Molecular Insights into Defense Responses of Vietnamese Maize Varieties to Fusarium verticillioides Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fusarium verticillioides Isolates
2.2. Multilocus DNA Sequence Based Identification of Fusarium verticillioides
2.3. Pathogenic Assay of Fusarium Verticillioides in Two Inbred Maize Lines
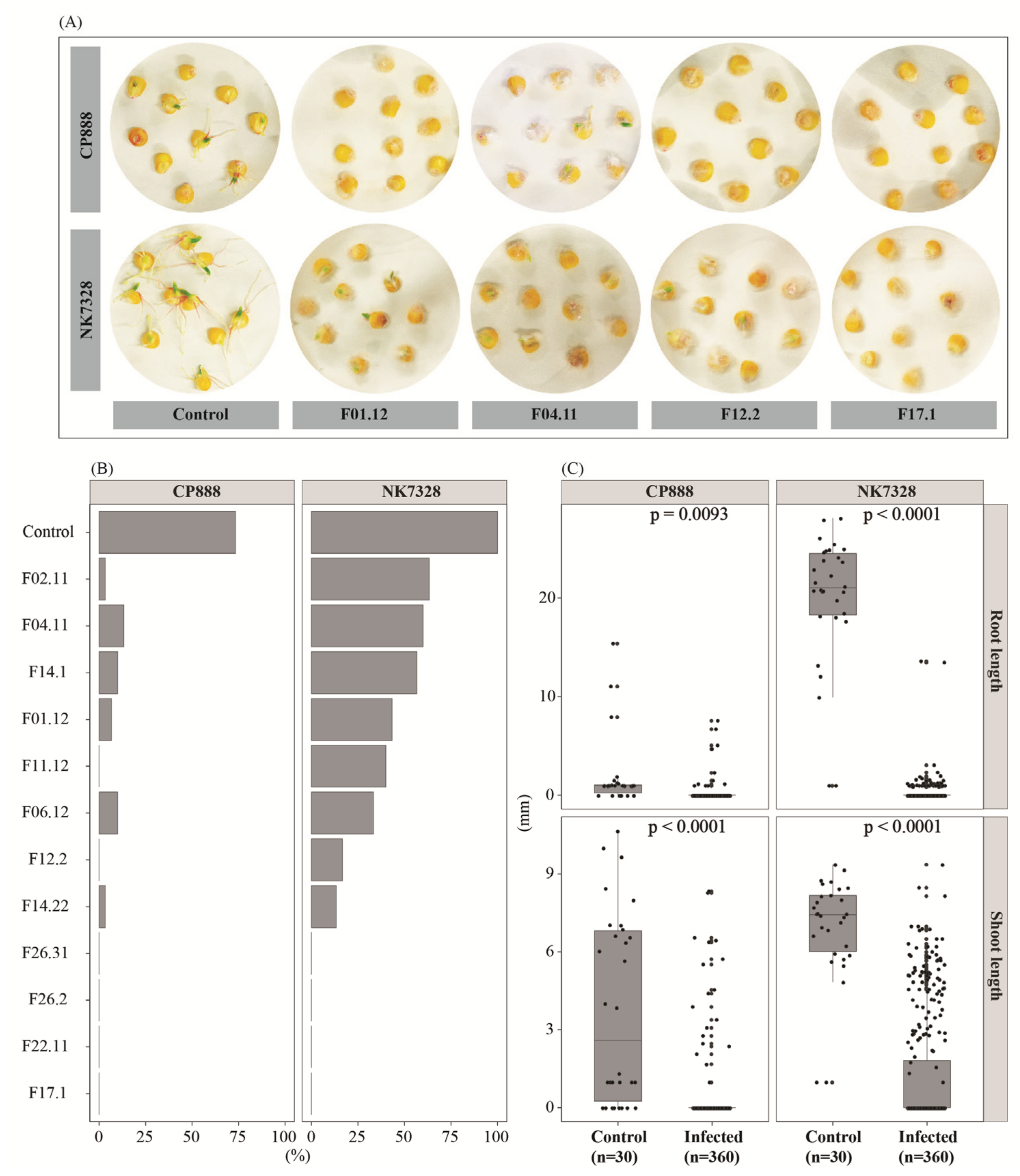
2.3.1. Seed Germination Assay
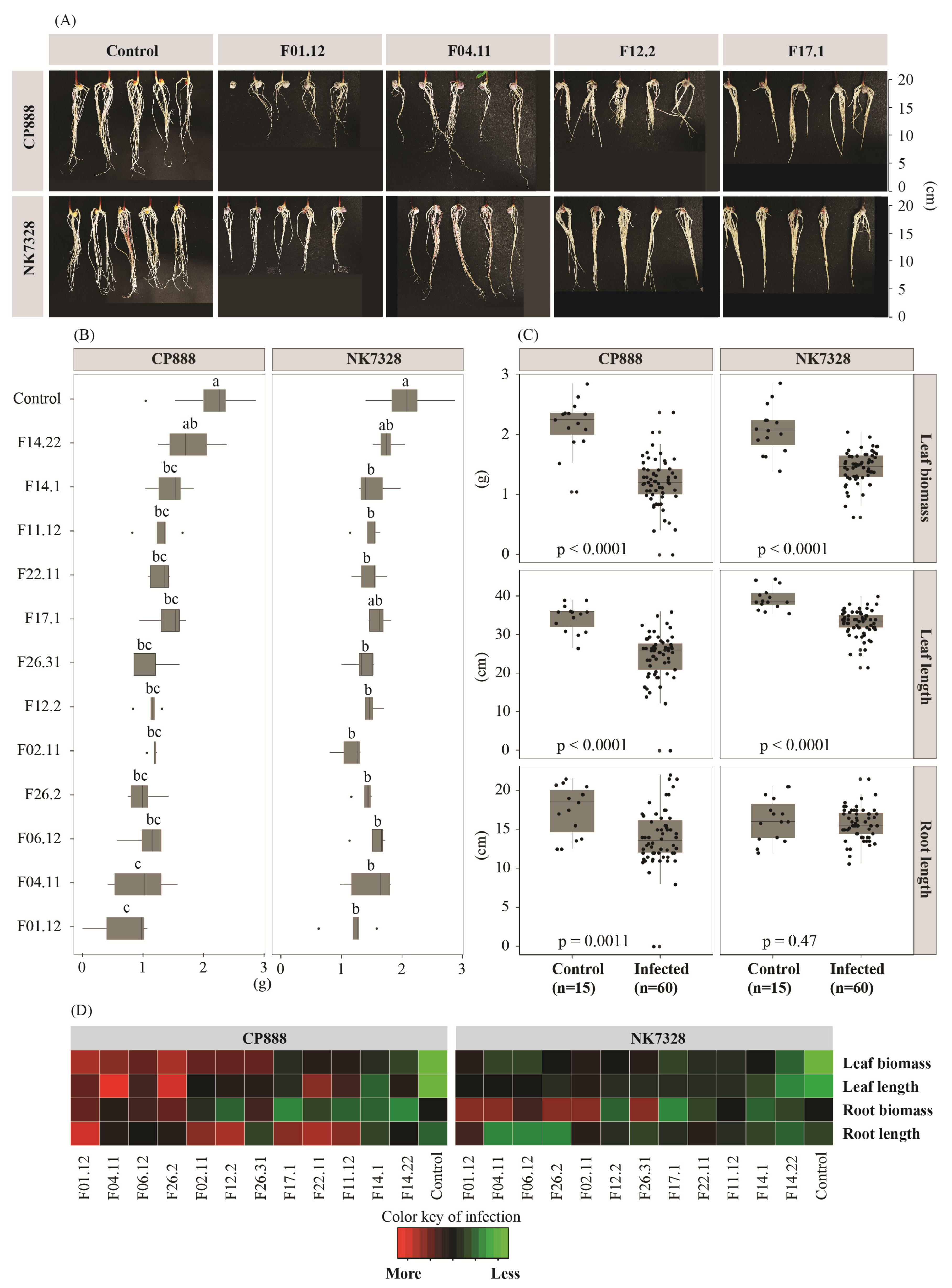
2.3.2. In-Planta Assay
2.4. RNA Extraction and RT-qPCR
2.5. Statistical Analysis
3. Results
3.1. Susceptibility of Two Maize Genotypes to Vietnamese Maize Fields’ Fusarium verticillioides Population on Germination Seeds
3.2. Susceptibility of Two Maize Genotypes to Vietnamese Maize Fields’ Fusarium verticillioides Population on Maize Seedlings
3.3. Differential Resistance Responses between Susceptible and Resistant Cultivars upon Infection by Fusarium verticillioides on Maize Seedlings
3.3.1. Salicylic Acid, Jasmonic Acid, and Abscisic Acid Dependent Responses
3.3.2. Transcriptional Regulation of Downstream Pathogenesis-Related Proteins
3.3.3. Transcriptional Regulation of Benzoxazinoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miller, J.D. Mycotoxins in small grains and maize: Old problems, new challenges. Food Addit. Contam. 2008, 25, 219–230. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Mycotoxin Regulations in 2003 and Current Developments. 2003. Available online: http://www.fao.org/3/y5499e/y5499e07.htm (accessed on 1 December 2020).
- Pascual, C.B.; Barcos, A.; Mandap, J.A.L.; Ocampo, E. Fumonisin-producing Fusarium species causing ear rot of corn in the Philippines. Philipp. J. Crop. Sci. 2016, 41, 12–21. [Google Scholar]
- Tran, M.T.; Ameye, M.; Phan, L.T.-K.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Impact of ethnic pre-harvest practices on the occurrence of Fusarium verticillioides and fumonisin B1 in maize fields from Vietnam. Food Control. 2021, 120, 107567. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, X.; Chen, G.; Sun, S.; Yang, Y.; Zhu, Z.; Duan, C. The Major Fusarium Species Causing Maize Ear and Kernel Rot and Their Toxigenicity in Chongqing, China. Toxins 2018, 10, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, J.I.; Miller, J.D. A concise history of mycotoxin research. J. Agric. Food Chem. 2016, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Advisory Group recommendations on priorities for the IARC Monographs. Lancet. Oncol. 2019, 20, 763–764. [Google Scholar] [CrossRef]
- Riley, R.T.; Enongene, E.; Voss, K.A.; Norred, W.P.; Meredith, F.I.; Sharma, R.P.; Spitsbergen, J.; Williams, D.E.; Carlson, D.B.; Merrill, A.H., Jr. Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ. Health Perspect. 2001, 109 (Suppl. S2), 301–308. [Google Scholar]
- Voss, K.; Smith, G.; Haschek, W. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Glenn, A.E.; Zitomer, N.C.; Zimeri, A.M.; Williams, L.D.; Riley, R.T.; Proctor, R.H. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol. Plant.-Microbe Interact. 2008, 21, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Zitomer, N.C.; Jones, S.; Bacon, C.; Glenn, A.E.; Baldwin, T.; Riley, R.T. Translocation of sphingoid bases and their 1-phosphates, but not fumonisins, from roots to aerial tissues of maize seedlings watered with fumonisins. J. Agric. Food Chem. 2010, 58, 7476–7481. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Plattner, R.D. Fumonisin B1-nonproducing strains of Fusarium verticillioides cause maize (Zea mays) ear infection and ear rot. J. Agric. Food Chem. 2000, 48, 5773–5780. [Google Scholar] [CrossRef]
- Tran, M.T.; Ameye, M.; Phan, L.T.-K.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Post-harvest contamination of maize by Fusarium verticillioides and fumonisins linked to traditional harvest and post-harvest practices: A case study of small-holder farms in Vietnam. Int. J. Food Microbiol. 2021, 339, 109022. [Google Scholar] [CrossRef] [PubMed]
- Maschietto, V.; Colombi, C.; Pirona, R.; Pea, G.; Strozzi, F.; Marocco, A.; Rossini, L.; Lanubile, A. QTL mapping and candidate genes for resistance to Fusarium ear rot and fumonisin contamination in maize. BMC Plant. Biol. 2017, 17, 20. [Google Scholar] [CrossRef] [Green Version]
- Mesterhazy, A.; Lemmens, M.; Reid, L.M. Breeding for resistance to ear rots caused by Fusarium spp. in maize—A review. Plant. Breed. 2012, 131, 1–19. [Google Scholar] [CrossRef]
- Hellmich, R.L.; Hellmich, K.A. Use and impact of Bt maize. Nat. Educ. Knowl. 2012, 3, 4. [Google Scholar]
- Campos-Bermudez, V.A.; Fauguel, C.M.; Tronconi, M.A.; Casati, P.; Presello, D.A.; Andreo, C.S. Transcriptional and metabolic changes associated to the infection by Fusarium verticillioides in maize inbreds with contrasting ear rot resistance. PLoS ONE 2013, 8, e61580. [Google Scholar] [CrossRef] [Green Version]
- Lambarey, H.; Moola, N.; Veenstra, A.; Murray, S.; Suhail Rafudeen, M. Transcriptomic analysis of a susceptible African maize line to Fusarium verticillioides infection. Plants 2020, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, T.; Baldwin, S.; Klos, K.; Bregitzer, P.; Marshall, J. Deletion of the benzoxazinoid detoxification gene NAT1 in Fusarium graminearum reduces deoxynivalenol in spring wheat. PLoS ONE 2019, 14, e0214230. [Google Scholar] [CrossRef] [Green Version]
- Cotton, T.A.; Pétriacq, P.; Cameron, D.D.; Al Meselmani, M.; Schwarzenbacher, R.; Rolfe, S.A.; Ton, J. Metabolic regulation of the maize rhizobiome by benzoxazinoids. ISME J. 2019, 13, 1647–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanubile, A.; Bernardi, J.; Marocco, A.; Logrieco, A.; Paciolla, C. Differential activation of defense genes and enzymes in maize genotypes with contrasting levels of resistance to Fusarium verticillioides. Environ. Exp. Bot. 2012, 78, 39–46. [Google Scholar] [CrossRef]
- De Zutter, N.; Ameye, M.; Debode, J.; De Tender, C.; Ommeslag, S.; Verwaeren, J.; Vermeir, P.; Audenaert, K.; De Gelder, L. Shifts in the rhizobiome during consecutive in planta enrichment for phosphate-solubilizing bacteria differentially affect maize P status. Microb. Biotechnol. 2021, 14, 1594–1612. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, R.-S.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar]
- Wang, Y.; Zhou, Z.; Gao, J.; Wu, Y.; Xia, Z.; Zhang, H.; Wu, J. The mechanisms of maize resistance to Fusarium verticillioides by comprehensive analysis of RNA-seq data. Front. Plant. Sci. 2016, 7, 1654. [Google Scholar] [CrossRef] [Green Version]
- Lanubile, A.; Ferrarini, A.; Maschietto, V.; Delledonne, M.; Marocco, A.; Bellin, D. Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genom. 2014, 15, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battilani, P.; Lanubile, A.; Scala, V.; Reverberi, M.; Gregori, R.; Falavigna, C.; Dall’asta, C.; Park, Y.S.; Bennett, J.; Borrego, E.J. Oxylipins from both pathogen and host antagonize jasmonic acid-mediated defence via the 9-lipoxygenase pathway in Fusarium verticillioides infection of maize. Mol. Plant. Pathol. 2018, 19, 2162–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant. Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.X.; Dehne, H.-W.; Steiner, U. Histopathological assessment of the infection of maize leaves by Fusarium graminearum, F. proliferatum, and F. verticillioides. Fungal Biol. 2016, 120, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.; McGee, D.; Carlton, W. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology 1997, 87, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef] [Green Version]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2 H-1, 4-benzoxazin-3 (4 H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef]
- Ding, X.; Yang, M.; Huang, H.; Chuan, Y.; He, X.; Li, C.; Zhu, Y.; Zhu, S. Priming maize resistance by its neighbors: Activating 1, 4-benzoxazine-3-ones synthesis and defense gene expression to alleviate leaf disease. Front. Plant. Sci. 2015, 6, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maag, D.; Köhler, A.; Robert, C.A.; Frey, M.; Wolfender, J.L.; Turlings, T.C.; Glauser, G.; Erb, M. Highly localized and persistent induction of Bx1-dependent herbivore resistance factors in maize. Plant. J. 2016, 88, 976–991. [Google Scholar] [CrossRef]
- Glenn, A.; Hinton, D.; Yates, I.; Bacon, C. Detoxification of corn antimicrobial compounds as the basis for isolating Fusarium verticillioides and some other Fusarium species from corn. Appl. Environ. Microbiol. 2001, 67, 2973–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glenn, A.; Gold, S.; Bacon, C. Fdb1 and Fdb2, Fusarium verticillioides loci necessary for detoxification of preformed antimicrobials from corn. Mol. Plant.-Microbe Interact. 2002, 15, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Murillo, I.; Cavallarin, L.; Segundo, B.S. Cytology of infection of maize seedlings by Fusarium moniliforme and immunolocalization of the pathogenesis-related PRms protein. Phytopathology 1999, 89, 737–747. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.; Balajee, S.A.; Schroers, H.-J.; Summerbell, R.C.; Robert, V.A.; Crous, P.W.; Zhang, N. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [Green Version]
- Tzin, V.; Hojo, Y.; Strickler, S.R.; Bartsch, L.J.; Archer, C.M.; Ahern, K.R.; Zhou, S.; Christensen, S.A.; Galis, I.; Mueller, L.A. Rapid defense responses in maize leaves induced by Spodoptera exigua caterpillar feeding. J. Exp. Bot. 2017, 68, 4709–4723. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Luca, P.; Adriano, M. Differential gene expression in kernels and silks of maize lines with contrasting levels of ear rot resistance after Fusarium verticillioides infection. J. Plant. Physiol. 2010, 167, 1398–1406. [Google Scholar]
- Lin, Y.; Zhang, C.; Lan, H.; Gao, S.; Liu, H.; Liu, J.; Cao, M.; Pan, G.; Rong, T.; Zhang, S. Validation of potential reference genes for qPCR in maize across abiotic stresses, hormone treatments, and tissue types. PLoS ONE 2014, 9, e95445. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.M.; Ameye, M.; Landschoot, S.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Molecular Insights into Defense Responses of Vietnamese Maize Varieties to Fusarium verticillioides Isolates. J. Fungi 2021, 7, 724. https://doi.org/10.3390/jof7090724
Tran TM, Ameye M, Landschoot S, Devlieghere F, De Saeger S, Eeckhout M, Audenaert K. Molecular Insights into Defense Responses of Vietnamese Maize Varieties to Fusarium verticillioides Isolates. Journal of Fungi. 2021; 7(9):724. https://doi.org/10.3390/jof7090724
Chicago/Turabian StyleTran, Trang Minh, Maarten Ameye, Sofie Landschoot, Frank Devlieghere, Sarah De Saeger, Mia Eeckhout, and Kris Audenaert. 2021. "Molecular Insights into Defense Responses of Vietnamese Maize Varieties to Fusarium verticillioides Isolates" Journal of Fungi 7, no. 9: 724. https://doi.org/10.3390/jof7090724





