528. [Google Scholar]

| * Extract (mg/mL or mg/g) | ||||||
|---|---|---|---|---|---|---|
| TE1 | TE2 | |||||
| Strain | MFC | MFCx2 | MFCx4 | MFC | MFCx2 | MFCx4 |
| Fusarium verticillioides | 0.78 | 1.56 | 3.12 | 1.56 | 3.12 | 6.25 |
| Fusarium graminearum | 0.78 | 1.56 | 3.12 | 1.56 | 3.12 | 6.25 |
| Penicillium verrucosum | 0.78 | 1.56 | 3.12 | 1.56 | 3.12 | 6.25 |
| Aspergillus flavus | 0.78 | 1.56 | 3.12 | 6.25 | 12.5 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stracquadanio, C.; Luz, C.; La Spada, F.; Meca, G.; Cacciola, S.O. Inhibition of Mycotoxigenic Fungi in Different Vegetable Matrices by Extracts of Trichoderma Species. J. Fungi 2021, 7, 445. https://doi.org/10.3390/jof7060445
Stracquadanio C, Luz C, La Spada F, Meca G, Cacciola SO. Inhibition of Mycotoxigenic Fungi in Different Vegetable Matrices by Extracts of Trichoderma Species. Journal of Fungi. 2021; 7(6):445. https://doi.org/10.3390/jof7060445
Chicago/Turabian StyleStracquadanio, Claudia, Carlos Luz, Federico La Spada, Giuseppe Meca, and Santa Olga Cacciola. 2021. "Inhibition of Mycotoxigenic Fungi in Different Vegetable Matrices by Extracts of Trichoderma Species" Journal of Fungi 7, no. 6: 445. https://doi.org/10.3390/jof7060445






