The Change in Fatty Acids and Sugars Reveals the Association between Trifoliate Orange and Endophytic Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Endophytic Fungi
2.2. Experimental Setup
2.3. Measurement of Root Fungal Colonization and Plant Biomass
2.4. Measurement of Sugars and FA Contents
2.5. Relative Expression of Root FA Desaturase Genes
2.6. Data Analysis
3. Results
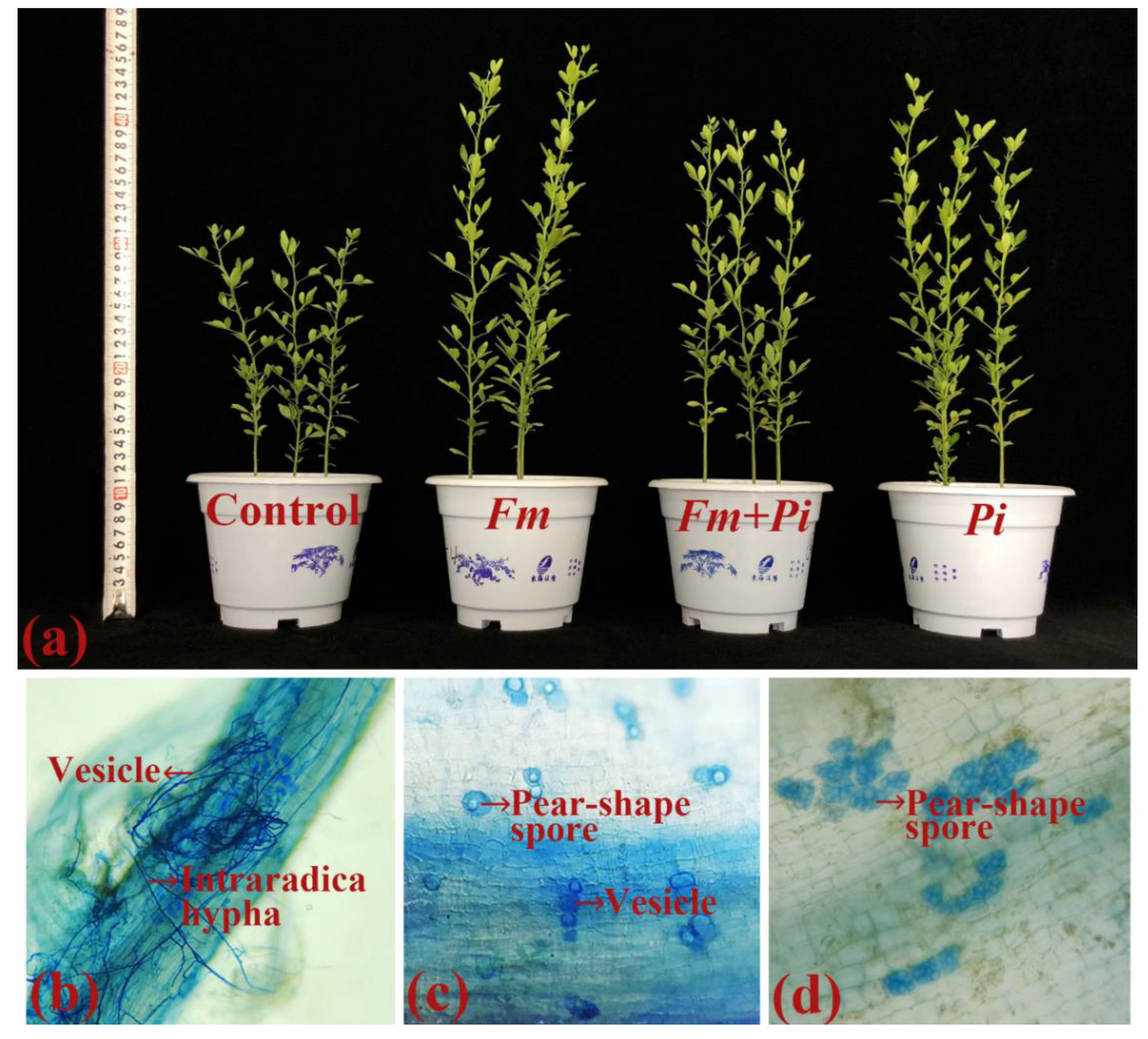
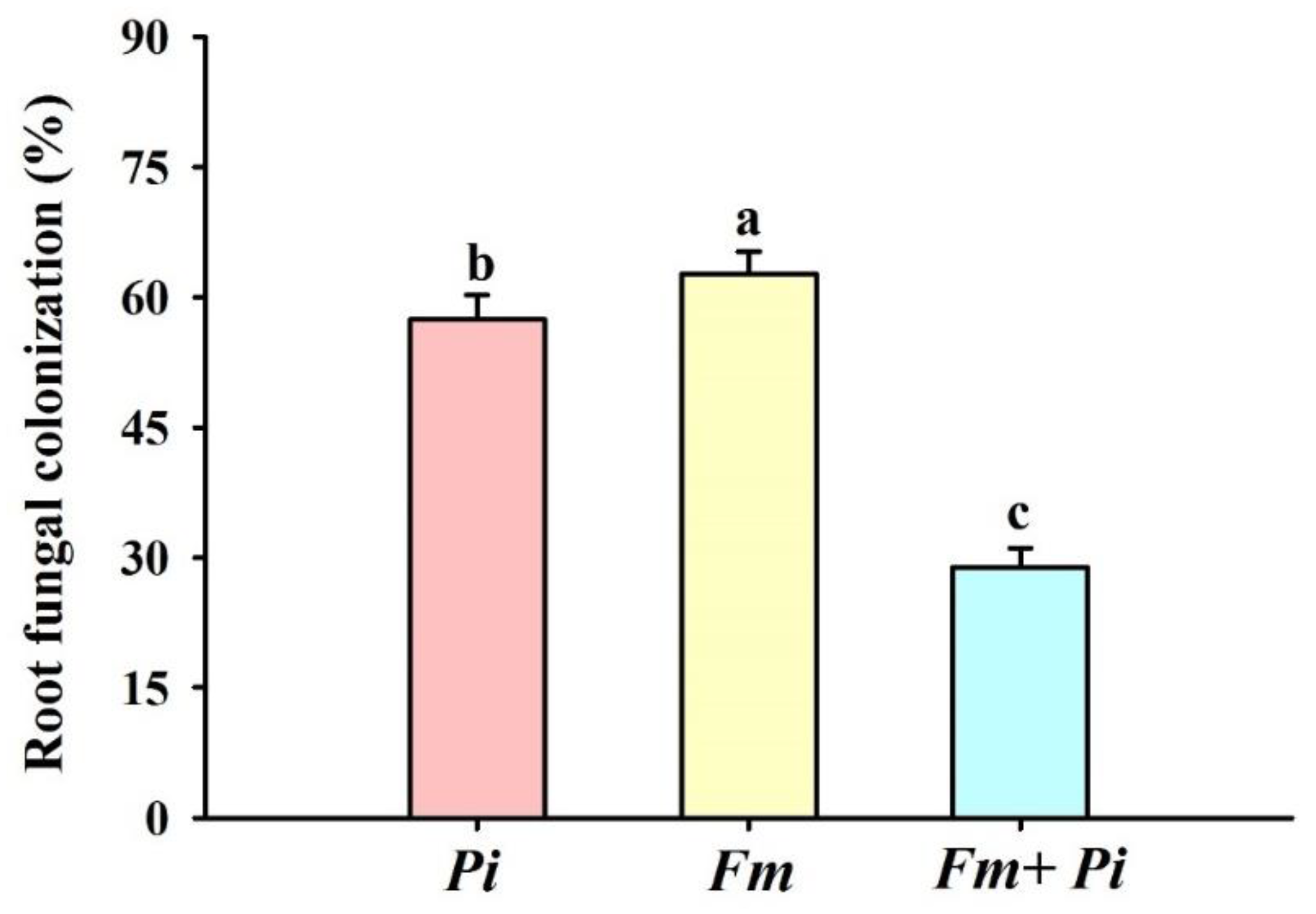
3.1. Root Colonization after Inoculation of Endophytic Fungi
3.2. Effects of Endophytic Fungi on Biomass Production
3.3. Effects of Endophytic Fungi on Sugar Concentrations of Leaves and Roots
3.4. Effects of Endophytic Fungi on FAs Contents in Roots
3.5. Effects of Endophytic Fungi on Relative Expression of FA Desaturase Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knapp, D.G.; Imrefi, I.; Boldpurev, E.; Csikos, S.; Akhmetova, G.; Berek-Nagy, P.J.; Otgonsuren, B.; Kovacs, G.M. Root-colonizing endophytic fungi of the dominant grass Stipa krylovii from a mongolian steppe grassland. Front. Microbiol. 2018, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Hadacek, F.; Kraus, G.F. Plant root carbohydrates affect growth behaviour of endophytic microfungi. FEMS Microbiol. Ecol. 2002, 41, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P. The future has roots in the past: The ideas and scientists that shaped mycorrhizal research. New Phytol. 2018, 220, 982–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.N.; Wang, W.X.; Xie, Q.J.; Liu, N.; Liu, L.X.; Wang, D.P.; Wang, E. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.S.; He, J.D.; Srivastava, A.K.; Zou, Y.N.; Kuca, K. Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiol. 2019, 39, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Srivastava, A.K.; Li, Y. Effect of mycorrhizal symbiosis on growth behavior and carbohdyrate metabolism of trifoliate orange under different substrate P levels. J. Plant Growth Regul. 2015, 34, 495–508. [Google Scholar] [CrossRef]
- Wu, Q.S.; Li, G.H.; Zou, Y.N. Improvement of root system architecture in peach (Prunus persica) seedlings by arbuscular mycorrhizal fungi, related to allocation of glucose/sucrose to root. Not. Bot. Horti Agrobot. 2011, 39, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.L.; He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhiza-released glomalin-related soil protein fractions contribute to soil total nitrogen in trifoliate orange. Plant Soil Environ. 2020, 66, 183–189. [Google Scholar] [CrossRef]
- Lee, Y.; Johnson, J.M.; Chien, C.; Sun, C.; Cai, D.; Lou, B.G.; Oelmuller, R.; Yeh, K. Growth promotion of Chinese cabbage and Arabidopsis by Piriformospora indica is not stimulated by mycelium-synthesized auxin. Mol. Plant-Microbe Interact. 2011, 24, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Franken, P. The plant strengthening root endophyte Piriformospora indica: Potential application and the biology behind. Appl. Microbiol. Biot. 2012, 96, 1455–1464. [Google Scholar] [CrossRef] [Green Version]
- Unnikumar, K.R.; Sree, K.S.; Varma, A. Piriformospora indica: A versatile root endophytic symbiont. Symbiosis 2013, 60, 107–113. [Google Scholar] [CrossRef]
- Yang, L.; Cao, J.L.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Piriformospora indica: A root endophytic fungus and its roles in plants. Not. Bot. Horti Agrobot. 2020, 48, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura, Y.; Akiyama, R.; Tanaka, S.; Yano, K.; Kameoka, H.; Marui, S.; Saito, M.; Kawaguchi, M.; Akiyama, K.; Saito, K. Myristate can be used as a carbon and energy source for the asymbiotic growth of arbuscular mycorrhizal fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 25779–25788. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.D.; Kumar, R.S.; Shyur, L.; Cheng, B.Y.; Tian, Z.H.; Oelmuller, R.; Yeh, K. Metabolomic compounds identified in Piriformospora indica-colonized Chinese cabbage roots delineate symbiotic functions of the interaction. Sci. Rep. 2017, 7, 9291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Y.N.; Tian, Z.H.; Wu, Q.S.; Kuča, K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 2021, 277, 109815. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, J.; Liu, Y.Z.; Zhao, X.L.; Deng, X.X.; Guo, L.L.; Gu, J.Q. A novel bud mutation that confers abnormal patterns of lycopene accumulation in sweet orange fruit (Citrus sinensis L. Osbeck). J. Exp. Bot. 2007, 58, 4161–4171. [Google Scholar] [CrossRef]
- Deshmukh, S.; Hueckelhoven, R.; Schaefer, P.; Imani, J.; Sharma, M.; Weiss, M.; Waller, F.; Kogel, K.H. The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc. Natl. Acad. Sci. USA 2006, 103, 18450–18457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bago, B.; Pfeffer, P.E.; Abubaker, J.; Jun, J.; Allen, J.; Brouillette, J.N.; Douds, D.; Lammers, P.; Shacharhill, Y. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant. Physiol. 2003, 131, 1496–1507. [Google Scholar] [CrossRef] [Green Version]
- Schubert, A.; Allara, P.; Morte, A. Cleavage of sucrose in roots of soybean (Glycine max) colonized by an arbuscular mycorrhizal fungus. New Phytol. 2004, 161, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.C.; Keck, M.; Godde, V.; Niehaus, K.; Kuster, H.; Hohnjec, N. Knockdown of the symbiotic sucrose synthase MtSucS1 affects arbuscule maturation and maintenance in mycorrhizal roots of Medicago truncatula. Plant. Physiol. 2010, 152, 1000–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roycewicz, P.; Malamy, J.E. Dissecting the effects of nitrate, sucrose and osmotic potential on Arabidopsis root and shoot system growth in laboratory assays. Philos. Trans. R. Soc. B 2012, 367, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.Y.; Li, H.; Zhang, Y. Photosynthesis and related physiological characteristics affected by exogenous glucose in wheat seedlings under water stress. Acta Agron. Sin. 2009, 35, 724–732. [Google Scholar] [CrossRef]
- Howe, G.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Curr. Opin. Plant. Biol. 2002, 5, 230–236. [Google Scholar] [CrossRef]
- Stumpe, M.; Carsjens, J.G.; Stenzel, I.; Gobel, C.; Lang, I.; Pawlowski, K.; Hause, B.; Feussner, I. Lipid metabolism in arbuscular mycorrhizal roots of Medicago truncatula. Phytochemistry 2005, 66, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S.; Kawashima, H.; Shinmen, Y.; Akimoto, K.; Yamada, H. Production of eicosapentaenoic acid by Mortierella fungi. J. Am. Oil Chem. Soc. 1988, 65, 1455–1459. [Google Scholar] [CrossRef]
- Cao, F.L.; Wang, H.L.; Yu, W.W.; Cheng, H. Progress of research on fatty acid desaturase and their coding genes in higher plant. J. Nanjing For. Univ. 2012, 36, 125–132. [Google Scholar]
- Dyer, J.M.; Mullen, R.T. Immunocytological localization of two plant fatty acid desaturases in the endoplasmic reticulum. FEBS Lett. 2001, 494, 44–47. [Google Scholar] [CrossRef]
- Hildebrand, D. Lipid Biosynthesis. In Plant Metabolism and Biotechnolog; Ashihara, H., Crozier, A., Komanine, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 27–66. [Google Scholar]
- Beilby, J.P. Fatty acid and sterol composition of ungerminated spores of the vesicular-arbuscular mycorrhizal fungus, Acaulospora laevis. Lipids 1980, 15, 949–952. [Google Scholar] [CrossRef]
- Cheeld, H.; Bhutada, G.; Beaudoin, F.; Eastmond, P.J. DES2 is a fatty acid Δ11 desaturase capable of synthesizing palmitvaccenic acid in the arbuscular mycorrhizal fungus Rhizophagus irregularis. FEBS Lett. 2020, 594, 1770–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, P.; Douds, D.; Bécard, G.; Shachar-Hill, Y. Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant. Physiol. 1999, 120, 587–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameoka, H.; Tsutsui, I.; Saito, K.; Kikuchi, Y.; Handa, Y.; Ezawa, T.; Hayashi, H.; Kawaguchi, M.; Akiyama, K. Stimulation of asymbiotic sporulation in arbuscular mycorrhizal fungi by fatty acids. Nat. Microbiol. 2019, 4, 1654–1660. [Google Scholar] [CrossRef]


| Genes | Gene IDs | Sequence (5’→3’)-Forward | Sequence (5’→3’)-Reverse |
|---|---|---|---|
| PtFAD2 | Orange1.1t02241 | AGGAGGCAAGAGTGGAGGATAAGG | GGAGCAGGTGGACGAATGTCTG |
| PtFAD6 | Cs8g17450 | CTGCACGGAGATACAGCTTGGC | GGAATGTGAGGAGCCGTATGATGC |
| PtΔ9 | orange1.1t03533 | TGCCTGCTCACTTGATGTACGATG | CTCCTCCAGCCTTCTGATTCTTGC |
| PtΔ15 | Cs6g08600 | CAAGAACTGGTCTAGCAGCCTCAG | ATGTGGCTGGACCTTGTGACTTAC |
| β-Actin | Cs1g05000 | CCGACCGTATGAGCAAGGAAA | TTCCTGTGGACAATGGATGGA |
| Treatments | Sucrose | Glucose | Fructose | |||
|---|---|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | Leaves | Roots | |
| Control | 58.86 ± 6.57 c | 25.54 ± 4.61 b | 66.30 ± 6.41 c | 86.16 ± 27.40 b | 112.81 ± 4.96 c | 114.80 ± 8.03 b |
| Fm | 73.47 ± 4.77 b | 34.95 ± 7.82 b | 155.45 ± 3.48 ab | 125.42 ± 7.68 a | 125.76 ± 2.61 ab | 138.09 ± 1.96 a |
| Pi | 83.11 ± 3.63 a | 57.25 ± 9.56 a | 171.42 ± 34.44 a | 141.84 ± 9.15 a | 130.50 ± 0.40 a | 141.34 ± 5.56 a |
| Fm + Pi | 66.55 ± 2.43 b | 52.01 ± 11.68 a | 85.43 ± 7.88 c | 129.45 ± 12.77 a | 123.56 ± 3.13 b | 132.53 ± 7.15 a |
| FA Types | FA Species | Treatments (μg/g FW) | |||
|---|---|---|---|---|---|
| Control | Pi | Fm | Fm + Pi | ||
| Saturated FAs | Methyl hexanoate (C6:0) | 1.47 ± 0.48 b | 2.13 ± 0.30 a | 1.96 ± 0.17 ab | 1.63 ± 0.09 ab |
| Methyl octanoate (C8:0) | 0.53 ± 0.05 b | 0.92 ± 0.21 a | 0.50 ± 0.02 b | 0.45 ± 0.01 b | |
| Methyl decanoate (C10:0) | 1.18 ± 0.17 ab | 1.44 ± 0.20 a | 1.04 ± 0.08 bc | 0.89 ± 0.06 c | |
| Methyl undecanoate (C11:0) | ND | ND | ND | ND | |
| Methyl laurate (C12:0) | 8.80 ± 0.57 a | 9.56 ± 0.69 a | 8.06 ± 1.27 ab | 7.31 ± 0.43 b | |
| Methyl tridecanoate (C13:0) | 1.32 ± 0.14 b | 1.48 ± 0.19 a | 1.39 ± 0.07 ab | 1.30 ± 0.14 ab | |
| Methyl myristate (C14:0) | 18.14 ± 0.90 c | 23.80 ± 1.70 a | 21.15 ± 1.10 b | 17.76 ± 1.18 c | |
| Methyl pentadecanoate (C15:0) | 11.38 ± 0.90 a | 13.86 ± 0.63 a | 12.84 ± 1.51 a | 11.30 ± 1.85 a | |
| Methyl palmitate (C16:0) | 1210.76 ± 114.10 a | 1379.62 ± 172.93 a | 1274.72 ± 14.76 a | 1227.51 ± 22.79 a | |
| Methyl heptadecanoate (C17:0) | 16.98 ± 2.19 a | 16.91 ± 1.59 a | 17.07 ± 3.18 a | 15.93 ± 2.23 a | |
| Methyl stearate (C18:0) | 767.37 ± 124.56 a | 823.26 ± 28.44 a | 791.65 ± 22.28 a | 757.79 ± 30.67 a | |
| Methyl arachidate (C20:0) | 12.89 ± 2.34 ab | 13.69 ± 0.82 a | 12.01 ± 0.13 ab | 10.80 ± 0.64 b | |
| Methyl heneicosadienoate (C21:0) | 2.11 ± 0.19 a | 2.14 ± 0.14 a | 1.92 ± 0.20 ab | 1.72 ± 0.22 b | |
| Methyl behenate (C22:0) | 13.62 ± 1.36 ab | 15.82 ± 1.68 a | 13.46 ± 1.90 ab | 12.02 ± 1.16 b | |
| Methyl tricosanoate (C23:0) | 13.22 ± 1.43 ab | 15.54 ± 1.74 a | 12.16 ± 1.84 b | 10.70 ± 1.01 b | |
| Methyl lignocerate (C24:0) | 26.45 ± 3.96 b | 36.00 ± 5.54 a | 28.76 ± 5.06 ab | 26.88 ± 3.24 b | |
| Unsaturated FAs | Methyl myristoleate (C14:1) | ND | ND | ND | ND |
| Methyl pentadecenoate (C15:1) | ND | ND | ND | ND | |
| Methyl palmitoleate (C16:1) | 9.97 ± 2.21 a | 9.15 ± 1.83 a | 7.61 ± 2.99 a | 8.90 ± 1.47 a | |
| Methyl heptadecenoate (C17:1) | 8.45 ± 1.77 a | 9.09 ± 0.32 a | 9.59 ± 0.34 a | 8.47 ± 0.15 a | |
| Methyl oleate (C18:1) | 175.21 ± 20.81 a | 162.46 ± 25.39 ab | 158.08 ± 2.69 ab | 125.60 ± 20.85 b | |
| Methyl linoleate (C18:2) | 548.39 ± 40.90 a | 542.91 ± 42.95 a | 490.88 ± 90.61 a | 459.15 ± 29.9 a | |
| Methyl gamma-Linolenate (C18:3N6) | 6.51 ± 0.56 b | 10.66 ± 1.35 a | 7.49 ± 2.45 b | 8.24 ± 0.39 ab | |
| Methyl linolenate (C18:3N3) | 149.23 ± 21.49 a | 159.62 ± 16.98 a | 133.37 ± 13.39 ab | 115.82 ± 6.63 b | |
| cis-11-Eicosenoic acid methyl ester (C20:1) | 8.68 ± 0.82 ab | 9.90 ± 1.01 a | 8.96 ± 0.13 ab | 7.84 ± 0.25 b | |
| cis-11,14-Eicosadienoic acid methyl ester (C20:2) | 2.31 ± 0.27 b | 3.48 ± 0.77 a | 2.79 ± 0.46 ab | 2.75 ± 0.34 ab | |
| Cis-11,14,-Eicosatrienotic acid methyl ester (C20:3N6) | 15.61 ± 0.78 b | 31.62 ± 6.99 a | 18.98 ± 2.30 b | 19.13 ± 1.19 b | |
| Arachidonate (C20:4N6) | 34.44 ± 2.96 b | 46.50 ± 6.40 a | 27.85 ± 6.81 b | 28.58 ± 0.01 b | |
| Cis-11,14,17-Eicosatrienoate acid methyl ester (C20:3N3) | 0.81 ± 0.08 b | 1.03 ± 0.07 a | 0.92 ± 0.05 ab | 0.82 ± 0.01 b | |
| Cis-5,8,11,14,17-eicosapentaenoate tic acid methyl ester (C20:5N3) | 1.56 ± 0.43 c | 4.53 ± 0.33 b | 4.02 ± 1.58 b | 6.721 ± 1.58 a | |
| Methyl erucate (C22:1N9) | 8.68 ± 1.90 b | 12.23 ± 0.86 a | 11.22 ± 1.72 ab | 10.99 ± 2.15 ab | |
| Cis-13,16-Docosadienotic acid methyl ester (C22:2) | ND | ND | ND | ND | |
| Cis-4,7,10,13,16,19-Docosahexaenotic acid methyl ester (C22:6N3) | ND | ND | ND | ND | |
| Methyl cis-15-tetracosenoate (C24:1) | 15.84 ± 2.56 b | 26.73 ± 6.12 a | 17.76 ± 2.96 b | 18.05 ± 1.99 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.-L.; Liu, R.-C.; Yang, L.; Zou, Y.-N.; Srivastava, A.K.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Giri, B.; Wu, Q.-S. The Change in Fatty Acids and Sugars Reveals the Association between Trifoliate Orange and Endophytic Fungi. J. Fungi 2021, 7, 716. https://doi.org/10.3390/jof7090716
Meng L-L, Liu R-C, Yang L, Zou Y-N, Srivastava AK, Kuča K, Hashem A, Abd_Allah EF, Giri B, Wu Q-S. The Change in Fatty Acids and Sugars Reveals the Association between Trifoliate Orange and Endophytic Fungi. Journal of Fungi. 2021; 7(9):716. https://doi.org/10.3390/jof7090716
Chicago/Turabian StyleMeng, Lu-Lu, Rui-Cheng Liu, Liu Yang, Ying-Ning Zou, Anoop Kumar Srivastava, Kamil Kuča, Abeer Hashem, Elsayed Fathi Abd_Allah, Bhoopander Giri, and Qiang-Sheng Wu. 2021. "The Change in Fatty Acids and Sugars Reveals the Association between Trifoliate Orange and Endophytic Fungi" Journal of Fungi 7, no. 9: 716. https://doi.org/10.3390/jof7090716
APA StyleMeng, L.-L., Liu, R.-C., Yang, L., Zou, Y.-N., Srivastava, A. K., Kuča, K., Hashem, A., Abd_Allah, E. F., Giri, B., & Wu, Q.-S. (2021). The Change in Fatty Acids and Sugars Reveals the Association between Trifoliate Orange and Endophytic Fungi. Journal of Fungi, 7(9), 716. https://doi.org/10.3390/jof7090716











