Narrative Review: Bioactive Potential of Various Mushrooms as the Treasure of Versatile Therapeutic Natural Product
Abstract
:1. Introduction
2. Pharmacological Actions of Mushroom
2.1. Mushrooms and Wound Healing
2.2. Mushrooms in Anti-HIV Action
2.3. Mushrooms and Anticancer Potentials
2.4. Mushrooms as Immunomodulators
2.5. Antioxidant and Antibacterial Action of Mushrooms
| The Scientific Name of the Mushroom | Antioxidant Compounds | References |
|---|---|---|
| Agaricus arvensis | β-Carotene, ascorbic acid, lycopene, phenolic compounds | [195,196] |
| Agaricus bisporus | Pyrogallol l-ergothioneine, α- and β-glucans Catechin, gallic acid, rutin, caffeic acid | [197,198,199] |
| Agaricus blazei | Benzoic acid, myricetin, quercetin, pyrogallol α- and β-Glucans | [200,201,202] |
| Agaricus romagnesii | Phenolic compounds, β-carotene | [203,204] |
| Agaricus silvaticus | Phenolic compounds, β-carotene | [205,206] |
| Agaricus silvicola | β-Carotene, ascorbic acid, lycopene, phenolic compounds | [207,208] |
| Agrocybe cylindracea | α-Tocopherol, β-tocopherol | [65] |
| Amanita rubescens | Phenolics compounds, flavonoids | [209,210] |
| Armillaria mellea | Antioxidant components, ascorbic acid, flavonoids, and phenolic compounds | [211,212,213] |
| Armillaria ostoyae | Phenolic compounds | [214] |
| Auricularia auricula-judae | Polysaccharides, phenolic compounds | [215,216,217] |
| Auriculariapolytricha | Phenolic compounds | [218,219] |
| Boletus badius | β-Carotene, α-tocopherol, phenolic compounds, flavonoids | [220,221] |
| Boletus edulis | β-Carotene, ascorbic acid, flavonoids, tocopherols | [222,223] |
| Calocybe gambosa | Phenolic compounds, flavonoids | [224] |
| Cantharellus cibarius | Phenolic compounds, flavonoids | [225,226,227] |
| Cantharellus clavatus | Phenolic compounds | [228] |
| Chlorophyllum rhacodes | Phenolic compounds | [229,230] |
| Clavaria vermicularis | Flavonoids, ascorbic acid | [231,232] |
| Clitocybe alexandri | Tocopherols, phenolic compounds | [233,234] |
| Clitocybe geotropa | Phenolic compounds | [235,236] |
| Coprinopsis atramentaria | β-Glucans | [237,238] |
| Coprinus comatus | β-Carotene, ascorbic acid, lycopene, phenolic compounds | [239] |
| Coriolus versicolor | Gallic, p-coumaric, protocatechin, caffeic, and vanillc acids | [240,241,242] |
| Cortinarius glaucopus | Tocopherols, phenolic compounds | [204] |
| Craterellus cornucopioides | Phenolic compounds, flavonoids | [243,244,245,246] |
| Fistulina hepatica | Tocopherols, phenolic compounds | [247,248] |
| Flammulina velutipes | Gallic acid, pyrogallol, homogentisic acid, 5-sulfosalicylic acid, protocatechuic acid, quercetin, caffeic acid | [249,250] |
| Ganoderma applanatum | Gallic, p-coumaric, protocatechin, caffeic, and vanillc acids | [251,252] |
| Ganoderma lucidum | Quercetin, kaempferol, Triterpenoids, polysaccharides | [253,254,255] |
| Ganoderma tsugae | Polysaccharides | [256,257] |
| Gomphus clavatus | Ergosterol, phenolic compounds | [258,259] |
| Grifola frondosa | Phenolic compounds, β-1,6 and β-1,3-glucan | [260] |
| Helvella crispa | Phenolic compounds | [261] |
| Hericium erinaceus | Phenolic compounds | [262] |
| Hydnum repandum | Tocopherols, phenolic compounds | [263,264] |
| I. obliquus | p-Hydroxybenzoic acid, quercetin, kaempferol | [265,266] |
| Laccaria laccata | Tocopherols, phenolic compounds | [267] |
| Lactarius citriolens | Free sugars, fatty acids, tocopherols, and phenolic acids | [268] |
| Lactarius deliciosus | Phenolic compounds, flavonoids | [269,270,271] |
| Lactarius piperatus | Phenolic compounds, flavonoids | [236,272] |
| Lactarius salmonicolor | Phenolic compounds | [273,274] |
| Lentinula edodes | Gallic acid, protocatechuic acid, catechin, tocopherols | [275,276,277] |
| Lepista nuda | β-Carotene, α-tocopherol | [278,279,280,281] |
| Leucopaxillus giganteus | β-carotene, ascorbic acid, lycopene, phenolic compounds | [282] |
| Macrolepiota procera | Phenolic compounds | [283] |
| Marasmius oreades | Flavonoids, ascorbic acid | [284,285] |
| Meripilus giganteus | Gallic, p-coumaric, protocatechin, caffeic, and vanillc acids | [286,287,288] |
| Phellinus igniarius | Hispidin | [289,290] |
| Phellinus linteus | β-Tocopherol, protocatechuic acid, gallic acid; pyrogallol; homogentisic acid, α- and β-glucans | [291] |
| Pleurotus ostreatus | β-Glucans gallic acid, homogentisic acid, naringin, myricetin, tocopherols, glycoproteins, β-D-Glucan (pleuran) Lectin | [292,293,294] |
| Pleurotus pulmonarius | Flavonoids, ascorbic acid | [295,296] |
| Pycnoporus sanguineus | Phenolic compounds | [297,298] |
| Ramaria botrytis | Tocopherols, phenolic compounds, ascorbic acid, β-carotene | [299,300,301] |
| Russula vinosa | Phenolic compounds | [302,303] |
| Schizophyllum commune | α- and β-Glucans, phenolic compounds | [304,305,306] |
| Sparassis crispa | Protocatechuic acid, benzoic acid, p-hydroxybenzoic acid | [307] |
2.6. Hepatoprotective Potentials of Mushrooms
2.7. Anti-Inflammatory Action of Mushroom
3. Clinical Trails
4. Recommendations and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alves, M.; Ferreira, I.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef] [Green Version]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumfield, M.; Abbott, K.; Duve, E.; Cassettari, T.; Marshall, S.; Fayet-Moore, F. Examining the health effects and bioactive components in Agaricus bisporus mushrooms: A scoping review. J. Nutr. Biochem. 2020, 84, 108453. [Google Scholar] [CrossRef] [PubMed]
- Ndung’u, S.W.; Otieno, C.A.; Onyango, C.; Musieba, F. Nutritional composition, physical qualities and sensory evaluation of wheat bread supplemented with Oyster Mushroom. Am. J. Food Technol. 2015, 10, 279–288. [Google Scholar] [CrossRef]
- Bernas, E. Monosodium glutamate equivalents and B-group vitamins in frozen mushrooms. Int. J. Food Prop. 2017, 20, 1613–1626. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1948. [Google Scholar] [CrossRef] [Green Version]
- Keflie, T.S.; Nölle, N.; Lambert, C.; Nohr, D.; Biesalski, H.K. Impact of the natural resource of UVB on the content of vitamin D2 in oyster mushroom (Pleurotus ostreatus) under subtropical settings. Saudi J. Biol. Sci. 2019, 26, 1724–1730. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, M.; Mujumdar, A.S. UV induced conversion during drying of ergosterol to vitamin D in various mushrooms: Effect of different drying conditions. Trends Food Sci. Technol. 2020, 105, 200–210. [Google Scholar] [CrossRef]
- Fischer, M.W.F.; Money, N.P. Why mushrooms form gills: Efficiency of the lamellate morphology. Fungal Biol. 2010, 114, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Yodkeeree, S.; Pornngarm, L. Skin wound-healing potential of polysaccharides from medicinal mushroom Auricularia auricula-judae (Bull.). J. Fungi 2021, 7, 247. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Veeraperumal, S.; Qiu, H.M.; Zeng, S.S.; Yao, W.Z.; Wang, B.P.; Liu, Y.; Cheong, K.L. Polysaccharides from Gracilaria lemaneiformis promote the HaCaT keratinocytes wound healing by polarised and directional cell migration. Carbohydr. Polym. 2020, 241, 116310. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Kumar, S.; Singh, I. Strategies and Therapies for Wound Healing: A Review. Curr. Drug Targets 2021, 22. (online ahead of print). [Google Scholar] [CrossRef]
- De Jesus, L.I.; Smiderle, F.R.; Ruthes, A.C.; Vilaplana, F.; Dal’Lin, F.T.; Maria-Ferreira, D.; Werner, M.F.; Van Griensven, L.J.L.D.; Iacomini, M. Chemical characterization and wound healing property of a β-D-glucan from edible mushroom Piptoporus betulinus. Int. J. Biol. Macromol. 2018, 117, 1361–1366. [Google Scholar] [CrossRef]
- Rao, K.M.; Suneetha, M.; Park, G.T.; Babu, A.G.; Han, S.S. Hemostatic, biocompatible, and antibacterial non-animal fungal mushroom-based carboxymethyl chitosan-ZnO nanocomposite for wound-healing applications. Int. J. Biol. Macromol. 2020, 155, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.H.; Qiu, Z.; Hashimoto, M.; Yamamoto, K.; Kimura, T. Effects of medicinal mushroom (Sparassis crispa) on wound healing in streptozotocin-induced diabetic rats. Am. J. Surg. 2009, 197, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Ö.; Gökşen Tosun, N.; Özgür, A.; Erden Tayhan, S.; Bilgin, S.; Türkekul, İ.; Gökce, İ. Microwave-assisted green synthesis of silver nanoparticles using crude extracts of Boletus edulis and Coriolus versicolor: Characterization, anticancer, antimicrobial and wound healing activities. J. Drug Deliv. Sci. Technol. 2021, 64, 102641. [Google Scholar] [CrossRef]
- Batterbury, M.; Tebbs, C.A.; Rhodes, J.M.; Grierson, I. Agaricus bisporus (edible mushroom lectin) inhibits ocular fibroblast proliferation and collagen lattice contraction. Exp. Eye Res. 2002, 74, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.F.; Yang, R.; Liu, B.; Gu, T.M.; Zhao, Z.L.; Shi, D.F.; Chang, D.Q. Chemical analysis of Agaricus blazei polysaccharides and effect of the polysaccharides on IL-1β mRNA expression in skin of burn wound-treated rats. Int. J. Biol. Macromol. 2010, 47, 155–157. [Google Scholar] [CrossRef]
- Da Silva, G.R.; Franklin, V.; Cambuí, J.M.; de Almeida, D.T.; Wadt, N.S.Y.; Cardoso, V.O.; Bach, E.E. Effect of Agaricus sylvaticus (Schaeffer) extract in rats skin wound healing. Biomed. J. Sci. Tech. Res. 2018, 10, 7598–7600. [Google Scholar]
- Khamrai, M.; Banerjee, S.L.; Kundu, P.P. A sustainable production method of mycelium biomass using an isolated fungal strain Phanerochaete chrysosporium (accession no: KY593186): Its exploitation in wound healing patch formation. Biocatal. Agric. Biotechnol. 2018, 16, 548–557. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, W.; Gao, H.; Chan, E.; Lan, J.; Zhou, S. Ganoderma lucidum polysaccharide fractions accelerate healing of acetic acid-induced ulcers in rats. J. Med. Food 2004, 7, 417–421. [Google Scholar] [CrossRef]
- Lin, H.J.; Chang, Y.S.; Lin, L.H.; Haung, C.F.; Wu, C.Y.; Ou, K.L. An immunomodulatory protein (Ling Zhi-8) from a Ganoderma lucidum induced acceleration of wound healing in rat liver tissues after monopolar electrosurgery. Evid. Based Complement. Altern. Med. 2014, 2014, 916531. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Kimura, T. Orally and topically administered Sparassis crispa (hanabiratake) improved healing of skin wounds in mice with streptozotocin-induced diabetes. Biosci. Biotechnol. Biochem. 2013, 77, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.A.; Fard, A.A.; Sabaratnam, V.; Wong, K.H.; Kuppusamy, U.R.; Abdullah, N.; Ismail, S. Potential activity of aqueous extract of culinary- medicinal Lion’s Mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) in accelerating wound healing in rats. Int. J. Med. Mushrooms 2011, 13, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Jang, K.H.; Jin, H.K. Polysaccharides isolated from Phellinus gilvus enhances dermal wound healing in streptozotocin-induced diabetic rats. J. Vet. Sci. 2005, 6, 161–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Lee, Y.M.; Park, J.P.; Lim, K.T.; Lee, S.J. Phytoglycoprotein isolated from Dioscorea batatas Decne promotes intestinal epithelial wound healing. Chin. J. Nat. Med. 2020, 18, 738–748. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Wu, P.; Zhao, Y.; Suo, X.; Xiao, A.; Ke, M.; He, X.; Tong, Z.; Chen, Y. Green fabrication of seedbed-like Flammulina velutipes polysaccharides-derived scaffolds accelerating full-thickness skin wound healing accompanied by hair follicle regeneration. Int. J. Biol. Macromol. 2021, 167, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Safaee-Ardakani, M.R.; Hatamian-Zarmi, A.; Sadat, S.M.; Mokhtari-Hosseini, Z.B.; Ebrahimi-Hosseinzadeh, B.; Rashidiani, J.; Kooshki, H. Electrospun Schizophyllan/polyvinyl alcohol blend nanofibrous scaffold as potential wound healing. Int. J. Biol. Macromol. 2019, 127, 27–38. [Google Scholar] [CrossRef]
- Nor Azlan, A.Y.H.; Katas, H.; Habideen, N.H.; Mh Busra, M.F. Dual-action of thermoresponsive gels containing DsiRNA-loaded gold nanoparticles for diabetic wound therapy: Characterization, in vitro safety and healing efficacy. Saudi Pharm. J. 2020, 28, 1420–1430. [Google Scholar] [CrossRef]
- Figueira, M.S.; Sá, L.A.; Vasconcelos, A.S.; Moreira, D.R.; Laurindo, P.S.O.C.; Ribeiro, D.R.G.; Santos, R.S.; Guzzo, P.; Dolabela, M.F.; Percario, S. Nutritional supplementation with the mushroom Agaricus sylvaticus reduces oxidative stress in children with HIV. Can. J. Infect. Dis. Med. Microbiol. 2014, 25, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djohan, Y.F.; Camara, C.; Mondé, A.A.; Koffi, G.; Niamké, G.; Déré, L.; Tiahou, G.; Djessou, P.; Sess, D. Interest of antioxidants in the care of the patients infected by the HIV: The experience of long term administration of Alternanthera pungens herb tea. Ann. Biol. Clin. 2009, 67, 563–568. [Google Scholar]
- Li, Y.R.; Liu, Q.H.; Wang, H.X.; Ng, T.B. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 51–57. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Y.; Li, S.; Zhao, J.; Zhang, G.; Wang, H.; Ng, T.B. A novel lectin with highly potent antiproliferative and HIV-1 reverse transcriptase inhibitory activities from the edible wild mushroom Russula delica. Glycoconj. J. 2010, 27, 259–265. [Google Scholar] [CrossRef]
- Singh, R.S.; Bhari, R.; Kaur, H.P. Mushroom lectins: Current status and future perspectives. Crit. Rev. Biotechnol. 2010, 30, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, L.; Fang, J.C.; Wong, J.H.; Ng, T.B.; Jiang, Y.; Wang, C.R.; Zhang, N.Y.; Wen, T.Y.; Qu, L.Y.; et al. Isolation and identification of a novel polysaccharide-peptide complex with antioxidant, anti-proliferative and hypoglycaemic activities from the abalone mushroom. Biosci. Rep. 2012, 32, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Collins, R.A.; Ng, T.B. Polysaccharopeptide from Coriolus versicolor has potential for use against human immunodeficiency virus type 1 infection. Life Sci. 1997, 60, 383–387. [Google Scholar] [CrossRef]
- Adotey, G.; Quarcoo, A.; Holliday, J.C.; Fofie, S.; Saaka, B. Effect of immunomodulating and antiviral agent of medicinal mushrooms (Immune Assist 24/7™) on CD4+ T-Lymphocyte Counts of HIV-infected patients. Int. J. Med. Mushrooms 2011, 13, 109–113. [Google Scholar] [CrossRef]
- Flórez-Sampedro, L.; Zapata, W.; Orozco, L.P.; Mejía, A.I.; Arboleda, C.; Rugeles, M.T. In vitro anti-HIV-1 activity of the enzymatic extract enriched with laccase produced by the fungi Ganoderma sp. and Lentinus sp. Vitae 2016, 23, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Rong, C.B.; Kong, C.; Liu, Y.; Xu, F.; Miao, Q.J.; Wang, S.X.; Wang, H.X.; Zhang, G.Q. A novel laccase with potent antiproliferative and HIV-1 reverse transcriptase inhibitory activities from mycelia of mushroom Coprinus comatus. BioMed Res. Int. 2014, 2014, 417461. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.Q.; Chen, Q.J.; Hua, J.; Liu, Z.L.; Sun, Y.; Xu, X.; Han, P.; Wang, H.X. An inulin-specific lectin with anti-HIV-1 reverse transcriptase, antiproliferative, and mitogenic activities from the edible mushroom Agaricus bitorquis. BioMed Res. Int. 2019, 2019, 1341370. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S. Anti-HIV-1 protease activity of the crude extracts and isolated compounds from Auricularia polytricha. BMC Complement. Altern. Med. 2019, 19, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef]
- El Dine, R.S.; El Halawany, A.M.; Ma, C.-M.; Hattori, M. Anti-HIV-1 protease activity of lanostane triterpenes from the vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 2008, 71, 1022–1026. [Google Scholar] [CrossRef]
- Lv, H.; Kong, Y.; Yao, Q.; Zhang, B.; Leng, F.W.; Bian, H.J.; Balzarini, J.; Van Damme, E.; Bao, J.K. Nebrodeolysin, a novel hemolytic protein from mushroom Pleurotus nebrodensis with apoptosis-inducing and anti-HIV-1 effects. Phytomedicine 2009, 16, 198–205. [Google Scholar] [CrossRef]
- Wang, C.R.; Zhou, R.; Ng, T.B.; Wong, J.H.; Qiao, W.T.; Liu, F. First report on isolation of methyl gallate with antioxidant, anti-HIV-1 and HIV-1 enzyme inhibitory activities from a mushroom (Pholiota adiposa). Environ. Toxicol. Pharmacol. 2014, 37, 626–637. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.X.; Ng, T.B. A peptide with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Russula paludosa. Peptides 2007, 28, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Chuchawankul, S. HIV-1 protease and reverse transcriptase inhibition by tiger milk mushroom (Lignosus rhinocerus) sclerotium extracts: In vitro and in silico studies. J. Tradit. Complement. Med. 2020, 10, 396–404. [Google Scholar] [CrossRef] [PubMed]
- El Dine, R.S.; El Halawany, A.M.; Ma, C.M.; Hattori, M. Inhibition of the dimerization and active site of HIV-1 protease by secondary metabolites from the Vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 2009, 72, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Liu, Y.; Zhang, G.Q.; Zhao, S.; Xu, F.; Geng, X.L.; Wang, H.X. Cordysobin, a novel alkaline serine protease with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Cordyceps sobolifera. J. Biosci. Bioeng. 2012, 113, 42–47. [Google Scholar] [CrossRef]
- Seniuk, O.F.; Gorovoj, L.F.; Beketova, G.V.; Savichuk, H.O.; Rytik, P.G.; Kucherov, I.I.; Prilutskay, A.B.; Prilutsky, A.I. Anti-infective properties of the melanin-glucan complex obtained from medicinal tinder bracket mushroom, Fomes fomentarius (L.: Fr.) Fr. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2011, 13, 7–18. [Google Scholar] [CrossRef]
- Bruggemann, R.; Orlandi, J.M.; Benati, F.J.; Faccin, L.C.; Mantovani, M.S.; Nozawa, C.; Linhares, R.E.C. Antiviral activity of Agaricus blazei Murrill ss. Heinem extract against human and bovine herpesviruses in cell culture. Braz. J. Microbiol. 2006, 37, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, R.; Le, M.H.; Nguyen, L.D.; Hofrichter, M. Laccase from the medicinal mushroom Agaricus blazei: Production, purification and characterization. Appl. Microbiol. Biotechnol. 2005, 67, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Nomura, A.; Yumen, T.; Mizuno, T.; Hagiwara, T.; Nakamura, T. Isolation and properties of a lectin from the fruiting bodies of Agaricus blazei. Carbohydr. Res. 2013, 183, 150–154. [Google Scholar] [CrossRef]
- Pavlova, N.I.; Savinova, O.V.; Nikolaeva, S.N.; Boreko, E.I.; Flekhter, O.B. Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia 2003, 74, 489–492. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Zhang, Y.; Zhang, N.; Fu, L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohydr. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phyther. Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef]
- Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Lindequist, U.; Niedermeyer, T.H.J.; Jülich, W.D. The pharmacological potential of mushrooms. Evid.-Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Zapora, E.; Wolkowycki, M.; Bakier, S.; Zjawiony, J.K. Phellinus igniarius: A pharmacologically active polypore mushroom. Nat. Prod. Commun. 2016, 11, 1043–1046. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ng, T.B. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 2001, 68, 2151–2158. [Google Scholar] [CrossRef]
- Wong, J.H.; Wang, H.X.; Ng, T.B. Marmorin, a new ribosome inactivating protein with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from the mushroom Hypsizigus marmoreus. Appl. Microbiol. Biotechnol. 2008, 81, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Ng, T.B. Isolation of a novel ubiquitin-like protein from Pleurotus ostreatus mushroom with anti-human immunodeficiency virus, translation-inhibitory, and ribonuclease activities. Biochem. Biophys. Res. Commun. 2000, 276, 587–593. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Sun, J.; Wang, H.X.; Ng, T.B. A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim. Pol. 2009, 56, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngai, P.H.K.; Zhao, Z.; Ng, T.B. Agrocybin, an antifungal peptide from the edible mushroom Agrocybe cylindracea. Peptides 2005, 26, 191–196. [Google Scholar] [CrossRef]
- Ho Wong, J.; Bun Ng, T.; Jiang, Y.; Liu, F.; Cho Wing Sze, S.; Yanbo Zhang, K. Purification and characterization of a laccase with inhibitory activity toward HIV-1 reverse transcriptase and tumor cells from an edible mushroom (Pleurotus cornucopiae). Protein Pept. Lett. 2010, 17, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.C.; Zhang, G.Q.; Ng, T.B.; Wang, H.X. A novel ribonuclease with potent HIV-1 reverse transcriptase inhibitory activity from cultured mushroom Schizophyllum commune. J. Microbiol. 2011, 49, 803–808. [Google Scholar] [CrossRef]
- Ngai, P.H.K.; Ng, T.B. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003, 73, 3363–3374. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Ng, T.B.; Wang, H. A novel lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from dried fruiting bodies of the monkey head mushroom hericium erinaceum. J. Biomed. Biotechnol. 2010, 2010, 716515. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.R.; Ng, T.B.; Li, L.; Fang, J.C.; Jiang, Y.; Wen, T.Y.; Qiao, W.T.; Li, N.; Liu, F. Isolation of a polysaccharide with antiproliferative, hypoglycemic, antioxidant and HIV-1 reverse transcriptase inhibitory activities from the fruiting bodies of the abalone mushroom Pleurotus abalonus. J. Pharm. Pharmacol. 2011, 63, 825–832. [Google Scholar] [CrossRef]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef]
- Arata, S.; Watanabe, J.; Maeda, M.; Yamamoto, M.; Matsuhashi, H.; Mochizuki, M.; Kagami, N.; Honda, K.; Inagaki, M. Continuous intake of the Chaga mushroom (Inonotus obliquus) aqueous extract suppresses cancer progression and maintains body temperature in mice. Heliyon 2016, 2, e00111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakata, T.; Yamada, T.; Taji, S.; Ohishi, H.; Wada, S.I.; Tokuda, H.; Sakuma, K.; Tanaka, R. Structure determination of inonotsuoxides A and B and in vivo anti-tumor promoting activity of inotodiol from the sclerotia of Inonotus obliquus. Bioorg. Med. Chem. 2007, 15, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Park, B.T.; Na, K.H.; Jung, E.C.; Park, J.W.; Kim, H.H. Antifungal and anticancer activities of a protein from the mushroom Cordyceps militaris. Korean J. Physiol. Pharmacol. 2009, 13, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Kosanić, M.; Ranković, B.; Rančić, A.; Stanojković, T. Evaluation of metal concentration and antioxidant, antimicrobial, and anticancer potentials of two edible mushrooms Lactarius deliciosus and Macrolepiota procera. J. Food Drug Anal. 2016, 24, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Han, K.H.; Song, K.Y.; Lee, K.H.; Jo, S.Y.; Lee, S.W.; Yoon, T.J. Activation of innate immunity by Lepiota procera enhances antitumor activity. Korean J. Pharmacogn. 2010, 41, 115–121. [Google Scholar]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-derived carbon dots for toxic metal Ion detection and as antibacterial and anticancer agents. ACS Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Zeng, D.; Zhao, J.; Luk, K.H.; Cheung, S.T.; Wong, K.H.; Chen, T. Potentiation of in vivo anticancer efficacy of Selenium nanoparticles by mushroom polysaccharides surface decoration. J. Agric. Food Chem. 2019, 67, 2865–2876. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Zhu, S. Purification, characterization, antioxidant and anticancer activities of novel polysaccharides extracted from Bachu mushroom. Int. J. Biol. Macromol. 2018, 107, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.A.; Zaghlol, G.M.; El-Garawani, I.M.; Ahmed, E.F.; Rateb, M.E.; Abdel Moneim, A.E. Ganoderma applanatum secondary metabolites induced apoptosis through different pathways: In vivo and in vitro anticancer studies. Biomed. Pharmacother. 2018, 101, 264–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shomali, N.; Onar, O.; Cihan, A.C.; Akata, I.; Yildirim, O. Antioxidant, anticancer, antimicrobial, and antibiofilm properties of the culinary-medicinal fairy ring mushroom, Marasmius oreades (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.H.; Leonard, J. In vitro effects on proliferation, apoptosis and colony inhibition in ER-dependent and ER-independent human breast cancer cells by selected mushroom species. Oncol. Rep. 2006, 15, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, L.; Liu, D.; Yang, X.; Li, S.; Gao, H.; Yao, X.; Wen, H.; Liu, H. Two new sesquiterpenes and six norsesquiterpenes from the solid culture of the edible mushroom Flammulina velutipes. Tetrahedron 2012, 68, 3012–3018. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, L.; Yang, X.; Li, L.; Li, S.; Gao, H.; Yao, X.S.; Wen, H.; Liu, H.W. Bioactive sesquiterpenoids from the solid culture of the edible mushroom Flammulina velutipes growing on cooked rice. Food Chem. 2012, 132, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.Y.K.; Fung, K.P.; Choy, Y.M. The isolation and characterization of an immunomodulatory and anti-tumor polysaccharide preparation from Flammulina velutipes. Immunopharmacology 1997, 35, 255–263. [Google Scholar] [CrossRef]
- Jiang, S.M.; Xiao, Z.M.; Xu, Z.H. Inhibitory activity of polysaccharide extracts from three kinds of edible fungi on proliferation of human hepatoma SMMC-7721 cell and mouse implanted S180 tumor. World J. Gastroenterol. 1999, 5, 404–407. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, K.; Liu, X.; Huang, Y.F.; Liu, B. In vitro antioxidant and antitumor activities of polysaccharides extracted from the mycelia of liquid-cultured Flammulina velutipes. Food Sci. Technol. Res. 2013, 19, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.; Zhong, H.; Tong, S.; Cao, X.; Firempong, C.K.; Liu, H.; Fu, M.; Yang, Y.; Feng, Y.; Zhang, H.; et al. Enhanced oral bioavailability of a sterol-loaded microemulsion formulation of Flammulina velutipes, a potential antitumor drug. Int. J. Nanomed. 2012, 7, 5067–5078. [Google Scholar]
- Ikekawa, T.; Miyano, T.; Okura, A.; Maruyama, H.; Kawamura, K.; Sawasaki, Y.; Shiratori, K.; Naito, K. Proflamin, a new antitumor agent: Preparation, physicochemical properties and antitumor Activity. Jpn. J. Cancer Res. GANN 1985, 76, 142–148. [Google Scholar] [PubMed]
- Lau, M.-F.; Chua, K.-H.; Sabaratnam, V.; Kuppusamy, U.R. In vitro and in silico anticancer evaluation of a medicinal mushroom, Ganoderma neo-japonicum Imazeki, against human colonic carcinoma cells. Biotechnol. Appl. Biochem. 2021, 68, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Dey, D.; Ghosh, D.; Lai, T.K.; Bhuvanesh, N.; Dolui, S.; Velayutham, R.; Acharya, K. Astrakurkurone, a sesquiterpenoid from wild edible mushroom, targets liver cancer cells by modulating Bcl-2 family proteins. IUBMB Life 2019, 71, 992–1002. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Nunes, F.M.; Rzeski, W. Branched mannans from the mushroom: Cantharellus cibarius enhance the anticancer activity of natural killer cells against human cancers of lung and colon. Food Funct. 2019, 10, 5816–5826. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, H.; Tong, X.; Qi, Y. An antitumour lectin from the edible mushroom Agrocybe aegerita. Biochem. J. 2003, 374, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Yin, Y.; Pan, Y.; Ye, X.; Xu, B.; Yu, W.; Zeng, H.; Sun, H. Anti-metastatic activity of Agrocybe aegerita galectin (AAL) in a mouse model of breast cancer lung metastasis. J. Funct. Foods 2018, 41, 163–170. [Google Scholar] [CrossRef]
- Yu, L.; Fernig, D.G.; Smith, J.A.; Milton, J.D.; Rhodes, J.M. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (Edible Mushroom) Lectin. Cancer Res. 1993, 53, 4627–4632. [Google Scholar]
- Feng, K.; Liu, Q.H.; Ng, T.B.; Liu, H.Z.; Li, J.Q.; Chen, G.; Sheng, H.Y.; Xie, Z.L.; Wang, H.X. Isolation and characterization of a novel lectin from the mushroom Armillaria luteo-virens. Biochem. Biophys. Res. Commun. 2006, 345, 1573–1578. [Google Scholar] [CrossRef]
- Sun, J.; Ng, T.B.; Wang, H.; Zhang, G. A novel hemagglutinin with antiproliferative activity against tumor cells from the hallucinogenic mushroom boletus speciosus. BioMed Res. Int. 2014, 2014, 340467. [Google Scholar] [CrossRef]
- Pohleven, J.; Obermajer, N.; Sabotič, J.; Anžlovar, S.; Sepčić, K.; Kos, J.; Kralj, B.; Štrukelj, B.; Brzin, J. Purification, characterization and cloning of a ricin B-like lectin from mushroom Clitocybe nebularis with antiproliferative activity against human leukemic T cells. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.B.; Ngai, P.H.K.; Xia, L. An agglutinin with mitogenic and antiproliferative activities from the mushroom Flammulina velutipes. Mycologia 2006, 98, 167–171. [Google Scholar] [CrossRef]
- Ngai, P.H.K.; Ng, T.B. A mushroom (Ganoderma capense) lectin with spectacular thermostability, potent mitogenic activity on splenocytes, and antiproliferative activity toward tumor cells. Biochem. Biophys. Res. Commun. 2004, 314, 988–993. [Google Scholar] [CrossRef]
- Kawagishi, H.; Nomura, A.; Mizuno, T.; Kimura, A.; Chiba, S. Isolation and characterization of a lectin from Grifola frondosa fruiting bodies. BBA Gen. Subj. 1990, 1034, 247–252. [Google Scholar] [CrossRef]
- Pires, A.d.R.A.; Ruthes, A.C.; Cadena, S.M.S.C.; Iacomini, M. Cytotoxic effect of a mannogalactoglucan extracted from Agaricus bisporus on HepG2 cells. Carbohydr. Polym. 2017, 170, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, L.; Zhao, T.; Zhang, Z.; Zhang, R.; Jin, J.; Cai, Y.; Wang, F. Restoration of the tumor-suppressor function to mutant p53 by Ganoderma lucidum polysaccharides in colorectal cancer cells. Oncol. Rep. 2017, 37, 594–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zhou, D.; Liu, Y.; Li, C.; Zhao, X.; Li, Y.; Li, W. Ganoderma lucidum polysaccharide inhibits prostate cancer cell migration via the protein arginine methyltransferase 6 signaling pathway. Mol. Med. Rep. 2018, 17, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Shi, S.; Chen, Q.; Lin, S.; Wang, R.; Wang, S.; Chen, C. Antitumor and immunomodulatory activities of Ganoderma lucidum polysaccharides in glioma-bearing rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Sun, D.; Meng, Q.; Guo, W.; Chen, Q.; Zhang, Y. Grifola frondosa polysaccharides induce breast cancer cell apoptosis via the mitochondrial-dependent apoptotic pathway. Int. J. Mol. Med. 2017, 40, 1089–1095. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Y.; Hou, X.X.; Li, Z.Y.; Shan, S.H.; Chang, M.C.; Feng, C.P.; Wei, Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at S-phage in colon cancer cells. Int. J. Biol. Macromol. 2020, 157, 288–295. [Google Scholar] [CrossRef]
- Jeff, I.B.; Fan, E.; Tian, M.; Song, C.; Yan, J.; Zhou, Y. In vivo anticancer and immunomodulating activities of mannogalactoglucan-type polysaccharides from Lentinus edodes (Berkeley) singer. Cent. Eur. J. Immunol. 2016, 41, 47–53. [Google Scholar] [CrossRef]
- Ya, G. A Lentinus edodes polysaccharide induces mitochondrial-mediated apoptosis in human cervical carcinoma HeLa cells. Int. J. Biol. Macromol. 2017, 103, 676–682. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym. 2020, 237, 116113. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wang, N.; Guo, J.; Yuan, L.; Yang, X. Chemical characterization of Pleurotus eryngii polysaccharide and its tumor-inhibitory effects against human hepatoblastoma HepG-2 cells. Carbohydr. Polym. 2016, 138, 123–133. [Google Scholar] [CrossRef]
- Uddin Pk, M.; Sayful Islam, M.; Pervin, R.; Dutta, S.; Talukder, R.I.; Rahman, M. Optimization of extraction of antioxidant polysaccharide from Pleurotus ostreatus (Jacq.) P. Kumm and its cytotoxic activity against murine lymphoid cancer cell line. PLoS ONE 2019, 14, e0209371. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, Y.J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 2007, 76, 447–480. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic Immunology (6th edition): Functions and Disorders of the Immune System; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; ISBN 9780323549431. [Google Scholar]
- Van de Velde, J.; Wilbers, R.H.P.; Westerhof, L.B.; van Raaij, D.R.; Stavrakaki, I.; Sonnenberg, A.S.; Bakker, J.; Schots, A. Assessing the immunomodulatory potential of high-molecular-weight extracts from mushrooms; an assay based on THP-1 macrophages. J. Sci. Food Agric. 2015, 95, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Cheung, C.C.H.; Law, H.K.W.; Lau, Y.L.; Chan, G.C.F. Ganoderma lucidum polysaccharides can induce human monocytic leukemia cells into dendritic cells with immuno-stimulatory function. J. Hematol. Oncol. 2008, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chanput, W.; Reitsma, M.; Kleinjans, L.; Mes, J.J.; Savelkoul, H.F.; Wichers, H.J. β-Glucans are involved in immune-modulation of THP-1 macrophages. Mol. Nutr. Food Res. 2012, 56, 822–833. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Alquini, G.; Tadra-Sfeir, M.Z.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J.L.D. Agaricus bisporus and Agaricus brasiliensis (1 → 6)-β-d-glucans show immunostimulatory activity on human THP-1 derived macrophages. Carbohydr. Polym. 2013, 94, 91–99. [Google Scholar] [CrossRef]
- Ellertsen, L.K.; Hetland, G.; Johnson, E.; Grinde, B. Effect of a medicinal extract from Agaricus blazei Murill on gene expression in a human monocyte cell line as examined by microarrays and immuno assays. Int. Immunopharmacol. 2006, 6, 133–143. [Google Scholar] [CrossRef]
- Castro-Alves, V.C.; Gomes, D.; Menolli, N.; Sforça, M.L.; do Nascimento, J.R.O. Characterization and immunomodulatory effects of glucans from Pleurotus albidus, a promising species of mushroom for farming and biomass production. Int. J. Biol. Macromol. 2017, 95, 215–223. [Google Scholar] [CrossRef]
- Lin, H.; She, Y.H.; Cassileth, B.R.; Sirotnak, F.; Rundles, S.C. Maitake beta-glucan MD-fraction enhances bone marrow colony formation and reduces doxorubicin toxicity in vitro. Int. Immunopharmacol. 2004, 4, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Jeon, H.; Jung, H.J.; Kim, B.; Shin, S.S.; Choi, J.J.; Lee, J.K.; Kang, C.Y.; Kim, S. Enhancement of repopulation and hematopoiesis of bone marrow cells in irradiated mice by oral administration of PG101, a water-soluble extract from Lentinus lepideus. Exp. Biol. Med. 2003, 228, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Hsu, M.L.; Hsu, H.C.; Tzeng, C.H.; Lee, S.S.; Shiao, M.S.; Ho, C.K. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int. J. Cancer 1997, 70, 699–705. [Google Scholar] [CrossRef]
- Harada, T.; Miura, N.; Adachi, Y.; Nakajima, M.; Yadomae, T.; Ohno, N. Effect of SCG, 1,3-β-D-Glucan from Sparassis crispa on the hematopoietic response in cyclophosphamide induced leukopenic mice. Biol. Pharm. Bull. 2002, 25, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.C.; Hsu, Y.J.; Chang, C.J.; Lin, C.S.; Martel, J.; Ojcius, D.M.; Ko, Y.F.; Lai, H.C.; Young, J.D. Immunomodulatory properties of medicinal mushrooms: Differential effects of water and ethanol extracts on NK cell-mediated cytotoxicity. Innate Immun. 2016, 22, 522–533. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Choi, M.W.; Xue, X.; Cheung, P.C.K. Immunomodulatory effect of structurally-characterized mushroom sclerotial polysaccharides isolated from Polyporus rhinocerus on bone marrow dendritic cells (BMDCs). J. Agric. Food Chem. 2019, 67, 12137–12143. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, P.C.K. Structure and immunomodulatory activity of microparticulate mushroom sclerotial β-Glucan prepared from Polyporus rhinocerus. J. Agric. Food Chem. 2019, 67, 9070–9078. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Zhang, L.; Zhang, Y.; Ding, K. Evaluation of water soluble β-d-glucan from Auricularia auricular-judae as potential anti-tumor agent. Carbohydr. Polym. 2010, 80, 977–983. [Google Scholar] [CrossRef]
- Firenzuoli, F.; Gori, L.; Lombardo, G. The medicinal mushroom Agaricus blazei murrill: Review of literature and pharmaco-toxicological problems. Evid.-Based Complement. Altern. Med. 2008, 5, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Lutsik-Kordovsky, M.D.; Stasyk, T.V.; Stoika, R.S. Analysis of cytotoxicity of lectin and non-lectin proteins from Amanita mushrooms. Exp. Oncol. 2001, 23, 43–45. [Google Scholar]
- Zheng, S.; Li, C.; Ng, T.B.; Wang, H.X. A lectin with mitogenic activity from the edible wild mushroom Boletus edulis. Process Biochem. 2007, 42, 1620–1624. [Google Scholar] [CrossRef]
- Yao, H.Y.; Zhang, L.H.; Shen, J.; Shen, H.J.; Jia, Y.L.; Yan, X.F.; Xie, Q.M. Cyptoporus polysaccharide prevents lipopolysaccharide-induced acute lung injury associated with down-regulating Toll-like receptor 2 expression. J. Ethnopharmacol. 2011, 137, 1267–1274. [Google Scholar] [CrossRef]
- Manna, D.; Pust, S.; Torgersen, M.L.; Cordara, G.; Künzler, M.; Krengel, U.; Sandvig, K. Polyporus squamosus Lectin 1a (PSL1a) exhibits cytotoxicity in mammalian cells by disruption of focal adhesions, inhibition of protein synthesis and induction of apoptosis. PLoS ONE 2017, 12, e0170716. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.W.; Guan, S.Y.; Duan, Z.W.; Shen, Y.H.; Fan, W.L.; Chen, L.J.; Zhang, L.; Zhang, L.; Li, T.L. Gene cloning of a novel fungal immunomodulatory protein from Chroogomphis rutilus and its expression in Pichia pastoris. J. Chem. Technol. Biotechnol. 2016, 91, 2761–2768. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Z.; Sun, L.; Liu, X.; Huang, Y.; Wang, F.; Xin, F. Characterization of a new fungal immunomodulatory protein, FIP-dsq2 from Dichomitus squalens. J. Biotechnol. 2017, 246, 45–51. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Y.; Wang, Y.; Chen, T.; Tang, H.; Wang, M. Purification, characterization and immuno-modulating properties of polysaccharides isolated from Flammulina velutipes mycelium. Am. J. Chin. Med. 2010, 38, 191–204. [Google Scholar] [CrossRef]
- Wang, P.H.; Hsu, C.I.; Tang, S.C.; Huang, Y.L.; Lin, J.Y.; Ko, J.L. Fungal immunomodulatory protein from Flammulina velutipes induces interferon-γ production through p38 mitogen-activated protein kinase signaling pathway. J. Agric. Food Chem. 2004, 52, 2721–2725. [Google Scholar] [CrossRef]
- Zhu, X.L.; Chen, A.F.; Lin, Z. Bin Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacol. 2007, 111, 219–226. [Google Scholar] [CrossRef]
- Matsui, K.; Kodama, N.; Nanba, H. Effects of maitake (Grifola frondosa) D-Fraction on the carcinoma angiogenesis. Cancer Lett. 2001, 172, 193–198. [Google Scholar] [CrossRef]
- Pacheco-Sanchez, M.; Boutin, Y.; Angers, P.; Gosselin, A.; Tweddell, R.J. A bioactive (1→3)-, (1→4)-β-D-glucan from Collybia dryophila and other mushrooms. Mycologia 2006, 98, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Chen, Y.; Lin, J.; Zhou, X. Cytokines expression induced by Ganoderma sinensis fungal immunomodulatory proteins (FIP-gsi) in mouse spleen cells. Appl. Biochem. Biotechnol. 2010, 162, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hsiao, Y.M.; Ou, C.C.; Lin, Y.W.; Chiu, Y.L.; Lue, K.H.; Chang, J.G.; Ko, J.L. GMI, a Ganoderma immunomodulatory protein, down-regulates tumor necrosis factor α-induced expression of matrix metalloproteinase 9 via NF-κB pathway in human alveolar epithelial A549 cells. J. Agric. Food Chem. 2010, 58, 12014–12021. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.M.; Huang, Y.L.; Tang, S.C.; Shieh, G.J.; Lai, J.Y.; Wang, P.H.; Ying, T.H.; Ko, J.L. Effect of a fungal immunomodulatory protein from Ganoderma tsugae on cell cycle and interferon-gamma production through phosphatidylinositol 3-kinase signal pathway. Process Biochem. 2008, 43, 423–430. [Google Scholar] [CrossRef]
- Bastiaan-Net, S.; Chanput, W.; Hertz, A.; Zwittink, R.D.; Mes, J.J.; Wichers, H.J. Biochemical and functional characterization of recombinant fungal immunomodulatory proteins (rFIPs). Int. Immunopharmacol. 2013, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Wang, X.F.; Bao, T.W.; Ran, L.; Lin, J.; Zhou, X.W. In vitro synthesis of a recombinant fungal immunomodulatory protein from Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W.Curt.:Fr.) P.Karst. (Aphyllophoromycetideae) and analysis of its immunomodulatory activity. Int. J. Med. Mushrooms 2010, 12, 347–358. [Google Scholar] [CrossRef]
- Lee, J.S.; Cho, J.Y.; Hong, E.K. Study on macrophage activation and structural characteristics of purified polysaccharides from the liquid culture broth of Hericium erinaceus. Carbohydr. Polym. 2009, 78, 162–168. [Google Scholar] [CrossRef]
- Won, D.P.; Lee, J.S.; Kwon, D.S.; Lee, K.E.; Shin, W.C.; Hong, E.K. Immunostimulating activity by polysaccharides isolated from fruiting body of Inonotus obliquus. Mol. Cells 2011, 31, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Cong, W.R.; Xu, H.; Liu, Y.; Li, Q.Z.; Li, W.; Zhou, X.W. Production and functional characterization of a novel fungal immunomodulatory protein FIP-SN15 shuffled from two genes of Ganoderma species. Appl. Microbiol. Biotechnol. 2014, 98, 5967–5975. [Google Scholar] [CrossRef]
- Koyama, Y.; Katsuno, Y.; Miyoshi, N.; Hayakawa, S.; Mita, T.; Muto, H.; Isemura, S.; Aoyagi, Y.; Isemura, M. Apoptosis induction by lectin isolated from the mushroom Boletopsis leucomelas in U937 cells. Biosci. Biotechnol. Biochem. 2002, 66, 784–789. [Google Scholar] [CrossRef]
- Morales, D.; Rutckeviski, R.; Villalva, M.; Abreu, H.; Soler-Rivas, C.; Santoyo, S.; Iacomini, M.; Smiderle, F.R. Isolation and comparison of α- and β-D-glucans from shiitake mushrooms (Lentinula edodes) with different biological activities. Carbohydr. Polym. 2020, 229, 115521. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.K.; Dey, B.; Maity, K.K.; Patra, S.; Mandal, S.; Maiti, S.; Maiti, T.K.; Sikdar, S.R.; Islam, S.S. Isolation and characterization of an immunoenhancing glucan from alkaline extract of an edible mushroom, Lentinus squarrosulus (Mont.) Singer. Carbohydr. Res. 2011, 346, 2039–2044. [Google Scholar] [CrossRef]
- Pushparajah, V.; Fatima, A.; Chong, C.H.; Gambule, T.Z.; Chan, C.J.; Ng, S.T.; Tan, C.S.; Fung, S.Y.; Lee, S.S.; Tan, N.H.; et al. Characterisation of a new fungal immunomodulatory protein from tiger milk mushroom, Lignosus rhinocerotis. Sci. Rep. 2016, 6, 30010. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, H.; Ng, T.B. Purification and characterization of a lectin with antiproliferative activity toward cancer cells from the dried fruit bodies of Lactarius flavidulus. Carbohydr. Res. 2011, 346, 2576–2581. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B.; Ooi, V.E.C.; Liu, W.K.; Chang, S.T. Actions of lectins from the mushroom Tricholoma mongolicum on macrophages, splenocytes and life-span in sarcoma-bearing mice. Anticancer Res. 1997, 17, 419–424. [Google Scholar]
- Ooi, V.E.C. Pharmacological studies on certain mushrooms from China. Int. J. Med. Mushrooms 2001, 3, 341–354. [Google Scholar] [CrossRef]
- Cordara, G.; Winter, H.C.; Goldstein, I.J.; Krengel, U.; Sandvig, K. The fungal chimerolectin MOA inhibits protein and DNA synthesis in NIH/3T3 cells and may induce BAX-mediated apoptosis. Biochem. Biophys. Res. Commun. 2014, 447, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.L.; Chen, Y.; Wang, S.S.; Kai, G.Q.; Fang, Y.M. Isolation, partial characterisation and immunomodulatory activities of polysaccharide from Morchella esculenta. J. Sci. Food Agric. 2011, 91, 2180–2185. [Google Scholar] [CrossRef]
- Su, C.A.; Xu, X.Y.; Liu, D.Y.; Wu, M.; Zeng, F.Q.; Zeng, M.Y.; Wei, W.; Jiang, N.; Luo, X. Isolation and characterization of exopolysaccharide with immunomodulatory activity from fermentation broth of Morchella conica. DARU J. Pharm. Sci. 2013, 21, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.J.; Zhang, J.S.; Yang, Y.; Tang, Q.J.; Jia, W.; Pan, Y.J. Purification, chemical modification and immunostimulating activity of polysaccharides from Tremella aurantialba fruit bodies. J. Zhejiang Univ. Sci. B 2010, 11, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Das, D.; Mondal, S.; Maiti, D.; Bhunia, B.; Maiti, T.K.; Islam, S.S. Structural studies of an immunoenhancing water-soluble glucan isolated from hot water extract of an edible mushroom, Pleurotus florida, cultivar Assam Florida. Carbohydr. Res. 2009, 344, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, X.; Zhang, L.; Kennedy, J.F. Flexible chain conformation of (1 → 3)-β-d-glucan from Poria cocos sclerotium in NaOH/urea aqueous solution. Carbohydr. Polym. 2009, 75, 586–591. [Google Scholar] [CrossRef]
- Gern, R.M.M.; Wisbeck, E.; Rampinelli, J.R.; Ninow, J.L.; Furlan, S.A. Alternative medium for production of Pleurotus ostreatus biomass and potential antitumor polysaccharides. Bioresour. Technol. 2008, 99, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.K.; Wang, H.X.; Ng, T.B. Purification and characterization of a novel lectin from the toxic wild mushroom Inocybe umbrinella. Toxicon 2009, 53, 360–366. [Google Scholar] [CrossRef]
- Mahajan, R.G.; Patil, S.I.; Mohan, D.R.K.; Shastry, P. Pleurotus eous mushroom lectin (PEL) with mixed carbohydrate inhibition and antiproliferative activity on tumor cell lines. J. Biochem. Mol. Biol. Biophys. 2002, 6, 341–345. [Google Scholar] [CrossRef]
- Li, S.Y.; Shi, L.J.; Ding, Y.; Nie, Y.; Tang, X.M. Identification and functional characterization of a novel fungal immunomodulatory protein from Postia placenta. Food Chem. Toxicol. 2015, 78, 64–70. [Google Scholar] [CrossRef]
- Chang, H.; Sheu, F. A novel fungal immunomodulatory protein (PCP) isolated from Poria cocos activates mouse peritoneal macrophage involved in toll-like receptor 4. FASEB J. 2007, 21, A738. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, J.; Wang, H.; Ng, T.B. First isolation and characterization of a novel lectin with potent antitumor activity from a Russula mushroom. Phytomedicine 2010, 17, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.R. The chemistry, nutritional value, immunopharmacology, and safety of the traditional food of medicinal split-gill fugus Schizophyllum commune Fr.:Fr. (Schizophyllaceae). A literature review. Int. J. Med. Mushrooms 2005, 7, 127–140. [Google Scholar] [CrossRef]
- Bimczok, D.; Wrenger, J.; Schirrmann, T.; Rothkötter, H.J.; Wray, V.; Rau, U. Short chain regioselectively hydrolyzed scleroglucans induce maturation of porcine dendritic cells. Appl. Microbiol. Biotechnol. 2009, 82, 321–331. [Google Scholar] [CrossRef]
- Han, X.Q.; Wu, X.M.; Chai, X.Y.; Chen, D.; Dai, H.; Dong, H.L.; Ma, Z.Z.; Gao, X.M.; Tu, P.F. Isolation, characterization and immunological activity of a polysaccharide from the fruit bodies of an edible mushroom, Sarcodon aspratus (Berk.) S. Ito. Food Res. Int. 2011, 44, 489–493. [Google Scholar] [CrossRef]
- Han, C.H.; Liu, Q.H.; Ng, T.B.; Wang, H.X. A novel homodimeric lactose-binding lectin from the edible split gill medicinal mushroom Schizophyllum commune. Biochem. Biophys. Res. Commun. 2005, 336, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, G.; Geng, X.; Zhao, Y.; Ng, T.B.; Zhao, L.; Wang, H. Isolation and characterization of a novel lectin from the edible mushroom Stropharia rugosoannulata. Molecules 2014, 19, 19880–19891. [Google Scholar] [CrossRef] [Green Version]
- Rau, U.; Kuenz, A.; Wray, V.; Nimtz, M.; Wrenger, J.; Cicek, H. Production and structural analysis of the polysaccharide secreted by Trametes (Coriolus) versicolor ATCC 200801. Appl. Microbiol. Biotechnol. 2009, 81, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Huang, T.S.; Hsu, M.L.; Chen, C.C.; Lin, W.S.; Lu, F.J.; Chang, W.H. Antitumor effects of the partially purified polysaccharides from Antrodia camphorata and the mechanism of its action. Toxicol. Appl. Pharmacol. 2004, 201, 186–193. [Google Scholar] [CrossRef]
- Wu, S.J.; Liaw, C.C.; Pan, S.Z.; Yang, H.C.; Ng, L.T. Phellinus linteus polysaccharides and their immunomodulatory properties in human monocytic cells. J. Funct. Foods 2013, 5, 679–688. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Sheu, F.; Chien, P.J.; Hsieh, K.Y.; Chin, K.L.; Huang, W.T.; Tsao, C.Y.; Chen, Y.F.; Cheng, H.C.; Chang, H.H. Purification, cloning, and functional characterization of a novel immunomodulatory protein from Antrodia camphorata (Bitter Mushroom) that exhibits TLR2-dependent NF-κB activation and M1 polarization within murine macrophages. J. Agric. Food Chem. 2009, 57, 4130–4141. [Google Scholar] [CrossRef]
- Li, F.; Wen, H.A.; Zhang, Y.J.; An, M.; Liu, X.Z. Purification and characterization of a novel immunomodulatory protein from the medicinal mushroom Trametes versicolor. Sci. China Life Sci. 2011, 54, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.Y.; Hua, K.F.; Wu, W.C.; Hsu, J.; Weng, S.T.; Lin, T.L.; Liu, C.Y.; Hseu, R.S.; Huang, C.T. Reishi immuno-modulation protein induces interleukin-2 expression via protein kinase-dependent signaling pathways within human T cells. J. Cell. Physiol. 2008, 215, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.J.; Song, A.; Lai, M.N.; Ng, L.T. Immunomodulatory properties of Xylaria nigripes in peritoneal macrophage cells of Balb/c mice. J. Ethnopharmacol. 2011, 138, 762–768. [Google Scholar] [CrossRef]
- Marty-Detraves, C.; Francis, F.; Baricault, L.; Fournier, D.; Paquereau, L. Inhibitory action of a new lectin from Xerocomus chrysenteron on cell-substrate adhesion. Mol. Cell. Biochem. 2004, 258, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Wang, H.; Ng, T.B. First report of a xylose-specific lectin with potent hemagglutinating, antiproliferative and anti-mitogenic activities from a wild ascomycete mushroom. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E. Antioxidant properties of mushroom mycelia obtained by batch cultivation and tocopherol content affected by extraction procedures. BioMed Res. Int. 2014, 2014, 974804. [Google Scholar] [CrossRef] [Green Version]
- Boonsong, S.; Klaypradit, W.; Wilaipun, P. Antioxidant activities of extracts from five edible mushrooms using different extractants. Agric. Nat. Resour. 2016, 50, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Yoon, K.N.; Alam, N.; Lee, K.R.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; Lee, T.S. Antioxidant and antityrosinase activities of various extracts from the fruiting bodies of Lentinus lepideus. Molecules 2011, 16, 2334–2347. [Google Scholar] [CrossRef] [PubMed]
- Im, K.H.; Nguyen, T.K.; Shin, D.B.; Lee, K.R.; Lee, T.S. Appraisal of antioxidant and anti-inflammatory activities of various extracts from the fruiting bodies of Pleurotus florida. Molecules 2014, 19, 3310–3326. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H. Bin Extraction of natural antioxidants from the Thelephora ganbajun mushroom by an ultrasound-assisted extraction technique and evaluation of antiproliferative activity of the extract against human cancer cells. Int. J. Mol. Sci. 2016, 17, 1664. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jia, J.; Ren, X.; Li, B.; Zhang, Q. Extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from Oudemansiella radicata mushroom. Int. J. Biol. Macromol. 2018, 120, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Meng, G.; Wu, Y.; Ma, H.F.; Cui, B.K.; Dai, Y.C. Medium composition optimization, structural characterization, and antioxidant activity of exopolysaccharides from the medicinal mushroom Ganoderma lingzhi. Int. J. Biol. Macromol. 2019, 124, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The Antibacterial activity of Coriolus versicolor methanol extract and its effect on ultrastructural changes of Staphylococcus aureus and Salmonella enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar] [CrossRef] [PubMed]
- Janeš, D.; Kreft, S.; Jurc, M.; Seme, K.; Štrukelj, B. Antibacterial activity in higher fungi (Mushrooms) and endophytic fungi from Slovenia. Pharm. Biol. 2008, 45, 700–706. [Google Scholar] [CrossRef]
- Kombrink, A.; Tayyrov, A.; Essig, A.; Stöckli, M.; Micheller, S.; Hintze, J.; van Heuvel, Y.; Dürig, N.; Lin, C.; Kallio, P.T.; et al. Induction of antibacterial proteins and peptides in the coprophilous mushroom Coprinopsis cinerea in response to bacteria. ISME J. 2019, 13, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.K.; Gautam, N. Evaluation of nutritional, nutraceutical, and antioxidant composition of eight wild culinary mushrooms (higher basidiomycetes) from the northwest Himalayas. Int. J. Med. Mushrooms 2016, 18, 539–546. [Google Scholar] [CrossRef]
- Barros, L.; Falcão, S.; Baptista, P.; Freire, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008, 111, 61–66. [Google Scholar] [CrossRef]
- Ghahremani-Majd, H.; Dashti, F. Chemical composition and antioxidant properties of cultivated button mushrooms (Agaricus bisporus). Hortic. Environ. Biotechnol. 2015, 56, 376–382. [Google Scholar] [CrossRef]
- Robaszkiewicz, A.; Bartosz, G.; Ławrynowicz, M.; Soszyński, M. The role of polyphenols, β-carotene, and lycopene in the antioxidative action of the extracts of dried, edible mushrooms. J. Nutr. Metab. 2010, 2010, 173274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; Van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Wu, S.; Li, F.; Jia, S.; Ren, H.; Gong, G.; Wang, Y.; Lv, Z.; Liu, Y. Drying effects on the antioxidant properties of polysaccharides obtained from Agaricus blazei Murrill. Carbohydr. Polym. 2014, 103, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Li, F.; Liu, Y.; Ren, H.; Gong, G.; Wang, Y.; Wu, S. Effects of extraction methods on the antioxidant activities of polysaccharides from Agaricus blazei Murrill. Int. J. Biol. Macromol. 2013, 62, 66–69. [Google Scholar] [CrossRef]
- Carneiro, A.A.J.; Ferreira, I.C.F.R.; Dueñas, M.; Barros, L.; Da Silva, R.; Gomes, E.; Santos-Buelga, C. Chemical composition and antioxidant activity of dried powder formulations of Agaricus blazei and Lentinus edodes. Food Chem. 2013, 138, 2168–2173. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Mleczek, M. Profile of phenolic and organic acids, antioxidant properties and ergosterol content in cultivated and wild growing species of Agaricus. Eur. Food Res. Technol. 2018, 244, 259–268. [Google Scholar] [CrossRef]
- Garrab, M.; Edziri, H.; El Mokni, R.; Mastouri, M.; Mabrouk, H.; Douki, W. Phenolic composition, antioxidant and anticholinesterase properties of the three mushrooms Agaricus silvaticus Schaeff., Hydnum rufescens Pers. and Meripilus giganteus (Pers.) Karst. in Tunisia. S. Afr. J. Bot. 2019, 124, 359–363. [Google Scholar] [CrossRef]
- Ribeiro, B.; de Pinho, P.G.; Andrade, P.B.; Oliveira, C.; Ferreira, A.C.S.; Baptista, P.; Valentão, P. Do bioactive carotenoids contribute to the color of edible mushrooms? Open Chem. Biomed. Methods J. 2011, 4, 14–18. [Google Scholar] [CrossRef]
- Öztürk, M.; Duru, M.E.; Kivrak, Ş.; Mercan-Doĝan, N.; Türkoglu, A.; Özler, M.A. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: A comparative study on the three most edible mushrooms. Food Chem. Toxicol. 2011, 49, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Buruleanu, L.C.; Radulescu, C.; Antonia Georgescu, A.; Dulama, I.D.; Nicolescu, C.M.; Lucian Olteanu, R.; Stanescu, S.G. Chemometric assessment of the interactions between the metal contents, antioxidant activity, total phenolics, and flavonoids in mushrooms. Anal. Lett. 2019, 52, 1195–1214. [Google Scholar] [CrossRef]
- Kouassi, K.A.; Kouadio, E.J.P.; Konan, K.H.; Dué, A.E.; Kouamé, L.P. Phenolic compounds, organic acid and antioxidant activity of Lactarius subsericatus, Cantharellus platyphyllus and Amanita rubescens, three edible ectomycorrhizal mushrooms from center of Côte d’Ivoire. Eurasian J. Anal. Chem. 2016, 11, 127–139. [Google Scholar]
- Lai, M.N.; Ng, L.T. Antioxidant and antiedema properties of solid-state cultured honey mushroom, Armillaria mellea (Higher Basidiomycetes), extracts and their polysaccharide and polyphenol contents. Int. J. Med. Mushrooms 2013, 15, 1–8. [Google Scholar] [CrossRef]
- Muszyńska, B.; Sułkowska-Ziaja, K.; Ekiert, H. Phenolic acids in selected edible basidiomycota species: Armillaria mellea, Boletus badius, Boletus edulis, Cantharellus cibarius, Lactarius deliciosus and Pleurotus ostreatus. Acta Sci. Pol. Hortorum Cultus 2013, 12, 107–116. [Google Scholar]
- Strapáč, I.; Baranová, M.; Smrčová, M.; Bedlovičová, Z. Antioxidant activity of honey mushrooms (Armillaria mellea). Folia Vet. 2016, 60, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Keleş, A.; Koca, I.; Gençcelep, H. Antioxidant properties of wild Edible mushrooms. J. Food Process. Technol. 2011, 2, 2–6. [Google Scholar]
- Cai, M.; Lin, Y.; Luo, Y.L.; Liang, H.H.; Sun, P.L. Extraction, antimicrobial, and antioxidant activities of crude polysaccharides from the wood ear medicinal mushroom Auricularia auricula-judae (higher basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 591–600. [Google Scholar] [CrossRef]
- Kho, Y.S.; Vikineswary, S.; Abdullah, N.; Kuppusamy, U.R.; Oh, H.I. Antioxidant capacity of fresh and processed fruit bodies and mycelium of Auricularia auricula-judae (Fr.) quél. J. Med. Food 2009, 12, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.C.; Oh, T.J. Antioxidant activities and antimicrobial effects of extracts from Auricularia auricula-judae. J. Korean Soc. Food Sci. Nutr. 2016, 45, 327–332. [Google Scholar] [CrossRef]
- Teoh, H.L.; Ahmad, I.S.; Johari, N.M.K.; Aminudin, N.; Abdullah, N. Antioxidant properties and yield of wood ear Mushroom, Auricularia polytricha (Agaricomycetes), cultivated on rubberwood sawdust. Int. J. Med. Mushrooms 2018, 20, 369–380. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Ma, X.; Chen, J.; Chen, Q.; Shi, X.; Hou, M.; Xue, P.; Kang, Y.; Xu, Z. Acid-active supramolecular anticancer nanoparticles based on cyclodextrin polyrotaxanes damaging both mitochondria and nuclei of tumor cells. Biomater. Sci. 2018, 6, 3126–3138. [Google Scholar] [CrossRef]
- Sun, L.; Bai, X.; Zhuang, Y. Effect of different cooking methods on total phenolic contents and antioxidant activities of four Boletus mushrooms. J. Food Sci. Technol. 2014, 51, 3362–3368. [Google Scholar] [CrossRef] [Green Version]
- Jaworska, G.; Pogoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Xiao, N.; He, P.; Sun, P. Chemical analysis and antioxidant activity in vitro of polysaccharides extracted from Boletus edulis. Int. J. Biol. Macromol. 2011, 49, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Nita, S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. BioMed Res. Int. 2013, 2013, 313905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Vamanu, E.; Nita, S. Bioactive compounds, antioxidant and anti-inflammatory activities of extracts from Cantharellus cibarius. Rev. Chim. 2014, 65, 372–379. [Google Scholar]
- Ebrahimzadeh, M.A.; Safdari, Y.; Khalili, M. Antioxidant activity of different fractions of methanolic extract of the golden chanterelle mushroom Cantharellus cibarius (higher basidiomycetes) from Iran. Int. J. Med. Mushrooms 2015, 17, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ding, X.; Hou, Y.; Hou, W.; Liu, L.; Xu, T.; Yang, D. Structural characterization, immune regulation and antioxidant activity of a new heteropolysaccharide from Cantharellus cibarius Fr. Int. J. Mol. Med. 2018, 41, 2744–2754. [Google Scholar] [CrossRef] [Green Version]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Triacylglycerol profile as a chemical fingerprint of mushroom species: Evaluation by principal component and linear discriminant analyses. J. Agric. Food Chem. 2012, 60, 10592–10599. [Google Scholar] [CrossRef]
- Šíma, J.; Vondruška, J.; Svoboda, L.; Šeda, M.; Rokos, L. The accumulation of risk and essential elements in edible mushrooms Chlorophyllum rhacodes, Suillus grevillei, Imleria badia, and Xerocomellus chrysenteron growing in the Czech Republic. Chem. Biodivers. 2019, 16, e1800478. [Google Scholar]
- Sharma, S.K.; Gautam, N. Chemical and bioactive profiling, and biological activities of coral fungi from northwestern Himalayas. Sci. Rep. 2017, 7, 46570. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Upadhyay, R.C.; Atri, N.S. Evaluation of nutraceutical components and antioxidant potential of north indian wild culinarymedicinal termitophilous mushrooms. Int. J. Med. Mushrooms 2013, 15, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.A.; Heleno, S.A.; Martins, A.; Almeida, G.M.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Wild mushrooms Clitocybe alexandri and Lepista inversa: In vitro antioxidant activity and growth inhibition of human tumour cell lines. Food Chem. Toxicol. 2010, 48, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Ersel, F.Y.; Cavas, L. Enzyme-based scavengers and lipid peroxidation in some wild edible Agaricales s.l. mushrooms from Mugla (Turkey). Int. J. Med. Mushrooms 2008, 10, 269–277. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B.; Semiz, D.K.; Solak, M.H. Evaluation of metal concentration and antioxidant activity of three edible mushrooms from Mugla, Turkey. Food Chem. Toxicol. 2010, 48, 1230–1233. [Google Scholar] [CrossRef]
- Kosanić, M.; Petrović, N.; Stanojković, T. Bioactive properties of Clitocybe geotropa and Clitocybe nebularis. J. Food Meas. Charact. 2020, 14, 1046–1053. [Google Scholar] [CrossRef]
- Schüffler, A. Secondary metabolites of basidiomycetes. In Physiology and Genetics the Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Anke, T., Schüffler, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 231–275. ISBN 978-3-319-71739-5. [Google Scholar]
- Heleno, S.A.; Ferreira, I.C.F.R.; Calhelha, R.C.; Esteves, A.P.; Martins, A.; Queiroz, M.J.R.P. Cytotoxicity of Coprinopsis atramentaria extract, organic acids and their synthesized methylated and glucuronate derivatives. Food Res. Int. 2014, 55, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Stilinović, N.; Čapo, I.; Vukmirović, S.; Rašković, A.; Tomas, A.; Popović, M.; Sabo, A. Chemical composition, nutritional profile and in vivo antioxidant properties of the cultivated mushroom Coprinus comatus. R. Soc. Open Sci. 2020, 7, 200900. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Salinaro, A.T.; Maiolino, L.; Calabrese, V. Nutritional mushroom treatment in meniere’s disease with Coriolus versicolor: A rationale for therapeutic intervention in neuroinflammation and antineurodegeneration. Int. J. Mol. Sci. 2020, 21, 284. [Google Scholar] [CrossRef] [Green Version]
- Stojanova, M.; Pantić, M.; Karadelev, M.; Čuleva, B.; Nikšić, M. Antioxidant potential of extracts of three mushroom species collected from the Republic of North Macedonia. J. Food Process. Preserv. 2021, 45, e15155. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Nikšić, M. The influence of mushroom Coriolus versicolor and hazelnuts enrichment on antioxidant activities and bioactive content of dark chocolate. Food Feed Res. 2020, 47, 23–32. [Google Scholar] [CrossRef]
- Costea, T.; Hudiţă, A.; Olaru, O.T.; Gălăţeanu, B.; Gîrd, C.E.; Mocanu, M.M. Chemical composition, antioxidant activity and cytotoxic effects of romanian Craterellus cornucopioides (L.) pers. mushroom. Farmacia 2020, 68, 340–347. [Google Scholar] [CrossRef]
- Liu, T.; Zang, N.; Zhou, N.; Li, W.; Xie, X.; Deng, Y.; Ren, L.; Long, X.; Li, S.; Zhou, L.; et al. Resveratrol inhibits the TRIF-dependent pathway by upregulating sterile alpha and armadillo motif protein, contributing to anti-inflammatory effects after respiratory syncytial virus infection. J. Virol. 2014, 88, 4229–4236. [Google Scholar] [CrossRef] [Green Version]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Radović-Jakovljević, M.; Ćirić, A.; Grujičić, D.; Milošević-Djordjević, O. Craterellus cornucopioides edible mushroom as source of biologically active compounds. Nat. Prod. Commun. 2019, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Duan, X.; Zhang, M.; Li, C.; Zhang, Z.; Liu, A.; Hu, B.; He, J.; Wu, D.; Chen, H.; et al. Cooking methods effect on the nutrients, bioaccessibility and antioxidant activity of Craterellus cornucopioides. LWT 2020, 131, 109768. [Google Scholar] [CrossRef]
- Velygodska, A.K.; Fedotov, O.V. The production and analysis of carotenoid preparations from some strains of xylotrophic Basidiomycetes. Biosyst. Divers. 2016, 24, 290–294. [Google Scholar] [CrossRef] [Green Version]
- Vaz, J.A.; Barros, L.; Martins, A.; Morais, J.S.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Phenolic profile of seventeen Portuguese wild mushrooms. LWT Food Sci. Technol. 2011, 44, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Krüzselyi, D.; Móricz, Á.M.; Vetter, J. Comparison of different morphological mushroom parts based on the antioxidant activity. LWT 2020, 127, 109436. [Google Scholar] [CrossRef]
- Ukaegbu, C.I.; Shah, S.R.; Hamid, H.A.; Alara, O.R.; Sarker, M.Z.I. Phenolic compounds of aqueous and methanol extracts of Hypsizygus tessellatus (brown and white var.) and Flammulina velutipes caps: Antioxidant and antiproliferative activities. Pharm. Chem. J. 2020, 54, 170–183. [Google Scholar] [CrossRef]
- Payamnoor, V.; Kavosi, M.R.; Nazari, J. Polypore fungi of Caucasian alder as a source of antioxidant and antitumor agents. J. For. Res. 2020, 31, 1381–1390. [Google Scholar] [CrossRef]
- Mohammadifar, S.; Gharaghoz, S.F.; Shayan, M.R.A.; Vaziri, A. Comparison between antioxidant activity and bioactive compounds of Ganoderma applanatum (Pers.) Pat. and Ganoderma lucidum (Curt.) P. Karst from Iran. Iran. J. Plant Physiol. 2020, 11, 3417–3424. [Google Scholar]
- Xu, Y.; Zhang, X.; Yan, X.H.; Zhang, J.L.; Wang, L.Y.; Xue, H.; Jiang, G.C.; Ma, X.T.; Liu, X.J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef]
- Cör, D.; Knez, Ž.; Hrnčič, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma Lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [Green Version]
- Uddin Pk, M.; Talukder, R.I.; Sarkar, M.K.I.; Rahman, T.; Pervin, R.; Rahman, M.; Zenat, E.A.; Akther, L. Effect of solvents on phytochemicals content and antioxidant activity of Ganoderma lucidum. Open Microbiol. J. 2019, 13, 10–15. [Google Scholar] [CrossRef]
- Ribeiro, B.; Valentão, P.; Baptista, P.; Seabra, R.M.; Andrade, P.B. Phenolic compounds, organic acids profiles and antioxidative properties of beefsteak fungus (Fistulina hepatica). Food Chem. Toxicol. 2007, 45, 805–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mau, J.L.; Tsai, S.Y.; Tseng, Y.H.; Huang, S.J. Antioxidant properties of hot water extracts from Ganoderma tsugae Murrill. LWT Food Sci. Technol. 2005, 38, 589–597. [Google Scholar] [CrossRef]
- Ding, X.; Hou, Y.; Zhu, Y.; Wang, P.; Fu, L.; Zhu, H.; Zhang, N.; Qin, H.; Qu, W.; Wang, F.; et al. Structure elucidation, anticancer and antioxidant activities of a novel polysaccharide from Gomphus clavatus Gray. Oncol. Rep. 2015, 33, 3162–3170. [Google Scholar] [CrossRef]
- Makropoulou, M.; Aligiannis, N.; Gonou-Zagou, Z.; Pratsinis, H.; Skaltsounis, A.L.; Fokialakis, N. Antioxidant and cytotoxic activity of the wild edible mushroom Gomphus clavatus. J. Med. Food 2012, 15, 216–221. [Google Scholar] [CrossRef]
- Zhang, J.C.; Kong, X.H.; Zhang, P.Q.; Liu, J.N.; Ma, Y.P.; Dai, X.D.; Han, Z.H.; Ma, Q.F.; Wang, X.Y.; Yu, L.P. Identification of a new fungal pathogen causing white villous disease on the fruiting body of the culinary-medicinal mushroom Auricularia auricula-judae (Agaricomycetes) in China. Int. J. Med. Mushrooms 2017, 19, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Choudhary, M.; Gupta, S.; Agrawal, D.C.; Dhar, M.K. Diversity and medicinal value of mushrooms from the Himalayan region, India. In Medicinal Mushrooms; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 371–389. ISBN 978-981-13-6381-8. [Google Scholar]
- Lew, S.Y.; Yow, Y.Y.; Lim, L.W.; Wong, K.H. Antioxidant-mediated protective role of Hericium erinaceus (Bull.: Fr.) pers. against oxidative damage in fibroblasts from friedreich’s ataxia patient. Food Sci. Technol. 2020, 40, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Tubić, J.; Grujičić, D.; Jakovljević, M.R.; Ranković, B.; Kosanić, M.; Stanojković, T.; Ćirić, A.; Milošević-Djordjević, O. Investigation of biological activities and secondary metabolites of Hydnum repandum acetone extract. Farmacia 2019, 67, 174–183. [Google Scholar] [CrossRef]
- Bakir, T.K.; Boufars, M.; Karadeniz, M.; Unal, S. Amino acid composition and antioxidant properties of five edible mushroom species from Kastamonu, Turkey. Afr. J. Tradit. Complement. Altern. Med. 2018, 15, 80–87. [Google Scholar]
- Ahmad, A.; Abuzinadah, M.; Alkreathy, H.; Kutbi, H.; Shaik, N.; Ahmad, V.; Saleem, S.; Husain, A. A novel polyherbal formulation containing thymoquinone attenuates carbon tetrachloride-induced hepatorenal injury in a rat model. Asian Pac. J. Trop. Biomed. 2020, 10, 147–155. [Google Scholar] [CrossRef]
- Burmasova, M.A.; Utebaeva, A.A.; Sysoeva, E.V.; Sysoeva, M.A. Melanins of Inonotus obliquus: Bifidogenic and antioxidant properties. Biomolecules 2019, 9, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egwim, E.; Elem, R. Proximate composition, phytochemical screening and antioxidant activity of ten selected wild edible Nigerian mushrooms. Am. J. Food Nutr. 2011, 1, 89–94. [Google Scholar] [CrossRef]
- Vieira, V.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Expanding current knowledge on the chemical composition and antioxidant activity of the genus Lactarius. Molecules 2014, 19, 20650–20663. [Google Scholar] [CrossRef]
- Rosa, G.B.; Sganzerla, W.G.; Ferreira, A.L.A.; Xavier, L.O.; Veloso, N.C.; da Silva, J.; de Oliveira, G.P.; Amaral, N.C.; de Veeck, A.P.L.; Ferrareze, J.P. Investigation of nutritional composition, antioxidant compounds, and antimicrobial activity of wild culinary–medicinal mushrooms Boletus edulis and Lactarius deliciosus (Agaricomycetes) from Brazil. Int. J. Med. Mushrooms 2020, 22, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Bozdogan, A.; Ulukanli, Z.; Bozok, F.; Eker, T.; Dogan, H.H.; Buyukalaca, S. Antioxidant potential of Lactarius deliciosus and Pleurotus ostreatus from amanos mountains. Adv. Life Sci. 2018, 5, 113–120. [Google Scholar]
- Xu, Z.; Fu, L.; Feng, S.; Yuan, M.; Huang, Y.; Liao, J.; Zhou, L.; Yang, H.; Ding, C. Chemical composition, antioxidant and antihyperglycemic activities of the wild Lactarius deliciosus from China. Molecules 2019, 24, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogarasi, M.; Diaconeasa, Z.M.; Pop, C.R.; Fogarasi, S.; Semeniuc, C.A.; Fărcaş, A.C.; Țibulcă, D.; Sălăgean, C.-D.; Tofană, M.; Socaci, S.A. Elemental composition, antioxidant and antibacterial properties of some wild edible mushrooms from Romania. Agronomy 2020, 10, 1972. [Google Scholar] [CrossRef]
- Athanasakis, G.; Aligiannis, N.; Gonou-Zagou, Z.; Skaltsounis, A.L.; Fokialakis, N. Antioxidant properties of the wild edible mushroom Lactarius salmonicolor. J. Med. Food 2013, 16, 760–764. [Google Scholar] [CrossRef]
- Zavastin, D.E.; Miron, A.; Gherman, S.P.; Boerescu, C.M.; Breaban, I.G.; Gavrilescu, C.M. Antioxidant activity, total phenolic and metals contents of Lactarius salmonicolor (R. HEIM & LECLAIR). Farmacia 2015, 63, 755–759. [Google Scholar]
- Chen, J.; Liu, D.; Liu, L.; Liu, P.; Xu, Q.; Xia, L.; Ling, Y.; Huang, D.; Song, S.; Zhang, D.; et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020, 49, 215–229. [Google Scholar]
- Garcia, J.; Afonso, A.; Fernandes, C.; Nunes, F.M.; Marques, G.; Saavedra, M.J. Comparative antioxidant and antimicrobial properties of Lentinula edodes Donko and Koshin varieties against priority multidrug-resistant pathogens. S. Afr. J. Chem. Eng. 2021, 35, 98–106. [Google Scholar]
- Garcia, I.M.; S., A.R.; Montero, D.; Arellano, M. Evaluation of total polyphenols and antioxidant capacity in mushroom extracts Pleurotus ostreatus and Lentinula edodes. Int. J. Curr. Pharm. Res. 2020, 12, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Pinto, S.; Barros, L.; Sousa, M.J.; Ferreira, I.C.F.R. Chemical characterization and antioxidant properties of Lepista nuda fruiting bodies and mycelia obtained by in vitro culture: Effects of collection habitat and culture media. Food Res. Int. 2013, 51, 496–502. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, Y.; Jia, J.; Ren, X.; Wang, Y. Extraction, purification and properties of water-soluble polysaccharides from mushroom Lepista nuda. Int. J. Biol. Macromol. 2019, 128, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Guo, H.B.; Li, Q.; Forsythe, A.; Chen, X.H.; Yu, X.D. Genetic diversity of Lepista nuda (Agaricales, Basidiomycota) in Northeast China as indicated by SRAP and ISSR markers. PLoS ONE 2018, 13, e0202761. [Google Scholar] [CrossRef]
- Mercan, N.; Duru, M.E.; Turko Glu, A.; Gezer, K.; Kivrak, I.; Turko Glu, H. Antioxidant and antimicrobial properties of ethanolic extract from Lepista nuda (Bull.) Cooke. Ann. Microbiol. 2006, 56, 339–344. [Google Scholar] [CrossRef]
- Niu, L.L.; Wu, Y.R.; Liu, H.P.; Wang, Q.; Li, M.Y.; Jia, Q. Optimization of extraction process, characterization and antioxidant activities of polysaccharide from Leucopaxillus giganteus. J. Food Meas. Charact. 2021, 15, 2842–2853. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Pinto da Silva, L.; Saidi, R.; Lamrani, Z.; Esteves da Silva, J.C.G.; Maouni, A. Chemical composition, bioactive compounds and antioxidant activity of two wild edible mushrooms Armillaria mellea and Macrolepiota procera from two countries (Morocco and Portugal). Biomolecules 2021, 11, 575. [Google Scholar] [CrossRef]
- Ramesh, C.; Pattar, M.G. Antimicrobial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of western ghats of Karnataka, India. Pharmacogn. Res. 2010, 2, 107–112. [Google Scholar]
- Queirós, B.; Barreira, J.C.M.; Sarmento, A.C.; Ferreira, I.C.F.R. In search of synergistic effects in antioxidant capacity of combined edible mushrooms. Int. J. Food Sci. Nutr. 2009, 60, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojković, D.S.; Kovačević-Grujičić, N.; Reis, F.S.; Davidović, S.; Barros, L.; Popović, J.; Petrović, I.; Pavić, A.; Glamočlija, J.; Ćirić, A.; et al. Chemical composition of the mushroom Meripilus giganteus Karst. and bioactive properties of its methanolic extract. LWT Food Sci. Technol. 2017, 79, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Maity, P.; Nandi, A.K.; Manna, D.K.; Pattanayak, M.; Sen, I.K.; Bhanja, S.K.; Samanta, S.; Panda, B.C.; Paloi, S.; Acharya, K.; et al. Structural characterization and antioxidant activity of a glucan from Meripilus giganteus. Carbohydr. Polym. 2017, 157, 1237–1245. [Google Scholar] [CrossRef]
- Sárközy, A.; Béni, Z.; Dékány, M.; Zomborszki, Z.P.; Rudolf, K.; Papp, V.; Hohmann, J.; Ványolós, A. Cerebrosides and steroids from the edible mushroom Meripilus giganteus with antioxidant potential. Molecules 2020, 25, 1395. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.N.; Ma, H.L.; Zhou, C.S.; Yan, Y.; Yin, X.L.; Yan, J.K. Enhanced production and antioxidant activity of endo-polysaccharides from Phellinus igniarius mutants screened by low power He-Ne laser and ultraviolet induction. Bioact. Carbohydr. Diet. Fibre 2018, 15, 30–36. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Li, Y.; Ji, H.; Liu, C.; Zhou, L.; Huang, Y.; Bai, C.; Jiang, Z.; Wu, X. Screening for proteins related to the biosynthesis of hispidin and its derivatives in Phellinus igniarius using iTRAQ proteomic analysis. BMC Microbiol. 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, Y.Y.; Ma, H.L.; Wang, Z. Bin Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrason. Sonochem. 2016, 29, 251–257. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhu, Y.F.; Guo, Z.Z.; Lou, Y.M.; He, S.G.; Guan, Y.; Zhu, L.J.; Liu, Z.Q.; Lu, L.L.; Liu, L. Aconitum alkaloids, the major components of Aconitum species, affect expression of multidrug resistance-associated protein 2 and breast cancer resistance protein by activating the Nrf2-mediated signalling pathway. Phytomedicine 2018, 44, 87–97. [Google Scholar] [CrossRef]
- Rahimah, S.B.; Djunaedi, D.D.; Soeroto, A.Y.; Bisri, T. The phytochemical screening, total phenolic contents and antioxidant activities in vitro of white oyster mushroom (Pleurotus ostreatus) preparations. Open Access Maced. J. Med. Sci. 2019, 7, 2404–2412. [Google Scholar] [CrossRef]
- Bakir, T.; Karadeniz, M.; Unal, S. Investigation of antioxidant activities of Pleurotus ostreatus stored at different temperatures. Food Sci. Nutr. 2018, 6, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.K.; Im, K.H.; Choi, J.; Shin, P.G.; Lee, T.S. Evaluation of antioxidant, anti-cholinesterase, and anti-inflammatory effects of culinary mushroom Pleurotus pulmonarius. Mycobiology 2016, 44, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Contato, A.G.; Inácio, F.D.; de Araújo, C.A.V.; Brugnari, T.; Maciel, G.M.; Haminiuk, C.W.I.; Bracht, A.; Peralta, R.M.; de Souza, C.G.M. Comparison between the aqueous extracts of mycelium and basidioma of the edible mushroom Pleurotus pulmonarius: Chemical composition and antioxidant analysis. J. Food Meas. Charact. 2020, 14, 830–837. [Google Scholar] [CrossRef]
- Gambato, G.; Todescato, K.; Pavão, E.M.; Scortegagna, A.; Fontana, R.C.; Salvador, M.; Camassola, M. Evaluation of productivity and antioxidant profile of solid-state cultivated macrofungi Pleurotus albidus and Pycnoporus sanguineus. Bioresour. Technol. 2016, 207, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, H.J.; Xu, C.P. Culture characterization of exopolysaccharides with antioxidant activity produced by Pycnoporus sanguineus in stirred-tank and airlift reactors. J. Taiwan Inst. Chem. Eng. 2014, 45, 2075–2080. [Google Scholar] [CrossRef]
- Bhanja, S.K.; Samanta, S.K.; Mondal, B.; Jana, S.; Ray, J.; Pandey, A.; Tripathy, T. Green synthesis of Ag@Au bimetallic composite nanoparticles using a polysaccharide extracted from Ramaria botrytis mushroom and performance in catalytic reduction of 4-nitrophenol and antioxidant, antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100341. [Google Scholar] [CrossRef]
- Han, S.R.; Kim, K.H.; Kim, H.J.; Jeong, S.H.; Oh, T.J. Comparison of biological activities using several solvent extracts from Ramaria botrytis. Indian J. Sci. Technol. 2016, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Li, H. Extraction, purification, characterization and antioxidant activities of polysaccharides from Ramaria botrytis (Pers.) Ricken. Chem. Cent. J. 2017, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Yan, S.F.; Chen, J. Structure and antioxidant activity of HAP-I in Russula vinosa. Mod. Food Sci. Technol. 2015, 31, 63–68. [Google Scholar]
- Liu, Q.; Tian, G.; Yan, H.; Geng, X.; Cao, Q.; Wang, H.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the wild edible mushroom Russula vinosa lindblad. J. Agric. Food Chem. 2014, 62, 8858–8866. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Mohd Fadzil, N.H.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Effects of different extracting conditions on anti-tyrosinase and antioxidant activities of Schizophyllum commune fruit bodies. Biocatal. Agric. Biotechnol. 2019, 19, 101116. [Google Scholar] [CrossRef]
- Wanna, C.; Sudhadham, M. The effect of coconut water and boiling on antioxidant activity and total phenolic contents in Schizophyllum commune Fr. Pharmacogn. J. 2018, 10, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Basso, V.; Schiavenin, C.; Mendonça, S.; de Siqueira, F.G.; Salvador, M.; Camassola, M. Chemical features and antioxidant profile by Schizophyllum commune produced on different agroindustrial wastes and byproducts of biodiesel production. Food Chem. 2020, 329, 127089. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.N.; Oh, Y.K.; Lee, Y.J.; Lee, Y.C. Effects of sparassis crispa in medical therapeutics: A systematic review and meta-analysis of randomized controlled trials. Int. J. Mol. Sci. 2018, 19, 1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitha, B.; Fijesh, P.V.; Janardhanan, K.K. Hepatoprotective activity of cultured mycelium of Morel mushroom, Morchella esculenta. Exp. Toxicol. Pathol. 2013, 65, 105–112. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, J.; Hu, J.; Liao, Q.; Zhou, R.; Zhang, P.; Chen, Z. Hepatoprotective effects of aqueous extract from lingzhi or reishi medicinal mushroom Ganoderma lucidum (Higher Basidiomycetes) on α-amanitin-induced liver injury in mice. Int. J. Med. Mushrooms 2013, 15, 383–391. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, M.; Geng, X.; Wang, H.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the edible mushroom Oudemansiella radicata. Molecules 2017, 22, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisar, J.; Mustafa, I.; Anwar, H.; Sohail, M.U.; Hussain, G.; Ullah, M.I.; Faisal, M.N.; Bukhari, S.A.; Basit, A. Shiitake culinary-medicinal mushroom, Lentinus edodes (Agaricomycetes): A species with antioxidant, immunomodulatory, and hepatoprotective activities in hypercholesterolemic rats. Int. J. Med. Mushrooms 2017, 19, 981–990. [Google Scholar] [CrossRef]
- Chang, C.W.; Lur, H.S.; Lu, M.K.; Cheng, J.J. Sulfated polysaccharides of Armillariella mellea and their anti-inflammatory activities via NF-κB suppression. Food Res. Int. 2013, 54, 239–245. [Google Scholar] [CrossRef]
- Talero, E.; Avila-Roman, J.; Motilva, V. Chemoprevention with phytonutrients and microalgae products in chronic inflammation and colon cancer. Curr. Pharm. Des. 2012, 18, 3939–3965. [Google Scholar] [CrossRef] [Green Version]
- Chien, R.C.; Lin, L.M.; Chang, Y.H.; Lin, Y.C.; Wu, P.H.; Asatiani, M.D.; Wasser, S.G.; Krakhmalnyi, M.; Agbarya, A.; Wasser, S.P.; et al. Anti-inflammation properties of fruiting bodies and submerged cultured mycelia of culinary-medicinal higher basidiomycetes mushrooms. Int. J. Med. Mushrooms 2016, 18, 999–1009. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, L.; Rakariyatham, K.; Han, Y.; Gao, Z.; Muinde Kimatu, B.; Hu, Q.; Xiao, H. Isolation of a novel bioactive protein from an edible mushroom Pleurotus eryngii and its anti-inflammatory potential. Food Funct. 2017, 8, 2175–2183. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, X.; Wang, W.; Xu, B. Optimization, characterization and antioxidant activities of selenized polysaccharides from Hypsizygus marmoreus. Am. J. Biochem. Biotechnol. 2019, 15, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Ahn, W.S.; Kim, D.J.; Chae, G.T.; Lee, J.M.; Bae, S.M.; Sin, J.I.; Kim, Y.W.; Namkoong, S.E.; Lee, I.P. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Therkelsen, S.P.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Cytokine levels after consumption of a medicinal Agaricus blazei Murill-based mushroom extract, AndoSan™, in patients with Crohn’s disease and ulcerative colitis in a randomized single-blinded Placebo-controlled study. Scand. J. Immunol. 2016, 84, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, J.M.; Wang, Q.; Kraft, C.; Slavin, J.L. Impact of Agaricus bisporus mushroom consumption on satiety and food intake. Appetite 2017, 117, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Wang, Q.; Gould, T.; Slavin, J. Impact of Agaricus bisporus mushroom consumption on gut health markers in healthy adults. Nutrients 2018, 10, 1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ba, D.M.; Gao, X.; Muscat, J.; Al-Shaar, L.; Chinchilli, V.; Zhang, X.; Ssentongo, P.; Beelman, R.B.; Richie, J.P. Association of mushroom consumption with all-cause and cause-specific mortality among American adults: Prospective cohort study findings from NHANES III. Nutr. J. 2021, 20, 38. [Google Scholar] [CrossRef]
- Li, J.; Zou, L.; Chen, W.; Zhu, B.; Shen, N.; Ke, J.; Lou, J.; Song, R.; Zhong, R.; Miao, X. Dietary mushroom intake may reduce the risk of breast cancer: Evidence from a meta-analysis of observational studies. PLoS ONE 2014, 9, e93437. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Stanilka, J.M.; Rowe, C.A.; Esteves, E.A.; Nieves, C., Jr.; Spaiser, S.J.; Christman, M.C.; Langkamp-Henken, B.; Percival, S.S. Immune Benefits from Mushroom Consumption. (20 July 2011–30 December 2013). Identifier NCT01398176. Available online: https://clinicaltrials.gov/ct2/show/NCT01398176 (accessed on 27 August 2021).
- Dai, X.; Stanilka, J.M.; Rowe, C.A.; Esteves, E.A.; Nieves, C., Jr.; Spaiser, S.J.; Christman, M.C.; Langkamp-Henken, B.; Percival, S.S. Consuming Lentinula edodes (Shiitake) mushrooms daily improves human immunity: A randomized dietary intervention in healthy young adults. J. Am. Coll. Nutr. 2015, 34, 478–487. [Google Scholar] [CrossRef]
- Wesa, K.M.; Cunningham-Rundles, S.; Klimek, V.M.; Vertosick, E.; Coleton, M.I.; Yeung, K.S.; Lin, H.; Nimer, S.; Cassileth, B.R. Does Maitake Mushroom Extract Enhance Hematopoiesis in Myelodysplastic Patients? (8 April 2010–19 May 2016). Identifier NCT01099917. Available online: https://clinicaltrials.gov/ct2/show/NCT01099917 (accessed on 27 August 2021).
- Wesa, K.M.; Cunningham-Rundles, S.; Klimek, V.M.; Vertosick, E.; Coleton, M.I.; Yeung, K.S.; Lin, H.; Nimer, S.; Cassileth, B.R. Maitake mushroom extract in myelodysplastic syndromes (MDS): A phase II study. Cancer Immunol. Immunother. 2015, 64, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, C.P. Effects of Pre-, Pro- & Anti-Biotics on Gut Microbiota. (11 August 2011–24 July 2017). Identifier NCT01414010. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01414010 (accessed on 27 August 2021).
- Chae, S.-W. A 12-week Human Trial to Compare the Efficacy and Safety of Polycan on Bone Metabolism. (26 July 2011–12 October 2012). Identifier NCT01402115. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01402115 (accessed on 27 August 2021).
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psychopharmacology of Psilocybin in Cancer Patients. (25 April 2007–19 July 2018). Identifier NCT00465595. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00465595 (accessed on 27 August 2021).
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.C. Effects of Lentinula edodes Bars on Dyslipidemia and Oxidative Stress in Cholesterol Individuals: Randomized Study. (5 December 2019–27 October 2020). Identifier NCT04186780. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04186780 (accessed on 27 August 2021).
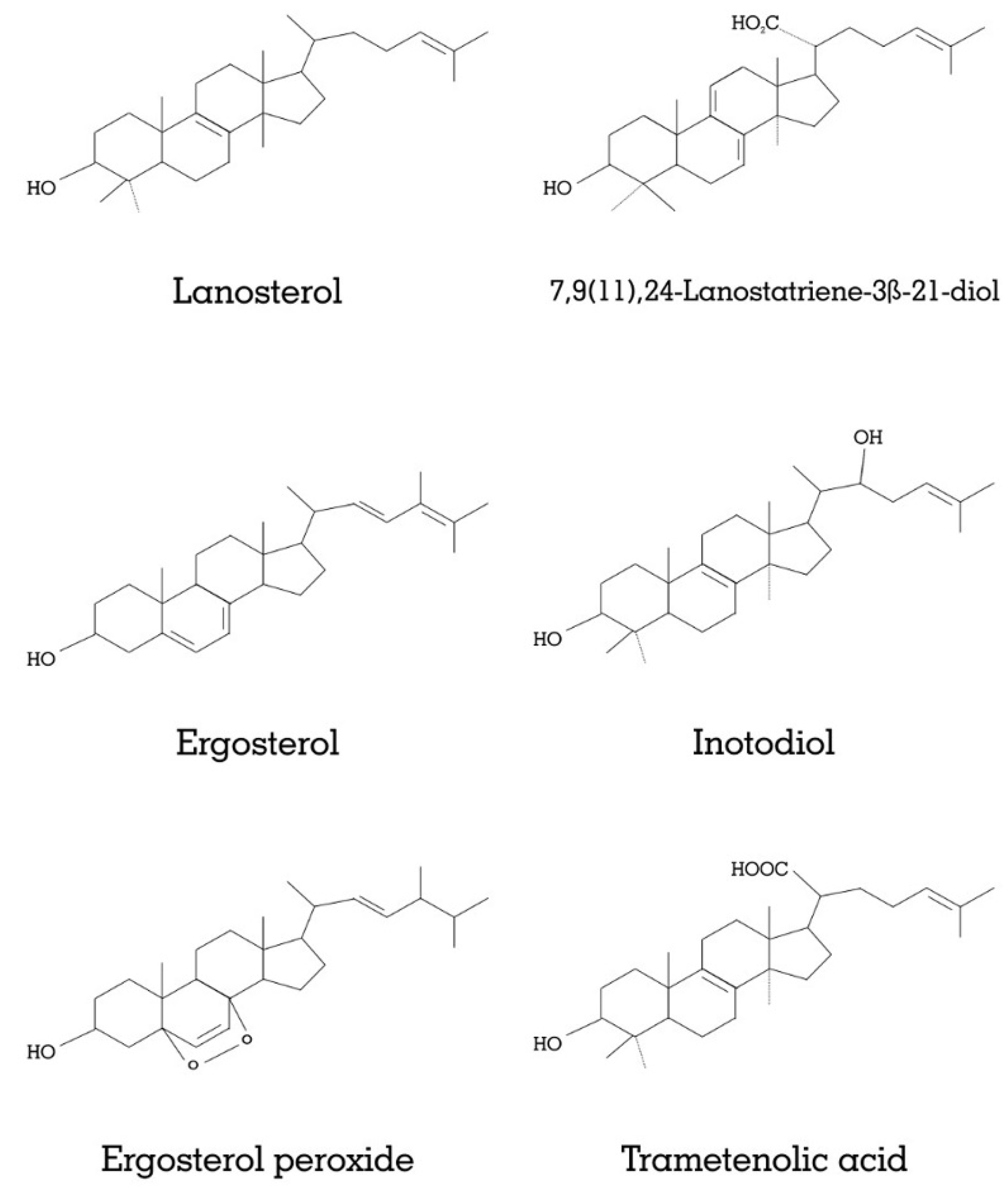
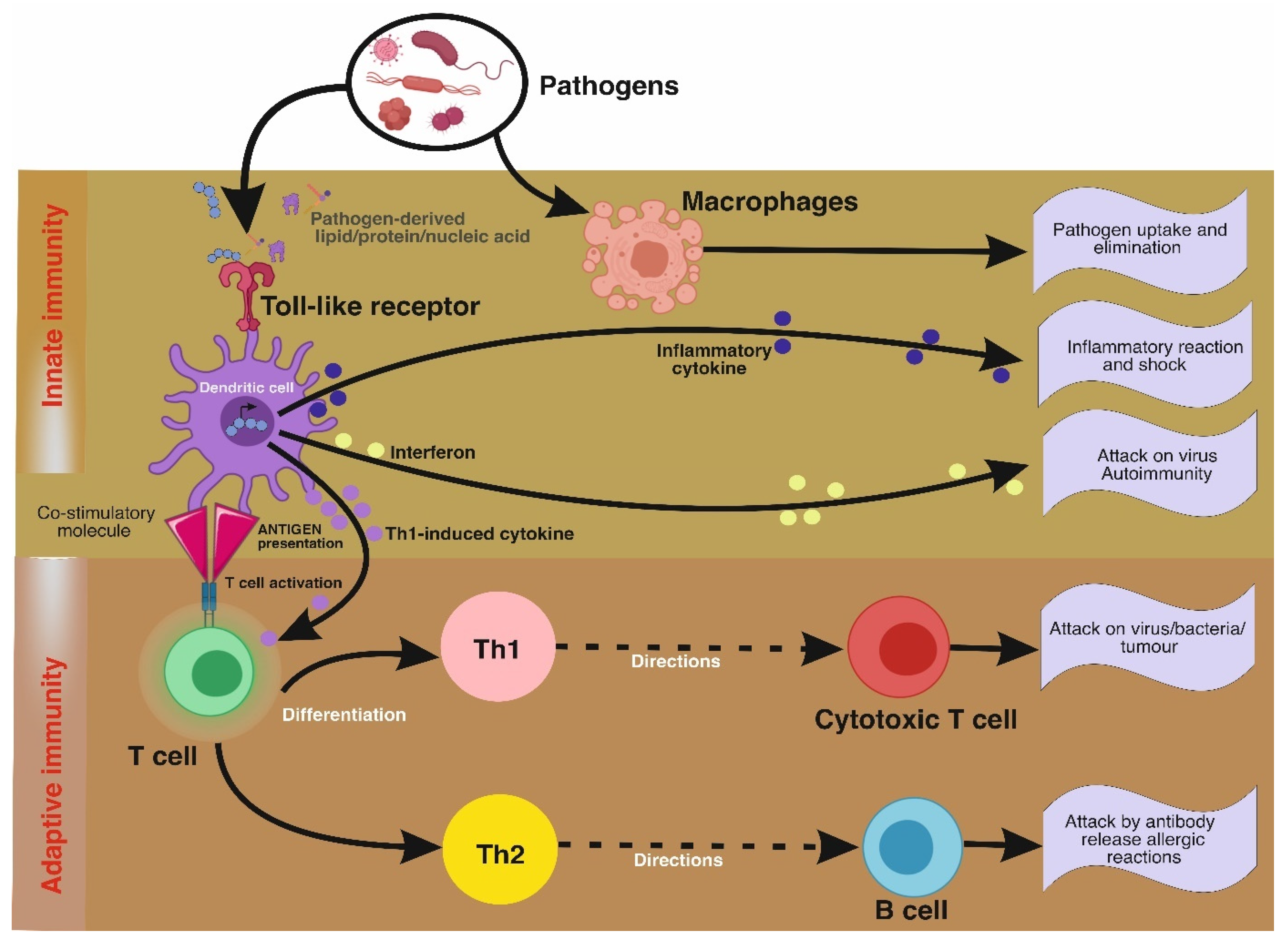
| Name of Mushroom | In-Vivo | In-Vitro | Outcome of Study | References |
|---|---|---|---|---|
| Coriolus versicolor and Boletus edulis | - | Cell lines: MCF-7, breast cancer cell lines; HT-29, human colorectal cancer cell line; HUH-7, human hepatoma cell lines; Antibacterial: (Pseudomonas aeruginosa, Klebsiella pneumonia, Staphylococcus aureus, and Enterococcus faecalis) and Antifungal (Candida albicans and Candida utilis). L929, murine fibroblast cell line. | The silver nanoparticles synthesized from mushrooms showed anticancer properties The silver nanoparticles synthesized from mushrooms showed anticancer properties The silver nanoparticles synthesized from mushroom showed wound healing | [17] |
| Agaricus bisporus | - | Human ocular fibroblasts | Use of Agaricus bisporus results in wound healing in a dose-dependent manner. | [18] |
| Agaricus blazei | Induced burn-wound-treated rats | - | The use of Agaricus blazei in the treatment of burns wounds induces the expression of IL-1 mRNA and increases the accumulation of macrophages in the wound area. | [19] |
| Agaricus Sylvaticus | Rats with wound | - | Phenolic component of mushroom was found associated to the wound healing properties | [20] |
| Phanerochaete chrysosporium | - | NIH 3T3, Murine Embryonic Fibroblast cell lines | Prepared curcumin loaded mycelium-based film capable of curing the injured tissue | [21] |
| Ganoderma lucidum | Indomethacin induced gastric mucosal lesions in rats | - | Polysaccharide fraction causes the healing of peptic ulcers in rats | [22] |
| Ganoderma lucidum | Sprague-Dawley rats induced with wound | - | Accelerated wound healing in rat liver tissues after Monopolar Electrosurgery | [23] |
| Sparassis crispa | Streptozotocin induced diabetic mice | - | Wound healing activity was observed on topical application of Sparassis crispa extract on wound | [24] |
| Hericium erinaceus | Male Sprague-Dawley rats induced with wound | - | Topical application of an aqueous extract of Hericium erinaceus showed wound healing action in rats | [25] |
| Phellinus gilvus | Streptozotocin induced diabetic rats | - | Isolated polysaccharides showed wound healing action | [26] |
| Dioscorea batatas Decne | - | INT-407 cells | The phytoglycoprotein isolated showed wound healing action at the intestinal epithelial wound | [27] |
| Flammulina velutipes | Female Sprague-Dawley rat | - | Flammulina velutipes polysaccharides scaffold showed skin wound healing and hair follicle regenerative action | [28] |
| Schizophyllum commune | NA | L929 fibroblasts cells | Electrospunned fiber with polyvinyl alcohol showed improved wound healing and promoted the migration of cells at the wound site | [29] |
| Lignosus rhinocerotis | NA | Human dermal cells | Gold nanoparticles synthesized with the mushroom extract and chitosan showed wound healing capability though non-cytotoxic | [30] |
| Name of Mushroom | Active Constituent | Anti-HIV Activity against | References |
|---|---|---|---|
| Russula paludosa | Fraction SU2 | HIV-1 RT | [47] |
| Agaricus bitorquis | Agaricus bitorquis Lectin | HIV-1 RT and leukemic cells | [41] |
| Lignosus rhinocerus | heliantriol F and 6 α-fluoroprogesterone | HIV-1 protease inhibitor; inhibition of HIV-1 induced syncytial formation and p24 production in the infected MOLT-4 cells. | [48] |
| Ganoderma colossum | Ganomycin I, Ganomycin B | HIV-1 protease | [49] |
| Cordyceps sobolifera | Cordysobin | HIV-1 RT | [50] |
| Fomes fomentarius | Water-soluble melanin-glucan complex; insoluble chitin-glucan-melanin complex | HIV-1 protease | [51] |
| A. subrufescens | β-glucan | HIV-1 RT | [52] |
| A. subrufescens | Laccase | HIV-1 RT | [53] |
| A. subrufescens | Lectin | HIV-1 RT | [54] |
| Inonotus obliquus | Terpenes | HIV-1 RT | [55] |
| I. obliquus | Polysaccharides | HIV-1 RT | [56] |
| I. obliquus | Terpenes | HIV-1 RT | [57] |
| I. obliquus | Polyphenols | HIV-1 RT | [58] |
| I. obliquus | Terpenes | HIV-1 RT | [59] |
| Phellinus igniarius | Terpenes | HIV-1 RT | [60] |
| Pleurotus abalonus | Polysaccharide–peptide complex LB-1b | HIV-1 RT | [36] |
| Flammulina velutipes | Velutin | HIV-1 RT | [61] |
| Hypsizigus marmoreus | Marmorin | HIV-1 RT | [62] |
| Pleurotus citrinopileatus | Lectin | HIV-1 RT | [33] |
| Russula delica | Dimeric lectin | HIV-1 RT | [34] |
| Pleurotus ostreatus | Glycoprotein | HIV-1 RT | [63] |
| Pholiota adiposa | Lectin | HIV-1 RT | [64] |
| Agrocybe cylindracea | Agrocybin | HIV-1 RT | [65] |
| Pleurotus cornucopiae | Laccase | HIV-1 RT | [66] |
| Schizophyllum commune | 20-kDa ribonuclease | HIV-1 RT | [67] |
| Lentinus edodes | Lentin | HIV-1 RT | [68] |
| Hericium erinaceum | Lentin | HIV-1 RT | [69] |
| Pleurotus abalonus | 120-kDa Polysaccharide | HIV-1 RT | [70] |
| Sl. No. | Name of Mushroom | Tested Chemical Constituent | Cell Lines Studied | References |
|---|---|---|---|---|
| 1 | Flammulina velutipes | Water extract | BT-20, MCF-7 and MDA-MB-231 | [83] |
| 2 | F. velutipes | Flammulinolide A, B, C, and F | Hela, HepG2 and KB cells | [84] |
| 3 | F. velutipes | Enokipodin B, D, and J, 2,5-cuparadiene-1,4-dione | HepG2, MCF-7, A549, and SGC7901 | [85] |
| 4 | F. velutipes | Alkaline-soluble polysaccharide | SC-180 mouse model | [86] |
| 5 | F. velutipes | Polysaccharides | S-180 mice tumor model and SMMC-7721 human hepatoma cells | [87] |
| 6 | F. velutipes | Polysaccharide | BEL-7402 cell | [88] |
| 7 | F. velutipes | Ergosterol, and 22, 23-dihydroergosterol | SGC, HepG2, A549, and U251 | [89] |
| 8 | F. velutipes | Proflamin | B-16 melanoma and Ca755 adenocarcinoma | [90] |
| 9 | Ganoderma neo-japonicum | Ethanolic extract | Human colonic carcinoma cells | [91] |
| 10 | Astraeus hygrometricus | Astrakurkurone | Hep 3B and Hep G2 | [92] |
| 11 | Cantharellus cibarius | Polysaccharides | NK92 cells | [93] |
| 12 | Agrocybe aegerita | Antitumor lectin | HeLa, SW480, SGC-7901, MGC80-3, BGC-823, HL-60, and mouse sarcoma S-180 | [94] |
| 13 | A. aegerita | A. aegerita galectin | 4T1 cells | [95] |
| 14 | Agaricus bisporus | Gal β-1,3-GalNAc-binding lectin | HT29 colon cancer cells | [96] |
| 15 | Armillaria luteo-virens | dimeric lectin | MBL2 cells, HeLa cells, and L1210 cells | [97] |
| 16 | Boletus speciosus | B. speciosus hemagglutinin | Hep G2 cells and L1210 | [98] |
| 17 | Clitocybe nebularis | Lectin | Human leukemic T cells | [99] |
| 18 | Flammulina velutipes | Hemagglutinin | Leukemia L1210 cells | [100] |
| 19 | Ganoderma capense | Lectin | L1210 and M1 cells and HepG2 cells | [101] |
| 20 | Grifola frondosa | N-acetylgalactosamine-specific lectin | HeLa cells | [102] |
| 21 | Hericium erinaceum | H. erinaceum agglutinin | HepG2 and MCF7 | [69] |
| 22 | A. bisporus | Mannogalactoglucan | HepG2 cells | [103] |
| 23 | Ganoderma lucidum | G. lucidum polysaccharides | HT29 cells | [104] |
| 24 | G. lucidum | G. lucidum polysaccharides | LNCaP human prostate cancer cells | [105] |
| 25 | G. lucidum | G. lucidum polysaccharides | K562 and RG2 cells | [106] |
| 26 | Grifola frondosa | G. frondosa polysaccharides | MCF-7 and MDA-MB-231 | [107] |
| 27 | Hericium erinaceus | HEFP-2b polysaccharide | HCT-116 cancer cells | [108] |
| 28 | Lentinus edodes | Mannogalactoglucan-type polysaccharides | Sarcoma 180 solid tumor | [109] |
| 29 | L. edodes | Homogeneous polysaccharide | Human cervical carcinoma HeLa cells | [110] |
| 30 | Cordyceps sinensis | C. sinensis polysaccharide | HCT116 cancer cell line | [111] |
| 31 | Pleurotus eryngii | P. eryngii polysaccharides | HepG-2 | [112] |
| 32 | Pleurotus ostreatus | P. ostreatus polysaccharide | Murine lymphoid cancer cell line | [113] |
| Source | Immunomodulatory Effect | References |
|---|---|---|
| Auricularia auricula-judae | Induces apoptosis of cancer cell | [129] |
| Agaricus blazei | Activates the NK cells, macrophages, dendritic cells, and granulocytes | [130] |
| Agaricus bisporus | Obstruct multiplying of L1210 and HT-29 cells | [41] |
| Agrocybe aegerita | Obstruct multiplying of 4T1, HeLa, SW480 SGC7901, MGC803, BGC823, HL-60, and S180 cells | [95] |
| Amanita phalloides | Obstruct multiplying of L1210 cells | [131] |
| Boletus edulis | Arouse mice splenocytes mitogenicity and obstruct multiplying of human hepatocyte carcinoma G2 (HepG2) and HT-29 cells | [132] |
| Boletus speciosus | Obstruct multiplying of HepG2 and L1210 cells | [98] |
| Cryptoporus volvatus | Diminutions of TLR2 and activate NF-κB | [133] |
| Cerioporus squamosus (syn. Polyporus squamosus) | Obstruct multiplying of HeLa cells | [134] |
| Clitocybe nebularis | Obstruct multiplying of human leukemic T cells | [99] |
| Chroogomphis rutilus | Arouse the proliferation of murine splenocytes and improved the secretion of IL-2 | [135] |
| Dichomitus squalens | Prompt apoptosis and interrupt the migration of A549 cells | [136] |
| Flammulina velutipes | Upsurges NO, IL-1 production, and TNF-α secretion | [137] |
| Flammulina velutipes | Excite mice splenocytes mitogenicity and obstruct multiplying of L1210 cells | [100] |
| Floccularia luteovirens (syn. Armillaria luteovirens) | Excite mice splenocytes mitogenicity and inhibit proliferation of L1210, Mouse myeloma MBL2 and HeLa cells | [97] |
| Flammulina velutipes | Excite mitogenesis in human peripheral lymphocytes, suppress systemic anaphylaxis reaction, improve transcription of IL-3, IFN-γ | [138] |
| Ganoderma lucidum | Excite TNF-α, IL-1, IFN-γ production, activates NF-κB | [139] |
| Grifola frondosa | Macrophage activation, induction of IL-1, IL-6, and TNF-α secretion | [140] |
| Gymnopus dryophilus (syn. Collybia dryophila) | Constrains NO production in activated macrophages | [141] |
| Ganoderma capense | Arouse mice splenocytes mitogenicity and inhibit proliferation of L1210, M1, HepG2 cells | [101] |
| Grifola frondosa | Constrain the proliferation of HeLa | [102] |
| Ganoderma sinensis | Augment production of IL-2, IL-3, IL-4, IFN-γ, TNF-α | [142] |
| Ganoderma microsporum | Downregulation of TNF-α | [143] |
| Ganoderma tsugae | Persuade cytokine secretion, cellular multiplication of human peripheral mononuclear cells (HPBMCs) enhancing IFN-γ expression | [144] |
| Ganoderma lucidum | Trigger THP-1 macrophages and induce proinflammatory cytokine transcription | [145] |
| Ganoderma lucidum | Augment transcription of IL-2, IL-3, IL-4, IFN-γ, TNF-α | [146] |
| Hericium erinaceus | Persuades NO production, increases expression of TNF-α, IL-1β, IL-12 | [147] |
| I. obliquus | Augment expression of IL-1β, IL-6, TNF-α, and inducible nitric oxide synthase (iNOS) in macrophages | [148] |
| Intrageneric shuffled library | Encourage U-251 MG cells apoptosis | [149] |
| Kurokawa leucomelas | Constrain the proliferation of U937 cells | [150] |
| Lentinula edodes (syn. Lentinus edodes) | Encourages non-specific cytotoxicity in macrophage and augment cytokine production | [151] |
| Lentinus squarrosulus | Stimulation of macrophages, splenocytes, and thymocytes | [152] |
| Lignosus rhinocerotis | Hinder the multiplication of HeLa, MCF7, and A549 cells | [153] |
| Lactarius flavidulus | Obstruct the multiplying of HepG2 and L1210 cells | [154] |
| Leucocalocybe mongolica (syn. Tricholoma mongolicum) | Hinder the production of S180 cells | [155] |
| Macrocybe gigantea | Upsurges phagocytic function of macrophages by activating macrophages to release mediators such as NO and TNF-α and inhibits S180 and HL-60 cells | [156] |
| Marasmius oreades | Hinder the proliferation of SW480, HepG2, and NIH-3T3 cells | [157] |
| Morchella esculenta | Macrophage activation, trigger NF-κB | [158] |
| Morchella conica | Encourages NO, IL-1β, IL-6 making | [159] |
| Naematelia aurantialba | Improves mouse spleen lymphocyte multiplication | [160] |
| Pleurotus sp.‘Florida’ | Arouses macrophages, splenocytes, and thymocytes | [161] |
| Poria cocos | Promotes the immune reaction; increases the expression of cytokines | [162] |
| Pleurotus ostreatus | Encourages IL-4 and IFN-γ production | [163] |
| Pseudosperma umbrinellum (syn Inocybe umbrinella) | Obstruct multiplication of HepG2 and MCF7 cells | [164] |
| Pleurotus eous | Obstruct multiplication of MCF7, K562, and HepG2 | [165] |
| Pleurotus citrinopileatus | Arouse mice splenocytes mitogenicity and obstruct multiplication of S180 cells | [33] |
| Pholiota adiposa | Obstruct multiplication of HepG2 and MCF7 cells | [64] |
| Postia placenta | Arouse mouse splenocyte cell proliferation and enhance interleukin-2 (IL-2) release, obstruct multiplication and persuade apoptotic effects on gastric tumor cells (MGC823) | [166] |
| Poria cocos | Enhance production of IL-1b, IL-6, IL-18, TNF-α, NO | [167] |
| Russula delica | Obstruct multiplication of HepG2 and MCF7 cells | [34] |
| Russula lepida | Obstruct multiplication of HepG2 and MCF7 cells | [168] |
| Schizophyllum commune | Instigation of T cell increases interleukin and TNF-α production | [169] |
| Sparassis crispa | Boosts IL-6 and IFN-γ production | [170] |
| Sarcodon aspratus | Upsurges the discharge of TNF-α and NO in macrophage | [171] |
| Schizophyllum commune | Excite mice splenocytes mitogenicity and obstruct multiplication of KB, HepG2, and S180 cells | [172] |
| Stropharia rugosoannulata | Obstruct multiplication of HepG2 and L1210 cells | [173] |
| Trametes versicolor | Upsurges the expression of cytokines; stimulates the macrophage phagocytes | [174] |
| Taiwanofungus camphoratus (syn. Antrodia camphorate) | Stimulation of IFN-γ, TNF-α | [175] |
| Tropicoporus linteus (syn. Phellinus linteus) | Instigation of murine B cells, Induces IL-12 and IFN-γ production | [176] |
| Tremella fuciformis | Encourages human monocytes to express interleukins | [177] |
| Taiwanofungus camphoratus (Syn. Antrodia camphorate) | Induce expression of different cytokines (IL-1b, IL-6, IL-12, TNF-α) | [178] |
| Trametes versicolor | Increase human peripheral blood lymphocytes, enhanced production of TNF-α, NO | [179] |
| Volvariella volvacea | Enhance expression of IL-2, IL-4, IFN-γ, TNF-α | [180] |
| Xylaria nigripes | Inhibits NO, IL-1β, IL-6, TNF-α, and IFN-γ production | [181] |
| Xerocomellus chrysenteron (syn. Xerocomus chrysenteron) | Inhibit the proliferation of NIH-3T3 and HeLa cells | [182] |
| Xylaria hypoxylon | Inhibit the proliferation of HepG2 cells | [183] |
| Clinical Trial No. | Intervention Model | Details of Trial and Outcome | References |
|---|---|---|---|
| NCT01398176 | Parallel Assignment | Consuming Lentinula edodes daily resulted in improvement in immunity and cellular proliferation. | [323,324] |
| NCT01099917 | Single Group Assignment | Administration of Maitake mushroom showed improvement in Hematopoiesis in Myelodysplastic Patients during Phase II evaluation | [325,326] |
| NCT01414010 | Parallel Assignment | The comparative analysis of administrating Trametes Versicolor, Saccharomyces Boulardii, and amoxicillin to subjects. The Trametes versicolor administration showed a significant reduction in bacterial percentage in the stool. | [327] |
| NCT01402115 | Parallel Assignment | Administration of polycan (a purified β-glucan from Aureobasidium pullulans) resulted in a reduction in bone loss and biomarkers present due to bone metabolism | [328] |
| NCT00465595 | Crossover Assignment | The use of Psilocybin This resulted in a decrease in mood anxiety and depression and increased well-being/life satisfaction | [329,330] |
| NCT04186780 | Parallel Assignment | Consumption of L. edodes Bars This resulted in a decrease in oxidative stress and Dyslipidemia in border line patients | [331] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, H.; Mishra, A.K.; Baig, A.A.; Mohanta, T.K.; Mohanta, Y.K.; Baek, K.-H. Narrative Review: Bioactive Potential of Various Mushrooms as the Treasure of Versatile Therapeutic Natural Product. J. Fungi 2021, 7, 728. https://doi.org/10.3390/jof7090728
Chopra H, Mishra AK, Baig AA, Mohanta TK, Mohanta YK, Baek K-H. Narrative Review: Bioactive Potential of Various Mushrooms as the Treasure of Versatile Therapeutic Natural Product. Journal of Fungi. 2021; 7(9):728. https://doi.org/10.3390/jof7090728
Chicago/Turabian StyleChopra, Hitesh, Awdhesh Kumar Mishra, Atif Amin Baig, Tapan Kumar Mohanta, Yugal Kishore Mohanta, and Kwang-Hyun Baek. 2021. "Narrative Review: Bioactive Potential of Various Mushrooms as the Treasure of Versatile Therapeutic Natural Product" Journal of Fungi 7, no. 9: 728. https://doi.org/10.3390/jof7090728
APA StyleChopra, H., Mishra, A. K., Baig, A. A., Mohanta, T. K., Mohanta, Y. K., & Baek, K.-H. (2021). Narrative Review: Bioactive Potential of Various Mushrooms as the Treasure of Versatile Therapeutic Natural Product. Journal of Fungi, 7(9), 728. https://doi.org/10.3390/jof7090728










