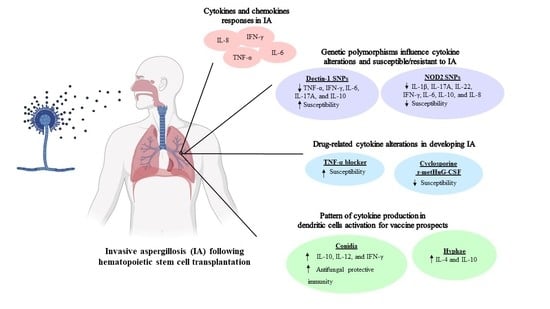
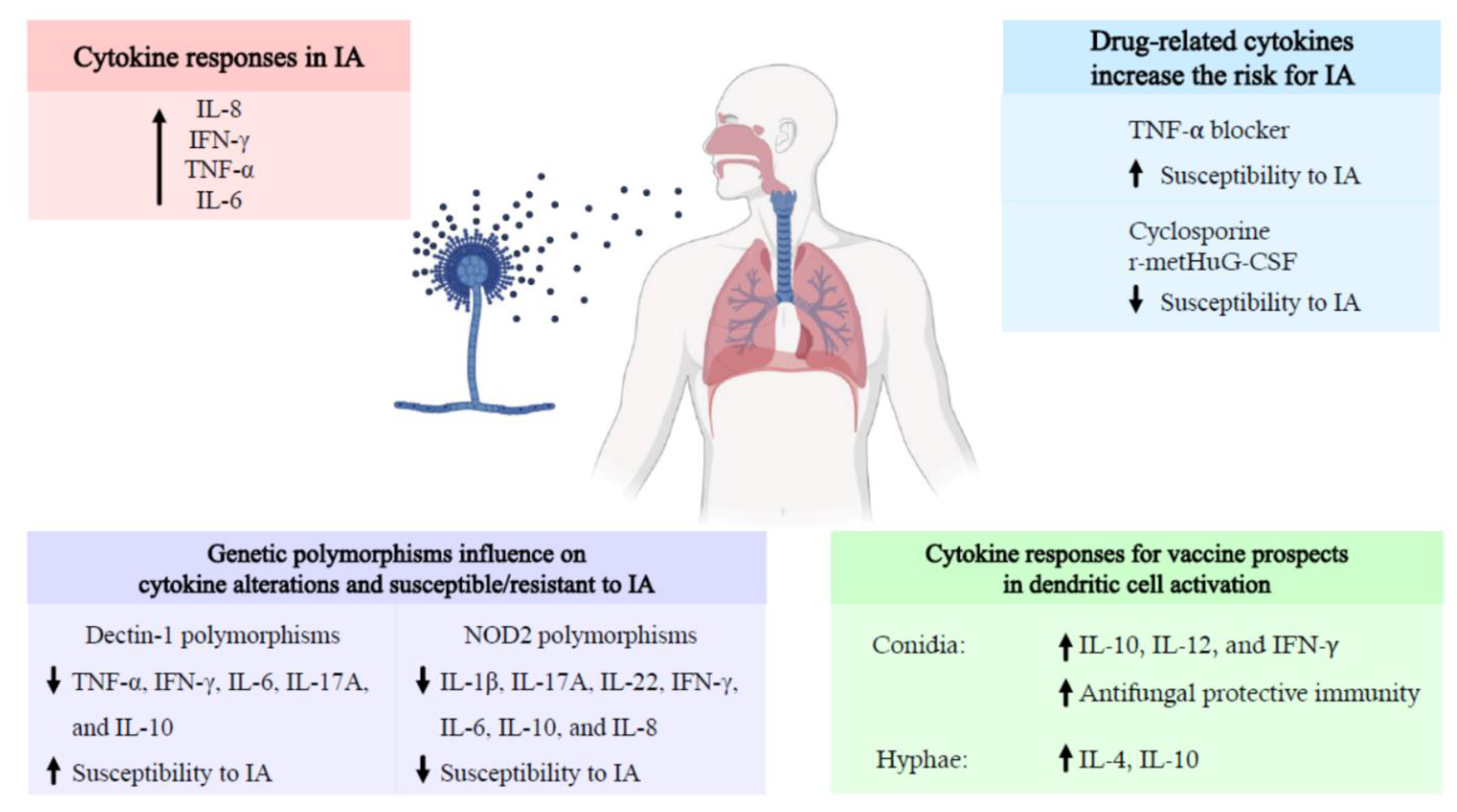
Cytokine and Chemokine Responses in Invasive Aspergillosis Following Hematopoietic Stem Cell Transplantation: Past Evidence for Future Therapy of Aspergillosis
Abstract
:1. Introduction
2. Cytokines and Chemokines Responses in Invasive Aspergillosis after Hematopoietic Stem Cell Transplantation
3. Genetic Polymorphisms in Hematopoietic Stem Cell Transplantation Patients Associated with Invasive Aspergillosis
4. Drug-Related Cytokine Alterations in Developing Invasive Aspergillosis in Hematopoietic Stem Cell Transplantation Patients
5. Pattern of Cytokine Production in Dendritic Cells (DCs) Activation for Vaccine Prospects against Invasive Aspergillosis in Hematopoietic Stem Cell Transplantation
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badiee, P.; Hashemizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204. [Google Scholar] [PubMed]
- Weaver, J.D.; Mullaney, E.J.; Lei, X.G. Altering the substrate specificity site of Aspergillus niger PhyB shifts the pH optimum to pH 3.2. Appl. Microbiol. Biotechnol. 2007, 76, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. Infection in solid-organ transplant recipients. N. Engl. J. Med. 2007, 357, 2601–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuehler, C.; Kuenzli, E.; Jaeger, V.K.; Baettig, V.; Ferracin, F.; Rajacic, Z.; Kaiser, D.; Bernardini, C.; Forrer, P.; Weisser, M.; et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation and association with occurrence and outcome of invasive aspergillosis. J. Infect. Dis. 2015, 212, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Wannemuehler, K.A.; Marr, K.A.; Hadley, S.; Kontoyiannis, D.P.; Walsh, T.J.; Fridkin, S.K.; Pappas, P.G.; Warnock, D.W. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: Interim results of a prospective multicenter surveillance program. Med. Mycol. 2005, 43 (Suppl. 1), S49–S58. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Paterson, D.L. Aspergillus infections in transplant recipients. Clin. Microbiol. Rev. 2005, 18, 44–69. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.J.; Schranz, J.; Teutsch, S.M. Aspergillosis case-fatality rate: Systematic review of the literature. Clin. Infect. Dis. 2001, 32, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Chotirmall, S.H.; Al-Alawi, M.; Mirkovic, B.; Lavelle, G.; Logan, P.M.; Greene, C.M.; McElvaney, N.G. Aspergillus-associated airway disease, inflammation, and the innate immune response. Biomed Res. Int. 2013, 2013, 723129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bader, N.; Sheppard, D.C. Aspergillosis and stem cell transplantation: An overview of experimental pathogenesis studies. Virulence 2016, 7, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Kirschning, C.J.; Nikolaus, T.; Wagner, H.; Heesemann, J.; Ebel, F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol. 2003, 5, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, S.; Moretti, S.; Perruccio, K.; Fallarino, F.; Bozza, S.; Montagnoli, C.; Mosci, P.; Lipford, G.B.; Pitzurra, L.; Romani, L. TLRs govern neutrophil activity in aspergillosis. J. Immunol. 2004, 173, 7406–7415. [Google Scholar] [CrossRef] [PubMed]
- Willment, J.A.; Brown, G.D. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008, 16, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, M.; Hayes, G.E.; Icheoku, U.J.; van Rhijn, N.; Denning, D.W.; Osherov, N.; Bignell, E.M. Anti-Aspergillus activities of the respiratory epithelium in health and disease. J. Fungi 2018, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Balloy, V.; Sallenave, J.M.; Wu, Y.; Touqui, L.; Latge, J.P.; Si-Tahar, M.; Chignard, M. Aspergillus fumigatus-induced interleukin-8 synthesis by respiratory epithelial cells is controlled by the phosphatidylinositol 3-kinase, p38 MAPK, and ERK1/2 pathways and not by the toll-like receptor-MyD88 pathway. J. Biol. Chem. 2008, 283, 30513–30521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, L.Y.; van de Veerdonk, F.; Marijnissen, R.J.; Cheng, S.C.; Khoo, A.L.; Hectors, M.; Lagrou, K.; Vonk, A.G.; Maertens, J.; Joosten, L.A.; et al. Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology 2010, 130, 46–54. [Google Scholar] [CrossRef]
- Cenci, E.; Mencacci, A.; Fe d’Ostiani, C.; Del Sero, G.; Mosci, P.; Montagnoli, C.; Bacci, A.; Romani, L. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 1998, 178, 1750–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reikvam, H.; Mosevoll, K.A.; Melve, G.K.; Gunther, C.C.; Sjo, M.; Bentsen, P.T.; Bruserud, O. The pretransplantation serum cytokine profile in allogeneic stem cell recipients differs from healthy individuals, and various profiles are associated with different risks of posttransplantation complications. Biol. Blood Marrow Transplant. 2012, 18, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Ceesay, M.M.; Kordasti, S.; Rufaie, E.; Lea, N.; Smith, M.; Wade, J.; Douiri, A.; Mufti, G.J.; Pagliuca, A. Baseline cytokine profiling identifies novel risk factors for invasive fungal disease among haematology patients undergoing intensive chemotherapy or haematopoietic stem cell transplantation. J. Infect. 2016, 73, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, S.F. Cytokine mediators of immunity and inflammation. Arch. Surg. 1993, 128, 1235–1241. [Google Scholar] [CrossRef]
- Shamriz, O.; Chandrakasan, S. Update on advances in hematopoietic cell transplantation for primary immunodeficiency disorders. Immunol. Allergy Clin. N. Am. 2019, 39, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Storek, J.; Geddes, M.; Khan, F.; Huard, B.; Helg, C.; Chalandon, Y.; Passweg, J.; Roosnek, E. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin. Immunopathol. 2008, 30, 425–437. [Google Scholar] [CrossRef]
- Ibrahim-Granet, O.; Philippe, B.; Boleti, H.; Boisvieux-Ulrich, E.; Grenet, D.; Stern, M.; Latge, J.P. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 2003, 71, 891–903. [Google Scholar] [CrossRef] [Green Version]
- Diamond, R.D.; Clark, R.A. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect. Immun. 1982, 38, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.S.; Rezvani, K. Immune reconstitution post allogeneic transplant and the impact of immune recovery on the risk of infection. Virulence 2016, 7, 901–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezger, M.; Kneitz, S.; Wozniok, I.; Kurzai, O.; Einsele, H.; Loeffler, J. Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J. Infect. Dis. 2008, 197, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Ok, M.; Latge, J.P.; Baeuerlein, C.; Ebel, F.; Mezger, M.; Topp, M.; Kurzai, O.; Killian, D.; Kapp, M.; Grigoleit, G.U.; et al. Immune responses of human immature dendritic cells can be modulated by the recombinant Aspergillus fumigatus antigen Aspf1. Clin. Vaccine Immunol. 2009, 16, 1485–1492. [Google Scholar] [CrossRef] [Green Version]
- Marischen, L.; Englert, A.; Schmitt, A.L.; Einsele, H.; Loeffler, J. Human NK cells adapt their immune response towards increasing multiplicities of infection of Aspergillus fumigatus. BMC Immunol. 2018, 19, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong-James, D.P.; Turnbull, S.A.; Teo, I.; Stark, J.; Rogers, N.J.; Rogers, T.R.; Bignell, E.; Haynes, K. Impaired interferon-gamma responses, increased interleukin-17 expression, and a tumor necrosis factor-alpha transcriptional program in invasive aspergillosis. J. Infect. Dis. 2009, 200, 1341–1351. [Google Scholar] [CrossRef] [Green Version]
- Namvar, S.; Warn, P.; Farnell, E.; Bromley, M.; Fraczek, M.; Bowyer, P.; Herrick, S. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin. Exp. Allergy 2015, 45, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A. Fungal infections in hematopoietic stem cell transplant recipients. Med. Mycol. 2008, 46, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Heidegger, S.; van den Brink, M.R.; Haas, T.; Poeck, H. The role of pattern-recognition receptors in graft-versus-host disease and graft-versus-leukemia after allogeneic stem cell transplantation. Front. Immunol. 2014, 5, 337. [Google Scholar] [CrossRef] [PubMed]
- Heldt, S.; Prattes, J.; Eigl, S.; Spiess, B.; Flick, H.; Rabensteiner, J.; Johnson, G.; Pruller, F.; Wolfler, A.; Niedrist, T.; et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: Performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J. Infect. 2018, 77, 235–241. [Google Scholar] [CrossRef]
- Goncalves, S.M.; Lagrou, K.; Rodrigues, C.S.; Campos, C.F.; Bernal-Martinez, L.; Rodrigues, F.; Silvestre, R.; Alcazar-Fuoli, L.; Maertens, J.A.; Cunha, C.; et al. Evaluation of bronchoalveolar lavage fluid cytokines as biomarkers for invasive pulmonary Aspergillosis in at-Risk patients. Front. Microbiol. 2017, 8, 2362. [Google Scholar] [CrossRef] [Green Version]
- Cunha, C.; Aversa, F.; Romani, L.; Carvalho, A. Human genetic susceptibility to invasive aspergillosis. PLoS Pathog. 2013, 9, e1003434. [Google Scholar] [CrossRef] [Green Version]
- Bartemes, K.R.; Kita, H. Innate and adaptive immune responses to fungi in the airway. J. Allergy Clin. Immunol. 2018, 142, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Galeas-Pena, M.; McLaughlin, N.; Pociask, D. The role of the innate immune system on pulmonary infections. Biol. Chem. 2019, 400, 443–456. [Google Scholar] [CrossRef]
- Re, F.; Strominger, J.L. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 2001, 276, 37692–37699. [Google Scholar] [CrossRef] [Green Version]
- Willment, J.A.; Lin, H.H.; Reid, D.M.; Taylor, P.R.; Williams, D.L.; Wong, S.Y.; Gordon, S.; Brown, G.D. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J. Immunol. 2003, 171, 4569–4573. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van der Vlist, M.; Wevers, B.; Bruijns, S.C.; Geijtenbeek, T.B. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol. 2009, 10, 203–213. [Google Scholar] [CrossRef]
- Mezger, M.; Steffens, M.; Beyer, M.; Manger, C.; Eberle, J.; Toliat, M.R.; Wienker, T.F.; Ljungman, P.; Hebart, H.; Dornbusch, H.J.; et al. Polymorphisms in the chemokine (C-X-C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem-cell transplantation and influence CXCL10 expression in monocyte-derived dendritic cells. Blood 2008, 111, 534–536. [Google Scholar] [CrossRef]
- Cunha, C.; Di Ianni, M.; Bozza, S.; Giovannini, G.; Zagarella, S.; Zelante, T.; D’Angelo, C.; Pierini, A.; Pitzurra, L.; Falzetti, F.; et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 2010, 116, 5394–5402. [Google Scholar] [CrossRef]
- Gresnigt, M.S.; Cunha, C.; Jaeger, M.; Goncalves, S.M.; Malireddi, R.K.S.; Ammerdorffer, A.; Lubbers, R.; Oosting, M.; Rasid, O.; Jouvion, G.; et al. Genetic deficiency of NOD2 confers resistance to invasive aspergillosis. Nat. Commun. 2018, 9, 2636. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Gresnigt, M.S.; Lecompte, T.; Bibert, S.; Manuel, O.; Joosten, L.A.; Rueger, S.; Berger, C.; Boggian, K.; Cusini, A.; et al. IL1B and DEFB1 Polymorphisms increase susceptibility to invasive mold infection after solid-organ transplantation. J. Infect. Dis. 2015, 211, 1646–1657. [Google Scholar] [CrossRef] [Green Version]
- Cunha, C.; Goncalves, S.M.; Duarte-Oliveira, C.; Leite, L.; Lagrou, K.; Marques, A.; Lupianez, C.B.; Mesquita, I.; Gaifem, J.; Barbosa, A.M.; et al. IL-10 overexpression predisposes to invasive aspergillosis by suppressing antifungal immunity. J. Allergy Clin. Immunol. 2017, 140, 867–870 e869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, L.Y.; de Boer, M.G.; van der Velden, W.J.; Plantinga, T.S.; van Spriel, A.B.; Jacobs, C.; Halkes, C.J.; Vonk, A.G.; Blijlevens, N.M.; van Dissel, J.T.; et al. The Y238X stop codon polymorphism in the human beta-glucan receptor dectin-1 and susceptibility to invasive aspergillosis. J. Infect. Dis. 2011, 203, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Zoran, T.; Weber, M.; Springer, J.; White, P.L.; Bauer, J.; Schober, A.; Loffler, C.; Seelbinder, B.; Hunniger, K.; Kurzai, O.; et al. Treatment with etanercept and low monocyte concentration contribute to the risk of invasive aspergillosis in patients post allogeneic stem cell transplantation. Sci. Rep. 2019, 9, 17231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, M.K.; Tansey, M.G. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J. Neuroinflamm. 2008, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Gattass, C.R.; King, L.B.; Luster, A.D.; Ashwell, J.D. Constitutive expression of interferon gamma-inducible protein 10 in lymphoid organs and inducible expression in T cells and thymocytes. J. Exp. Med. 1994, 179, 1373–1378. [Google Scholar] [CrossRef]
- Guo, Y.; Kasahara, S.; Jhingran, A.; Tosini, N.L.; Zhai, B.; Aufiero, M.A.; Mills, K.A.M.; Gjonbalaj, M.; Espinosa, V.; Rivera, A.; et al. During Aspergillus infection, monocyte-derived DCs, neutrophils, and plasmacytoid DCs enhance innate immune defense through CXCR3-dependent crosstalk. Cell Host Microbe 2020, 28, 104–116 e104. [Google Scholar] [CrossRef]
- Tramsen, L.; Schmidt, S.; Roeger, F.; Schubert, R.; Salzmann-Manrique, E.; Latge, J.P.; Klingebiel, T.; Lehrnbecher, T. Immunosuppressive compounds exhibit particular effects on functional properties of human anti-Aspergillus Th1 cells. Infect. Immun. 2014, 82, 2649–2656. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.B.; Nishimura, R.D.; Zimmerman, R.P.; Tran, L.; Shiplacoff, J.; Tormey, M.; Contreras, R.; Juillard, G.F. Filgrastim (r-metHuG-CSF) and its potential use in the reduction of radiation-induced oropharyngeal mucositis: An interim look at a randomized, double-blind, placebo-controlled trial. Cytokines Cell. Mol. Ther. 1999, 5, 175–180. [Google Scholar] [PubMed]
- Pursell, K.; Verral, S.; Daraiesh, F.; Shrestha, N.; Skariah, A.; Hasan, E.; Pitrak, D. Impaired phagocyte respiratory burst responses to opportunistic fungal pathogens in transplant recipients: In vitro effect of r-metHuG-CSF (Filgrastim). Transpl. Infect. Dis. 2003, 5, 29–37. [Google Scholar] [CrossRef]
- Kandalla, P.K.; Sarrazin, S.; Molawi, K.; Berruyer, C.; Redelberger, D.; Favel, A.; Bordi, C.; de Bentzmann, S.; Sieweke, M.H. M-CSF improves protection against bacterial and fungal infections after hematopoietic stem/progenitor cell transplantation. J. Exp. Med. 2016, 213, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Sato, K.; Akiyama, H.; Sao, H.; Okamoto, S.; Kobayashi, N.; Hara, M.; Kawa, K.; Motoyoshi, K.; Japan Marrow Donor Program. M-CSF attenuates severity of chronic GVHD after unrelated BMT. Bone Marrow Transplant. 2012, 47, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Cell mediated immunity to fungi: A reassessment. Med. Mycol. 2008, 46, 515–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigot, J.; Guillot, L.; Guitard, J.; Ruffin, M.; Corvol, H.; Balloy, V.; Hennequin, C. Bronchial epithelial cells on the front line to fight lung infection-causing Aspergillus fumigatus. Front. Immunol. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Espinosa, V.; Rivera, A. First line of defense: Innate cell-mediated control of pulmonary Aspergillosis. Front. Microbiol. 2016, 7, 272. [Google Scholar] [CrossRef] [Green Version]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [Green Version]
- Perruccio, K.; Bozza, S.; Montagnoli, C.; Bellocchio, S.; Aversa, F.; Martelli, M.; Bistoni, F.; Velardi, A.; Romani, L. Prospects for dendritic cell vaccination against fungal infections in hematopoietic transplantation. Blood Cells Mol. Dis. 2004, 33, 248–255. [Google Scholar] [CrossRef]
- Bozza, S.; Gaziano, R.; Spreca, A.; Bacci, A.; Montagnoli, C.; di Francesco, P.; Romani, L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 2002, 168, 1362–1371. [Google Scholar] [CrossRef] [Green Version]
- Bozza, S.; Perruccio, K.; Montagnoli, C.; Gaziano, R.; Bellocchio, S.; Burchielli, E.; Nkwanyuo, G.; Pitzurra, L.; Velardi, A.; Romani, L. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 2003, 102, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Grazziutti, M.; Przepiorka, D.; Rex, J.H.; Braunschweig, I.; Vadhan-Raj, S.; Savary, C.A. Dendritic cell-mediated stimulation of the in vitro lymphocyte response to Aspergillus. Bone Marrow Transplant. 2001, 27, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Bellocchio, S.; Bozza, S.; Montagnoli, C.; Perruccio, K.; Gaziano, R.; Pitzurra, L.; Romani, L. Immunity to Aspergillus fumigatus: The basis for immunotherapy and vaccination. Med. Mycol. 2005, 43 (Suppl. 1), S181–S188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, M.; Santiago, L.; Vidal-Garcia, M.; Redrado, S.; Lanuza, P.; Comas, L.; Domingo, M.P.; Rezusta, A.; Galvez, E.M. Preparations for invasion: Modulation of host lung immunity during pulmonary Aspergillosis by Gliotoxin and other fungal secondary metabolites. Front. Immunol. 2018, 9, 2549. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; De Luca, A.; Bonifazi, P.; Montagnoli, C.; Bozza, S.; Moretti, S.; Belladonna, M.L.; Vacca, C.; Conte, C.; Mosci, P.; et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 2007, 37, 2695–2706. [Google Scholar] [CrossRef]
- Zelante, T.; Bozza, S.; De Luca, A.; D’Angelo, C.; Bonifazi, P.; Moretti, S.; Giovannini, G.; Bistoni, F.; Romani, L. Th17 cells in the setting of Aspergillus infection and pathology. Med. Mycol. 2009, 47 (Suppl. 1), S162–S169. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.B.; Taborda, C.P.; Nosanchuk, J.D. Advances in Fungal Peptide Vaccines. J. Fungi 2020, 6, 119. [Google Scholar] [CrossRef]
- Claudia, M.; Bacci, A.; Silvia, B.; Gaziano, R.; Spreca, A.; Romani, L. The interaction of fungi with dendritic cells: Implications for Th immunity and vaccination. Curr. Mol. Med. 2002, 2, 507–524. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, W.; Hayek, S.S.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Association between early treatment with Tocilizumab and mortality among critically Ill patients with COVID-19. JAMA Intern. Med. 2021, 181, 41–51. [Google Scholar] [CrossRef]
| Models | Samples | Methods | Major Findings | Interpretation | Ref. | ||
|---|---|---|---|---|---|---|---|
| Cytokines | Chemokines | Others | |||||
| In vitro | |||||||
| iDC + A. fumigatus-small germinating conidia (6 h of stimulation) | Infected iDCs | qRT-PCR |
|
|
| A. fumigatus germ tubes induced the expression of genes associated with recognition and phagocytosis in iDCs with a time-dependent manner. | [25] |
| iDC + A. fumigatus antigen Aspf1 | Infected iDCs | qRT-PCR |
|
|
| Aspf1, a member of a family of conserved RNases, induces a pro-inflammatory cytokine response. | [26] |
| NK cells obtained from PBMCs + A. fumigatus hyphae (6 h of stimulation) | Infected NK cells | qRT-PCR |
|
|
| NK cells reveal the expression and release of immunomodulatory molecules involved in antifungal immune responses. | [27] |
| In vivo | |||||||
| Mice CD1 strain infected by intranasal instillation with A. fumigatus conidia (N = 24) | Mouse whole-lung homogenates |
| Immunpetent mice: Infted vs. Saline controls
| Susceptibility to IA is associated with a high level of TNF-α at the site of infection and the upregulation of a network of TNF-α–related genes. | [28] | ||
| Immunosuppressed mice: Infected vs. Saline controls
| |||||||
| Immunocompetent vs. Immunosuppressed mice
| |||||||
| BALB/c mice infected by intranasal instillation with A. fumigatus proteases, Asp f 5 and Asp f 13 (N = 20) | Mouse lung homogenates |
| Infected vs. PBS controls
| A. fumigatus secreted allergen proteases, Asp f 5 and Asp f 13, are important for induction of Th2 cytokines secretion and increased IgE levels, which are fundamental features of allergic asthma and an indication of disease severity. | [29] | ||
| Clinical study | |||||||
| Adult hematology patients with proven/probable IFD (N = 172) | Serum | ELISA |
|
| − | High IL-2R and CCL2 concentrations as indicators for the risk of developing IFD. | [18] |
| Adult hematology patients with probable/possible IA (N = 43) |
| ELISA | Serum
| BAL
|
| High serum IL-8 levels were highly specific and highly sensitive for the diagnosis of IA. | [32] |
| Patients diagnosed with IA (N = 48) |
| ELISA | BAL
| BAL
|
| Alveolar cytokines might be useful in supporting current diagnostic approaches for IPA biomarkers. IL-8 was the best performing analyte with the most relevant discriminator between cases of IPA and controls. | [33] |
| Models | Polymorphism | Major Findings | Interpretation | Ref. | |
|---|---|---|---|---|---|
| Cytokines | Others | ||||
| In vitro | |||||
| PBMCs | Dectin-1 Y238X Stop Codon Polymorphism + heat-killed A. fumigatus hyphae + live A. fumigatus conidia |
|
| Dectin-1 Y238X resulted in the reduction of pro-inflammatory cytokines due to the Dectin-1 receptor, which is known to play a role in fungal cell wall β-glucan recognition. | [40] |
| BEAS-2B (Respiratory epithelial cells) | Dectin-1 blockade by siRNA + Stimuli (β-glucan or Aspergillus conidia) |
| − | Dectin-1 expressed on epithelial cells contributes to the production of cytokines. | [41] |
| PBMCs from allogeneic HSCT | NOD2 genetic variation - P268S (TT-genotype) + A. fumigatus conidia - complete NOD deficiency + A. fumigatus conidia | Infected in TT-genotype compared with infected in CC-and CT-genotype
| Aspergillus infected compared with uninfected
| Human NOD2 deficiency reduces Aspergillus-induced inflammatory cytokines. | [42] |
| Human PMBCs from solid-organ transplant recipients |
| IL1B rs16944 SNP
|
| Both IL1B rs16944 and IL1RN rs419598 SNPs effect Aspergillus-induced cytokine release. | [43] |
| Macrophages from healthy blood donors | IL10 SNP with GG genotype + A. fumigatus conidia |
|
| IL-10 overexpression influences IA by suppressing antifungal immunity. | [44] |
| In vivo | |||||
| BALB/c mice with HSCT + Aspergillus (N = 16) | Dectin-1 knockout mice |
|
| Dectin-1 modulates immunity and tolerance via IFN-γ / IL-10 production, and both cytokines activate the protection of Th1/Treg antifungal responses. | [41] |
| Nod2-deficient (Nod2-/-) C57BL/6 mice + Aspergillus (lethal dose) (N = 22) | Nod2-/- deficient mice (Splenocytes) |
|
| NOD2 augments Aspergillus-induced cytokine responses and results in resistance to Aspergillus infection. | [42] |
| Clinical study | |||||
| Patients who developed IA post HSCT (N = 71) Non-HSCT patients with IA (N = 21) | Y238X Stop Codon Polymorphism |
|
| Dectin-1 Y238X heterozygosity had a limited influence on susceptibility to IA. | [45] |
| Hematological patients undergoing allogeneic HSCT (N = 310) | NOD2 genetic variation - P268S SNP |
|
| Genetic deficiency of NOD2 results in an alteration of cytokine production in response to Aspergillus infection. | [42] |
| An allograft with IA (N = 81) or without IA (N = 58) | CXCL10 genetic variation - C+11101T - C+1642G - A1101G |
|
| Polymorphisms in CXCL10 altered chemokine secretion and increased the risk of IA after alloSCT. | [40] |
| Models | Study Protocol | Study Pethods | Major Findings | Interpretation | Ref. | ||
|---|---|---|---|---|---|---|---|
| Cytokines | Chemokines | Others | |||||
| In vitro | |||||||
| Human MDM | A. fumigatus (MOI 0.5) with 2 µg/mL of TNF-α blocker, Etanercept for 6 h |
|
|
|
| Etanercept lowered inflammatory cytokines and chemokines as well as downregulated genes involved in TNF-α signaling, which offers new data regarding risk factors for IA and the administration of etanercept. | [46] |
| PBMCs from healthy volunteers (N = 8) | Generation of anti-Aspergillus Th1 cells + Cyclosporine |
|
| − |
| Cyclosporine suppresses human anti-Aspergillus Th1 cells. | [50] |
| Neutrophils | + A. fumigatus +/− r-metHuG-CSF | FACS analysis | − | − |
| G-CSF enhances the activities of neutrophils against Aspergillus spp. | [52] |
| In vivo | |||||||
| Irradiated and HS/PC-transplanted mice (N = 13) | 10 µg of recombinant M-CSF + A. fumigatus |
| − | − |
| M-CSF has a beneficial effect against severe infections after transplantation. | [53] |
| Clinical study | |||||||
| Serum samples from patients with probable IA (N = 8) | TNF-α blocker treatment | ELISA |
| − | − | TNF-α blocker reduces CXCL10 serum concentrations in patients with probable IA. | [46] |
| Models | Study protocol | Major Findings in DCs Pulsedwith Fungal Morphotypes | Interpretation | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Conidia | Conidial RNA | Hyphae | Hyphal RNA | Others | ||||
| In vitro | ||||||||
| Murine DCs | A. fumigatus application for 24 h |
|
|
|
|
| Murine DCs - mainly produced IL-12 in response to conidia or the corresponding RNA - produced IL-4/IL-10 in response to hyphae or the corresponding RNA. | [59] |
| Human Myeloid DCs (MDCs) | 1. A. fumigatus application for 24 h 2. A. fumigatus + activated cytokines producing CD4+ Th cells | Group 1
| Group 2
| Group 1
| Group 2
| − | MDCs mainly produced IL-12 after Aspergillus infection. Upon pulsing with conidia, MDCs mainly activated IFN-γ producing CD4+ Th1 cells. | [59] |
| Human plasmacytoid DCs (PDCs) | 1. A. fumigatus application for 24 h 2. A. fumigatus + activated cytokines producing CD4+ Th cells | Group 1
| Group 1
| Group 2
| Group 1
| − | PDCs produced IL-10 and IFN-α in response to Aspergillus fumigatus. Upon pulsing with conidia, PDCs mainly activated IFN-γ- and IL-10-producing CD4+ cells. | [59] |
| Murine lung myeloid DCs | A. fumigatus application for 24 h |
| − |
| − | − | Upon exposure to A. fumigatus conidia or hyphae, pulmonary DC differentially produce IL-12 and IL-4/IL-10. | [60] |
| Murine DCs | A. fumigatus application for 24 h |
|
|
|
| − | Murine DC produced mainly IL-12 in response to conidia and IL-4 and IL-10 in response to hyphae. | [61] |
| Human DCs | A. fumigatus application for 24 h |
| − | − | − |
| DCs produced IL-12 in response to A. fumigatus conidia. | [62] |
| Cocultures of autologous DCs with lymphocytes | A. fumigatus application for 24 h |
| − | − | − | − | A. fumigatus stimulation of lymphocytes through autologous DC results in a type-1 polarization (protection against aspergillosis). | |
| Human DCs | 1. recombinant A. fumigatus antigens + 18-kDa RNase Aspf1 2. recombinant A. fumigatus antigens + putative glycosidase Crf1 | − | − | − | − | Group 1
| The interactions between human immature dendritic cells and A. fumigatus antigens triggered the increased level of expression of genes encoding pro-inflammatory cytokines and chemokines. | [26] |
| Adoptive transferred Aspergillus-pulsed DCs in vivo | ||||||||
| Murine splenic DCs | Pulsed with Aspergillus conidia and administered into recipient HSCT mice | − |
| − | − |
| Adoptively transferred fungus RNA-transfected dendritic cells induce Th1-mediated resistance to fungal infections in mice with allogeneic HSCT. | [59] |
| Murine splenic DCs | Pulsed with Aspergillus and administered into recipient HSCT mice |
|
|
|
| − | Adoptively transferred fungus pulsed dendritic cells induce TH priming to the fungus in vivo. | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thammasit, P.; Sripetchwandee, J.; Nosanchuk, J.D.; Chattipakorn, S.C.; Chattipakorn, N.; Youngchim, S. Cytokine and Chemokine Responses in Invasive Aspergillosis Following Hematopoietic Stem Cell Transplantation: Past Evidence for Future Therapy of Aspergillosis. J. Fungi 2021, 7, 753. https://doi.org/10.3390/jof7090753
Thammasit P, Sripetchwandee J, Nosanchuk JD, Chattipakorn SC, Chattipakorn N, Youngchim S. Cytokine and Chemokine Responses in Invasive Aspergillosis Following Hematopoietic Stem Cell Transplantation: Past Evidence for Future Therapy of Aspergillosis. Journal of Fungi. 2021; 7(9):753. https://doi.org/10.3390/jof7090753
Chicago/Turabian StyleThammasit, Patcharin, Jirapas Sripetchwandee, Joshua D. Nosanchuk, Siriporn C. Chattipakorn, Nipon Chattipakorn, and Sirida Youngchim. 2021. "Cytokine and Chemokine Responses in Invasive Aspergillosis Following Hematopoietic Stem Cell Transplantation: Past Evidence for Future Therapy of Aspergillosis" Journal of Fungi 7, no. 9: 753. https://doi.org/10.3390/jof7090753