Arbuscular Mycorrhizae Mitigate Aluminum Toxicity and Regulate Proline Metabolism in Plants Grown in Acidic Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup, Plant Materials, and Growth Conditions
2.2. Photosynthetic Rate
2.3. Mycorrhizal Parameters
2.4. Determination of Aluminum Content in Soil and Plant Tissue
2.5. Amino Acid and Nitrogen Measurements
2.6. Enzyme Activity Assays
2.7. Statistical Analysis
3. Results
3.1. AMF Colonization and Hyphal Growth
3.2. Plant Biomass, Photosynthesis, and Al Accumulation
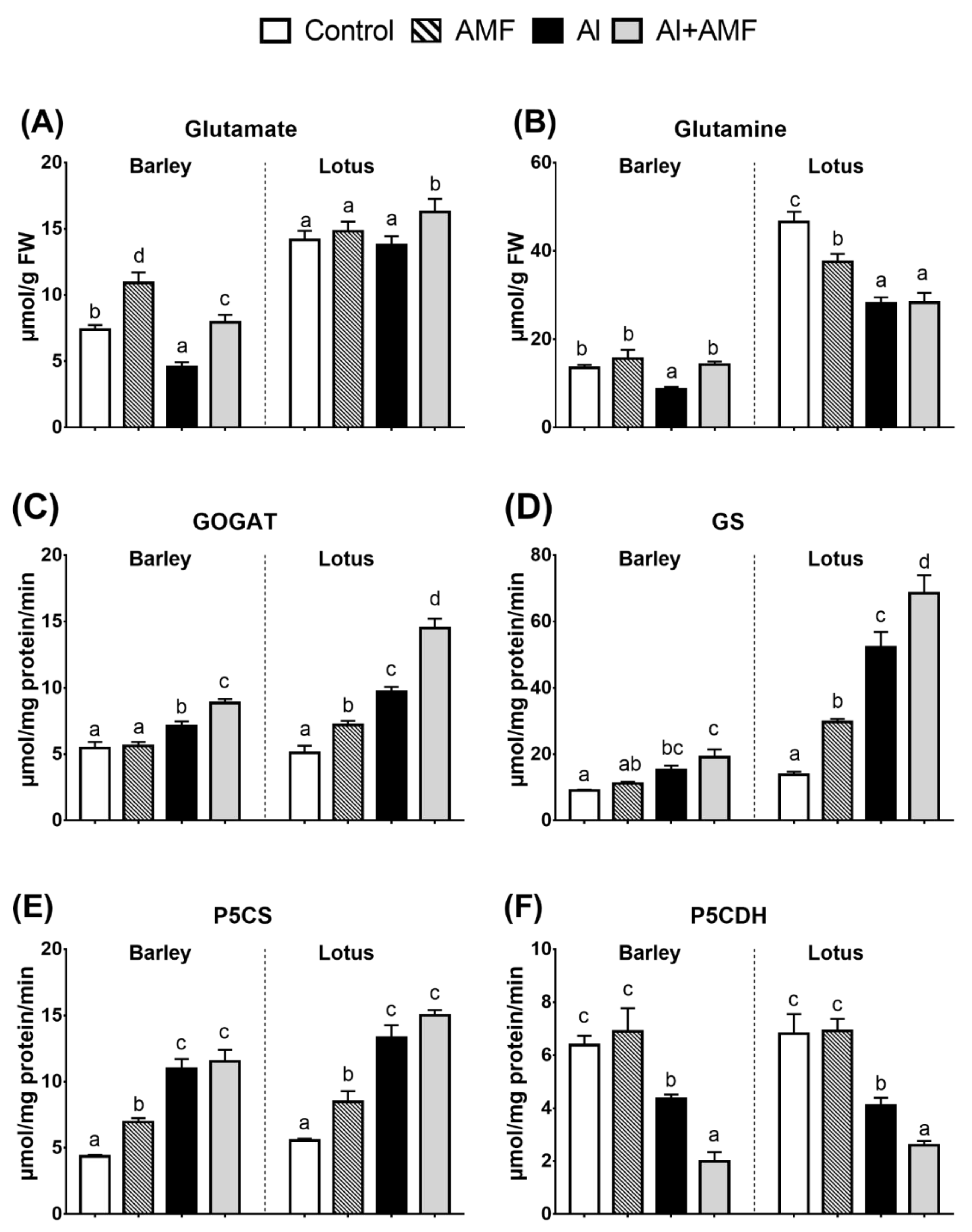
3.3. Proline Biosynthesis: Glutamine Pathway
3.4. Proline Biosynthesis: Ornithine Pathway
3.5. P5C–Pro Metabolism Cycle
3.6. Nitrogen (N) Assimilation
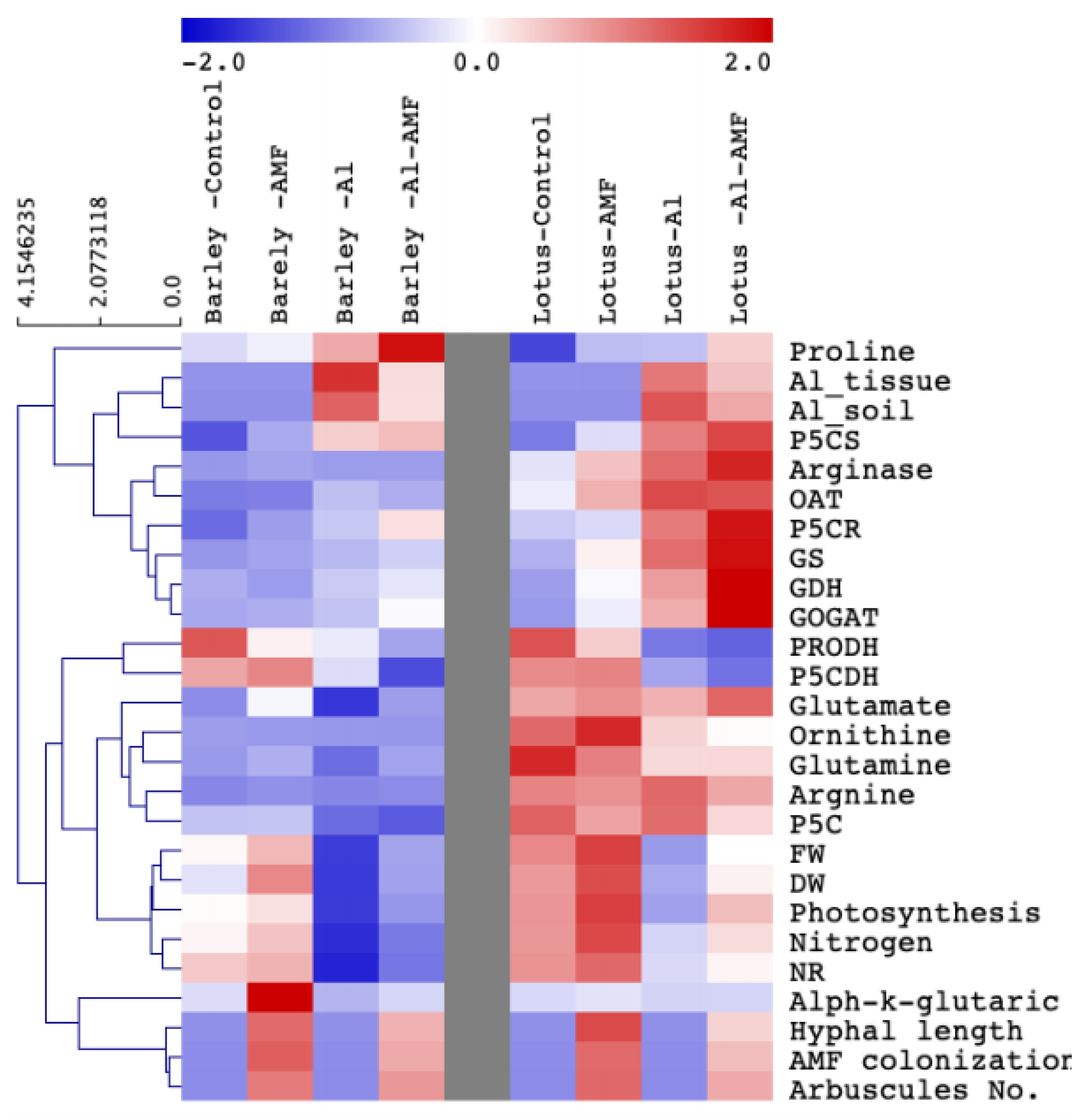
3.7. Hierarchical Clustering of Proline-Metabolism-Related Parameters
4. Discussion
4.1. Al Greatly Inhibits Growth and Photosynthesis in Both Plants, with Lotus Bring More Tolerant
4.2. AMF Decreased Al Accumulation and Alleviated its Negative Impact on Growth and Photosynthesis
4.3. Proline Metabolism in Lotus Is More Responsive to Al Than in Barley
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Gupta, V.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, A.; Saleh, A.M.; Habeeb, T.H.; Hassan, Y.M.; Zrieq, R.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; Matos, M.; AbdElgawad, H. Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci. Total Environ. 2019, 693, 133636. [Google Scholar] [CrossRef] [PubMed]
- Dal Molin, S.J.; Ernani, P.R.; Gerber, J.M. Soil acidification and nitrogen release following application of nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 2020, 51, 2551–2558. [Google Scholar] [CrossRef]
- Pierre, W.H.; Webb, J.R.; Shrader, W.D. Quantitative Effects of Nitrogen Fertilizer on the Development and Downward Movement of Soil Acidity in Relation to Level of Fertilization and Crop Removal in a Continuous Corn Cropping System1. Agron. J. 1971, 63, 291–297. [Google Scholar] [CrossRef]
- Yamamoto, Y. Aluminum toxicity in plant cells: Mechanisms of cell death and inhibition of cell elongation. Soil Sci. Plant Nutr. 2019, 65, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Egerton-Warburton, L. Aluminum-Tolerant Pisolithus Ectomycorrhizas Confer Increased Growth, Mineral Nutrition, and Metal Tolerance to Eucalyptus in Acidic Mine Spoil. Appl. Environ. Soil Sci. 2015, 2015, 803821. [Google Scholar] [CrossRef] [Green Version]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Rehman, R.U.; Hakeem, K.R.; Alharby, H.F. Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiol. Biochem. 2019, 144, 178–186. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Kochian, L.; Piñeros, M.; Hoekenga, O. The Physiology, Genetics and Molecular Biology of Plant Aluminum Resistance and Toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Panda, S.K.; Baluška, F.; Matsumoto, H. Aluminum stress signaling in plants. Plant Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, M.J.; McGraw, R.L. Lotus adaptation, use, and management. Trefoil Sci. Technol. Lotus 1999, 28, 97–119. [Google Scholar]
- Ma, J.F.; Zheng, S.J.; Li, X.F.; Takeda, K.; Matsumoto, H. A rapid hydroponic screening for aluminium tolerance in barley. Plant Soil 1997, 191, 133–137. [Google Scholar] [CrossRef]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Del Gallo, M.; Kitouni, M.; Barik, D.P.; et al. Arbuscular mycorrhizal symbiosis: Plant growth improvement and induction of resistance under stressful conditions. J. Plant Nutr. 2021, 1993–2028. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Higo, M.; Isobe, K.; Kang, D.-J.; Ujiie, K.; Drijber, R.A.; Ishii, R. Inoculation with arbuscular mycorrhizal fungi or crop rotation with mycorrhizal plants improves the growth of maize in limed acid sulfate soil. Plant Prod. Sci. 2010, 13, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Lv, J.; Li, G.; Zhang, Z.; Zhang, J.; et al. Chromium immobilization by extra-and intraradical fungal structures of arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higo, M.; Kang, D.-J.; Isobe, K. First report of community dynamics of arbuscular mycorrhizal fungi in radiocesium degradation lands after the Fukushima-Daiichi Nuclear disaster in Japan. Sci. Rep. 2019, 9, 8240. [Google Scholar] [CrossRef]
- Seguel, A.; Cumming, J.R.; Klugh-Stewart, K.; Cornejo, P.; Borie, F. The role of arbuscular mycorrhizas in decreasing aluminium phytotoxicity in acidic soils: A review. Mycorrhiza 2013, 23, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Vergara, C.; Araujo, K.E.C.; de Souza, S.R.; Schultz, N.; Saggin, J.O.J.; Sperandio, M.V.L.; Zilli, J.É. Plant-mycorrhizal fungi interaction and response to inoculation with different growth-promoting fungi. Pesqui. Agropecuária Bras. 2019, 54. [Google Scholar] [CrossRef] [Green Version]
- Kishor, K.; Polavarapu, B.; Hima, K.P.; Sunita, M.S.L.; Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef] [Green Version]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Shinde, S.; Villamor, J.G.; Lin, W.; Sharma, S.; Verslues, P.E. Proline Coordination with Fatty Acid Synthesis and Redox Metabolism of Chloroplast and Mitochondria. Plant Physiol. 2016, 172, 1074–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavi, K.P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant. Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef] [Green Version]
- Ku, H.-M.; Tan, C.-W.; Su, Y.-S.; Chiu, C.-Y.; Chen, C.-T.; Jan, F.-J. The effect of water deficit and excess copper on proline metabolism in Nicotiana benthamiana. Biol. Plant. 2012, 56, 337–343. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; El-Keblawy, A.; Ali, D.F.I.; Ibrahim, H.M.; El-Sheikh, M.A.; Sharma, A.; Alhaj Hamoud, Y.; Shaghaleh, H.; Brestic, M.; Skalicky, M.; et al. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar. Agriculture 2021, 11, 194. [Google Scholar] [CrossRef]
- Abdelgawad, H.; Farfan-vignolo, E.R.; De Vos, D.; Asard, H. Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 2015, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Andrade, G.; Mihara, K.L.; Linderman, R.G.; Bethlenfalvay, G.J. Bacteria from rhizosphere and hyphosphere soils of different arbuscular-mycorrhizal fungi. Plant Soil 1997, 192, 71–79. [Google Scholar] [CrossRef]
- Giannakoula, A.; Moustakas, M.; Mylona, P.; Papadakis, I.; Yupsanis, T. Aluminum tolerance in maize is correlated with increased levels of mineral nutrients, carbohydrates and proline, and decreased levels of lipid peroxidation and Al accumulation. J. Plant Physiol. 2008, 165, 385–396. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Peshev, D.; Zinta, G.; den Ende, W.; Janssens, I.A.; Asard, H. Climate extreme effects on the chemical composition of temperate grassland species under ambient and elevated CO2: A comparison of fructan and non-fructan accumulators. PLoS ONE 2014, 9, e92044. [Google Scholar] [CrossRef] [PubMed]
- Temple, S.J.; Kunjibettu, S.; Roche, D.; Sengupta-Gopalan, C. Total glutamine synthetase activity during soybean nodule development is controlled at the level of transcription and holoprotein turnover. Plant Physiol. 1996, 112, 1723–1733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Tanaka, T.; Ikura, M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 1995, 2, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Majerus, V.; Kinet, J.M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 1999, 105, 450–458. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Schelbert, S.; Park, S.-Y.; Han, S.-H.; Lee, B.-D.; Andrès, C.B.; Kessler, F.; Hörtensteiner, S.; Paek, N.-C. STAY-GREEN and Chlorophyll Catabolic Enzymes Interact at Light-Harvesting Complex II for Chlorophyll Detoxification during Leaf Senescence in Arabidopsis. Plant Cell 2012, 24, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Charest, C.; Ton, P.C. Cold acclimation of wheat (Triticum aestivum): Properties of enzymes involved in proline metabolism. Physiol. Plant. 1990, 80, 159–168. [Google Scholar] [CrossRef]
- Nuzum, C.T.S.P.J. The Urea Cycle; Grisolía, S., Mayor, F.B.R., Eds.; John William and Sons: New York, NY, USA, 1976. [Google Scholar]
- AbdElgawad, H.; Abuelsoud, W.; Madany, M.M.Y.; Selim, S.; Zinta, G.; Mousa, A.S.M.; Hozzein, W.N. Actinomycetes Enrich Soil Rhizosphere and Improve Seed Quality as well as Productivity of Legumes by Boosting Nitrogen Availability and Metabolism. Biomolecules 2020, 10, 1675. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, H.P.; Da Matta, F.M.; Da Matta, J.C. Responses of the photosynthetic apparatus to aluminum stress in two sorghum cultivars. J. Plant Nutr. 2002, 25, 821–832. [Google Scholar] [CrossRef]
- Saleh, A.M.; Hassan, Y.M.; Habeeb, T.H.; Alkhalaf, A.A.; Hozzein, W.N.; Selim, S.; Abdelgawad, H. Interactive effects of mercuric oxide nanoparticles and future climate CO 2 on maize plant. J. Hazard. Mater. 2021, 401, 123849. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.J. Nonstructural carbohydrate and prunasin composition of peach seedlings fertilized with different nitrogen sources and aluminum. Sci. Hortic. 2002, 94, 21–32. [Google Scholar] [CrossRef]
- Jiang, H.-X.; Chen, L.-S.; Zheng, J.-G.; Han, S.; Tang, N.; Smith, B.R. Aluminum-induced effects on Photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008, 28, 1863–1871. [Google Scholar] [CrossRef]
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46. [Google Scholar] [CrossRef]
- Kohlbrenner, W.E.; Cross, R.L. The mode of inhibition of oxidative phosphorylation by efrapeptin (A23871): Measurement of substrate effects on rates of inactivation by a tight-binding inhibitor. Arch. Biochem. Biophys. 1979, 198, 598–607. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Pereira, W.E.; de Siqueira, D.L.; Martínez, C.A.; Puiatti, M. Gas exchange and chlorophyll fluorescence in four citrus rootstocks under aluminium stress. J. Plant Physiol. 2000, 157, 513–520. [Google Scholar] [CrossRef]
- Leal-Alvarado, D.A.; Espadas-Gil, F.; Sáenz-Carbonell, L.; Talavera-May, C.; Santamaría, J.M. Lead accumulation reduces photosynthesis in the lead hyper-accumulator Salvinia minima Baker by affecting the cell membrane and inducing stomatal closure. Aquat. Toxicol. 2016, 171, 37–47. [Google Scholar] [CrossRef]
- Li, X.; He, M.; Guo, J.; Cao, T. Upregulation of circular RNA circ-ERBB2 predicts unfavorable prognosis and facilitates the progression of gastric cancer via miR-503/CACUL1 and miR-637/MMP-19 signaling. Biochem. Biophys. Res. Commun. 2019, 511, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Daly, P.; Sharma, A.; Shaghaleh, H.; Hamoud, Y.A.; El-Esawi, M.A.; Pan, R.; Wan, Q.; et al. Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J. Sci. Food Agric. 2021, 101, 2027–2041. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bryla, D.; Lee, J. Salt Exclusion and Mycorrhizal Symbiosis Increase Tolerance to NaCl and CaCl2 Salinity in “Siam Queen” Basil. Hortic. Sci. Publ. Am. Soc. Hortic. Sci. 2017, 52, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Cumming, J.R.; Ning, J. Arbuscular mycorrhizal fungi enhance aluminium resistance of broomsedge (Andropogon virginicus L.). J. Exp. Bot. 2003, 54, 1447–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Liang, Y.; Ang, E.L.; Zhao, H. Delta Integration CRISPR-Cas (Di-CRISPR) in Saccharomyces cerevisiae. In Microbial Metabolic Engineering: Methods and Protocols; Santos, C.N.S., Ajikumar, P.K., Eds.; Springer: New York, NY, USA, 2019; pp. 73–91. ISBN 978-1-4939-9142-6. [Google Scholar]
- Rufyikiri, G.; Nootens, D.; Dufey, J.E.; Delvaux, B. Effect of aluminium on bananas (Musa spp.) cultivated in acid solutions. I. Plant growth and chemical composition. Fruits 2000, 55, 367–379. [Google Scholar]
- Lux, H.B.; Cumming, J.R. Mycorrhizae confer aluminum resistance to tulip-poplar seedlings. Can. J. For. Res. 2001, 31, 694–702. [Google Scholar] [CrossRef]
- Yang, W.; Weng, P.J.; Gao, Y. A new paradigm of DNA synthesis: Three-metal-ion catalysis. Cell Biosci. 2016, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, H.; Zhao, C.; Li, S.; Kong, L.; Wu, W.; Kong, W.; Liu, Y.; Wei, Y.; Zhu, J.-K.; et al. The inhibition of protein translation mediated by AtGCN1 is essential for cold tolerance in Arabidopsis thaliana. Plant. Cell Environ. 2017, 40, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.-S.; Srivastava, A.K.; Zou, Y.-N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arab. Book 2010, 8, e0140. [Google Scholar] [CrossRef] [Green Version]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Yang, J.; Zhang, N.; Ma, C.; Qu, Y.; Si, H.; Wang, D. Prediction and verification of microRNAs related to proline accumulation under drought stress in potato. Comput. Biol. Chem. 2013, 46, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, L.-M.; Lin, C.-C.; Kao, C.-H. Regulation of proline accumulation in detached rice leaves exposed to excess copper. Plant Sci. 2001, 160, 283–290. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Pavlíková, D.; Tlustoš, P. The changes of contents of selected free amino acids associated with cadmium stress in Noccaea caerulescens and Arabidopsis halleri. Plant Soil Environ. 2013, 59, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Dwivedi, N. Proline Catalyzed α-Aminoxylation Reaction in the Synthesis of Biologically Active Compounds. Acc. Chem. Res. 2013, 46, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, E.; López-Lefebre, L.R.; García, P.C.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Proline metabolism in response to highest nitrogen dosages in green bean plants (Phaseolus vulgaris L. cv. Strike). J. Plant Physiol. 2001, 158, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Singh, V.P.; Prasad, S.M. Nitrogen modifies NaCl toxicity in eggplant seedlings: Assessment of chlorophyll a fluorescence, antioxidative response and proline metabolism. Biocatal. Agric. Biotechnol. 2016, 7, 76–86. [Google Scholar] [CrossRef]
- de Sousa, A.; AbdElgawad, H.; Fidalgo, F.; Teixeira, J.; Matos, M.; Hamed, B.A.; Selim, S.; Hozzein, W.N.; Beemster, G.T.S.; Asard, H. Al exposure increases proline levels by different pathways in an Al-sensitive and an Al-tolerant rye genotype. Sci. Rep. 2020, 10, 16401. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcón, R.; Palma, J.M. Superoxide dismutase activity in arbuscular mycorrhizal Lactuca sativa plants subjected to drought stress. New Phytol. 1996, 134, 327–333. [Google Scholar] [CrossRef]
- Ruíz-Sánchez, M.; Armada, E.; Muñoz, Y.; de Salamone, I.E.G.; Aroca, R.; Ruíz-Lozano, J.M.; Azcón, R. Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Yooyongwech, S.; Phaukinsang, N.; Cha-Um, S.; Supaibulwattana, K. Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. Grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul. 2013, 69. [Google Scholar] [CrossRef]
- Moradi, T.Z.; Iranbakhsh, A.; Mehregan, I.; Ahmadvand, R. Impact of arbuscular mycorrhizal fungi (AMF) on gene expression of some cell wall and membrane elements of wheat (Triticum aestivum L.) under water deficit using transcriptome analysis. Physiol. Mol. Biol. Plants 2020, 26, 143–162. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Barley | Lotus | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | AMF | Al | Al + AMF | Control | AMF | Al | Al + AMF | |
| AM colonization (% per root) | 0 | 58.35 ± 1a | 0 | 43.0 ± 1.45b | 0 | 55.9 ± 1.6a | 0 | 38.8 ± 1.7b |
| Hyphal length (mm/g soil) | 0 | 1943 ± 98a | 0 | 1411.2 ± 12b | 0 | 2168 ± 198a | 0 | 1161 ± 101b |
| Arbuscules (number/cm root) | 0 | 4.8 ± 0.703a | 0 | 4.4 ± 0.34a | 0 | 5.1 ± 0.7a | 0 | 4.1 ± 0.3b |
| Parameter | Barley | Lotus | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | AMF | Al | Al + AMF | Control | AMF | Al | Al + AMF | |
| Fresh weight | 2.03 ± 0.1c | 2.4 ± 0.15d | 0.82 ± 0.03a | 1.43 ± 0.14b | 2.68 ± 0.05c | 3.11 ± 0.2d | 1.38 ± 0.12a | 1.97 ± 0.21b |
| Dry weight | 0.44 ± 0.02b | 0.67 ± 0.06c | 0.18 ± 0.02a | 0.34 ± 0.07b | 0.64 ± 0.01c | 0.76 ± 0.08d | 0.35 ± 0.01a | 0.51 ± 0.08b |
| Rate of photosynthesis | 0.14 ± 0.01c | 0.15 ± 0.01c | 0.04 ± 0.02a | 0.08 ± 0.01b | 0.19 ± 0.01b | 0.23 ± 0.01bc | 0.09 ± 0.01a | 0.17 ± 0.02b |
| Al in tissues | ND | ND | 164.84 ± 22.95b | 76.48 ± 2.53a | ND | ND | 128.62 ± 4.32b | 90.11 ± 11.41a |
| Al in soil | ND | ND | 15.51 ± 0.38b | 08.86 ± 1.19a | ND | ND | 16.24 ± 1.62b | 11.72 ± 1.02a |
| Parameter | Barley | Lotus | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | AMF | Al | Al + AMF | Control | AMF | Al | Al + AMF | |
| Nitrogen | 218.3 ± 12c | 238 ± 17c | 133 ± 15a | 163 ± 21b | 255 ± 19c | 285 ± 26c | 198 ± 16a | 228 ± 13b |
| Nitrate reductase | 443 ± 28b | 453 ± 98b | 311 ± 32a | 351 ± 12a | 468 ± 31c | 488 ± 41c | 378 ± 18a | 421 ± 27b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, M.O.; Saleh, A.M.; Sobrinho, R.L.; Sheteiwy, M.S.; El-Sawah, A.M.; Mohammed, A.E.; Elgawad, H.A. Arbuscular Mycorrhizae Mitigate Aluminum Toxicity and Regulate Proline Metabolism in Plants Grown in Acidic Soil. J. Fungi 2021, 7, 531. https://doi.org/10.3390/jof7070531
Alotaibi MO, Saleh AM, Sobrinho RL, Sheteiwy MS, El-Sawah AM, Mohammed AE, Elgawad HA. Arbuscular Mycorrhizae Mitigate Aluminum Toxicity and Regulate Proline Metabolism in Plants Grown in Acidic Soil. Journal of Fungi. 2021; 7(7):531. https://doi.org/10.3390/jof7070531
Chicago/Turabian StyleAlotaibi, Modhi O., Ahmed M. Saleh, Renato L. Sobrinho, Mohamed S. Sheteiwy, Ahmed M. El-Sawah, Afrah E. Mohammed, and Hamada Abd Elgawad. 2021. "Arbuscular Mycorrhizae Mitigate Aluminum Toxicity and Regulate Proline Metabolism in Plants Grown in Acidic Soil" Journal of Fungi 7, no. 7: 531. https://doi.org/10.3390/jof7070531






